Abstract
Purpose: This study aimed to determine the efficacy of the integrative group-based cognitive rehabilitation programme, REHACOP, on improving cognitive functions in multiple sclerosis (MS).
Methods: Fourty-two MS patients were randomized to the treatment programme REHACOP (n = 21) or waiting list control condition (n = 21). The REHACOP group received cognitive rehabilitation in group format for three months focused on attention, processing speed, learning and memory, language, executive functioning, and social cognition. Patients completed a neuropsychological assessment at baseline and follow-up, which included tests of attention, processing speed, working memory, verbal memory, verbal fluency, and executive functioning. Repeated measures multivariate analysis of covariance (MANCOVA) was used to determine the efficacy of the cognitive rehabilitation programme.
Results: Group × Time interactions revealed significant improvements in the REHACOP group as compared with the control group for processing speed (p = 0.011, np2 = 0.16), working memory (p = 0.014, np2 = 0.15), verbal memory (p = 0.025, np2 = 0.13), and executive functioning (p = 0.024, np2 = 0.13), showing medium–large effect sizes.
Conclusions: Patients receiving REHACOP showed improvements in several cognitive domains. This preliminary study thus provides evidence supporting the efficacy of this integrative group-based cognitive rehabilitation intervention in MS. Future research should confirm these findings, examine the impact of the treatment on everyday life functioning and explore the presence of brain changes associated with cognitive rehabilitation.
This study provides initial evidence for integrative group-based cognitive rehabilitation efficacy in MS patients through the implementation of the REHACOP cognitive rehabilitation programme.
Patients received cognitive rehabilitation for three months (3 one-hour-sessions per week) focused on training attention, learning and memory, language, executive functioning, and social cognition.
Patients attending REHACOP sessions showed medium to large and statistically significant improvements in processing speed, working memory, verbal memory, and executive functioning.
Implications for rehabilitation
Introduction
Multiple sclerosis (MS) is a chronic, autoimmune, and neurodegenerative disorder that is characterized by the inflammation and progressive demyelination of the central nervous system. It is well-known that MS often results in cognitive impairment, even at early stages of the disease, with a prevalence ranging from 43% to 70% of patients.[Citation1] Patients’ neuropsychological profile involves deficits in attention, working memory, processing speed, verbal memory, verbal fluency, and executive functioning.[Citation1]
The presence of cognitive impairment in MS has been associated with difficulties in daily life activities,[Citation2,Citation3] unemployment,[Citation4] declining engagement in social and leisure activities,[Citation3,Citation5] as well as reduced quality of life,[Citation6–8] and health perception.[Citation9] Moreover, pharmacological treatments have shown inconsistent findings in improving cognitive deficits in MS.[Citation10] This inconsistency regarding the effects of pharmacological treatments, coupled with the wide range of daily life aspects negatively impacted by the disorder, highlights the need for using a potentially more effective approach for developing interventions that address cognitive deficits in MS patients.
While there is some evidence to support cognitive rehabilitation in persons with MS, this literature is marred by numerous methodological limitations.[Citation11–13] These include: the lack of an objective cognitive status assessment at baseline, lack of appropriate controls, using inappropriate randomization methods, inconsistency among cognitive rehabilitation targets, and outcome measures, etc.[Citation13] However, there are several well-designed studies that have yielded positive results. For instance, Chiaravalloti et al.[Citation14,Citation15] provided class I evidence showing that a specific intervention can significantly improve neuropsychological performance, everyday functional activity and result in increased functional brain activation and connectivity.
Traditionally, cognitive interventions for persons with MS have focused on a single or a few specific domains despite the diverse cognitive functions that may be impacted by the disease. However, recent studies have started to implement more integrative approaches (interventions that aim to improve overall cognition) with promising results.[Citation16–18] In addition, few studies have implemented group cognitive rehabilitation in MS despite the fact that the group format offers several clinical advantages over the traditional 1-on-1 approach such as increased efficiency, decreased cost, and social interaction among patients during therapy.[Citation19–22] Therefore, there is a need for rigorous studies that implement integrative group-based cognitive interventions in MS.
The REHACOP is an integrative cognitive rehabilitation programme,[Citation23] that trains several cognitive functions including attention, processing speed, learning and memory (working memory), language, and executive functioning. Its efficacy in group format has previously been demonstrated for schizophrenia,[Citation24] and Parkinson’s disease.[Citation25] Schizophrenia, Parkinson’s disease, and MS are very dissimilar neurological diseases characterized by particular etiologies, illness courses as well as symptomatology.[Citation26–28] Cognitive impairment also differs among these pathologies in several features (e.g., prevalence, progression, severity, etc.).[Citation1,Citation29,Citation30] Nevertheless, patients who are diagnosed with any of these disorders may present cognitive decline in attention, processing speed, working memory, episodic memory, verbal fluency, and executive functioning.[Citation1,Citation29,Citation30] Thus, it is expected that treatment with REHACOP would result in improved cognitive performance in MS as well.
The aim of the current study was to determine the efficacy of the REHACOP integrative group-based cognitive rehabilitation programme for improving cognitive functioning in MS. The primary outcome measures were several Z scores assessing the impact of the REHACOP on attention, processing speed, working memory, verbal memory, verbal fluency, and executive functioning in MS patients.
Methods
Participants
Forty-two outpatients with clinically definite MS were recruited from Cruces and Basurto University Hospitals, in Biscay, Spain. Patients were randomly assigned to the treatment programme REHACOP (n = 21) or waiting list control condition (n = 21). Both groups’ socio-demographic and clinical characteristics are provided in .
Table 1. Socio-demographic and clinical characteristics of the sample at baseline.
All patients met criteria for the diagnosis of MS according to McDonald et al. [Citation31] Additional study inclusion criteria were: (i) patients aged between 20 and 60 years; (ii) with relapsing-remitting, secondary progressive or primary progressive MS; and (iii) with or without cognitive deficits. Exclusion criteria were as follows: (i) the presence of dementia as defined by a Mini Mental State Examination Test [Citation32] score lower than 24; (ii) having suffered an exacerbation during the month prior to the cognitive assessment; (iii) being treated with corticosteroids during study participation; (iv) the presence of another relevant neurological disorder; (v) history of stroke or traumatic brain injury resulting in a loss of consciousness for more than 30 min; or (vi) the presence of psychiatric disorders.
Procedure
This study was approved by the Ethics Committee at the Health Department of the Basque Mental Health System in Spain. The trial was registered in clinicaltrials.gov (NCT02287454). All patients completed the written informed consent prior to their involvement in the study. Individual numeric identifiers were assigned to each patient to ensure confidentiality.
A priori power analysis was conducted to determine the sample size based on a previous study [Citation33] using the G*Power 3 software.[Citation34] A sample size of 42 subjects, 21 in each group, was sufficient to attain an effect size of 0.88 to detect between-group differences in each cognitive domain, with 80% power and a 0.5% level of significance. The study design was a non-blinded parallel-group randomized trial with equal randomization. Patients were informed of the opportunity to participate in the study by their neurologists. The recruitment and enrollment were conducted in several periods from January to March 2013, from January to April 2014, and from May to September 2015. All patients underwent a neurologic and neuropsychological assessment at baseline and 3 month follow-up. Patients were randomly assigned to each study condition using an online computer-generated random number at a ratio of 1:1. The REHACOP group received cognitive rehabilitation for three months, which included three one-hour-sessions per week (total number of sessions = 39). Five patients from the experimental condition also participated in private cognitive rehabilitation during their participation in the study, attending a mean of 10 sessions (45 min each) mainly focused on short-term memory. Post-treatment assessment (finished by July 2013, July 2014, and December 2015) was performed within the first week after completing the intervention. The control group was assigned to a waiting list, which provided an opportunity to participate in the cognitive rehabilitation programme once they completed the study.
Instruments
Patients completed an extensive neuropsychological battery both prior to and within one week of completing the treatment phase. This included tests of attention, processing speed, working memory, verbal memory, verbal fluency, and executive functioning. Additionally, several clinical scales were administered to examine the equality of both groups on other relevant variables at baseline.
Attention. The Brief Test of Attention (BTA) was used to measure patients’ attentional abilities.[Citation35] This test consists of two parallel forms, numbers and letters, in which the same 10 sequences of random digits and characters were auditory presented at the rate of one stimulus per second. Patients were instructed to count how many digits or characters were read aloud in the numbers and letters forms, respectively.
Processing speed. The Symbol Digit Modalities Test (SDMT),[Citation36] the Trail Making Test A (TMT-A),[Citation37] and the Salthouse Perceptual Comparison Test (PCT) [Citation38] were utilized to quantify information processing speed. In the SDMT several symbols are presented in a sheet of paper, each of which is associated with a specific number. Patients were asked to assign the corresponding number to several symbols using the reference key. The time limit for the completion of the test is 90 s. The TMT-A consists of 25 circles numbered from 1 to 25 and distributed over a sheet of paper. Patients were instructed to draw lines to connect the numbers in ascending order as fast as possible. The time for completion of the test was registered. The PCT required patients to compare pairs of three and six letter sequences to one another and to write an “I” if both sequences were identical or a “D” if they were different. The time limit for each form is 30 s.
Working memory. The Backward Digits subtest (BD) of the Wechsler Adult Intelligence Scale III was employed to assess working memory.[Citation39] The BD is composed by seven sequences of numbers that the examiner reads aloud at the rate of one digit per second. Patients were instructed to repeat aloud the sequences in reverse order.
Verbal learning and memory. This cognitive domain was assessed with the Hopkins Verbal Learning Test-Revised (HVLT-R) learning and long-term recall scores.[Citation40] The HVLT-R consists of a list of 12 nouns that the examiner read three times at a rate of one word per second. Patients were required to recall them immediately after each reading. In the delayed recall, completed 20 min later, patients were asked to freely recall the list of nouns. Different alternate, equivalent forms of the HVLT-R were administered at the baseline and follow-up assessment to avoid a learning effect.
Verbal fluency. Semantic and phonemic fluency was assessed by the Calibrated Ideational Fluency Assessment (CIFA).[Citation41] In the category-cued task patients were asked to report words related to animals and supermarket semantic categories for the period of 1 min each. In the letter-cued task patients were instructed to report as many words beginning with the letter "P" as they could during 3 min.
Executive functioning. This cognitive domain was measured by the Stroop Color-Word Test, color-word and interference scores.[Citation42] In the color-word response inhibition trial, a sheet with color words printed in incongruent colors is presented to the examinee. Patients were instructed to name the color ink in which each word was presented. The time limit for this trial is 45 s.
Disability status. The Expanded Disability Status Scale (EDSS) was used to rate patients’ disability status.[Citation43] Higher scores represent a higher degree of disability.
Premorbid IQ and cognitive reserve. The premorbid IQ was tested by the Accentuation Reading Test (TAP),[Citation44] the National Adult Reading Test Spanish version. Cognitive reserve was estimated with the Cognitive Reserve Questionnaire (CRQ),[Citation45] a 15-item multiple-choice questionnaire that includes questions about education/culture, working activity, leisure and hobbies, as well as physical and social activities. Higher scores indicate a better premorbid IQ and greater cognitive reserve.
Depressive symptoms. The 15-item Geriatric Depression Scale (GDS) assessed patients’ depressive symptoms.[Citation46] Higher scores represent a higher degree of depression.
Fatigue. The Fatigue Severity Scale (FSS) [Citation47] and mental fatigue Visual Analog Scale (VAS) were employed to explore physical and mental fatigue, respectively. In both scales higher scores show a higher degree of fatigue.
Intervention
REHACOP is an integrative cognitive rehabilitation programme based on the principles of restoration, compensation and optimization. REHACOP uses a bottom-up approach and a final integration with top-down tasks as part of the activities of daily living module. That is, treatment begins with remediation of basic cognitive processes, gradually advancing to more complex cognitive domains, and finishes with daily living complex tasks that integrate the utilization of several more basic cognitive domains. This cognitive rehabilitation programme allows for individual or group format and is composed of up to 300 paper and pen tasks divided into eight consecutive modules: attention, learning and memory, language, executive functions, social cognition, social skills, activities of daily living, and psycho-education. Processing speed is also trained in the first four modules, because several tasks are timed. Tasks within each module are hierarchically arranged by ability subtypes and difficulty levels to ensure an increasing level of cognitive demand.[Citation23]
MS patients in the REHACOP group attended cognitive rehabilitation at the Multiple Sclerosis Association of Biscay (ADEMBI). Cognitive rehabilitation was implemented in group format. Four groups, with between 5 and 8 patients each, were conducted. Two neuropsychologists, trained in the administration of the protocol, conducted the cognitive rehabilitation using the same materials and instructions. Patients attended each session with the remaining members of their group. The neuropsychologists provided instructions of each paper–pencil task to the whole group, and subsequently, patients individually performed the task. The correction processes were carried out with the collaboration of the entire group. Once the correction process was finished, patients could share with the remaining members of the group the difficulties encountered and the strategies employed during the task.
The cognitive rehabilitation schedule included: four weeks of training in attention (sustained, selective, alternating, and divided attention); three weeks focused on learning and memory (verbal and visual memory stimulation was combined with learning and compensatory strategies; training was also provided in working memory); three weeks focused on language (verbal fluency, syntax, grammar, vocabulary, and comprehension); three weeks exercising executive functioning (objectives planning and attainment, verbal reasoning, categorization, and conceptualisation); and one week of training in social cognition (social reasoning, theory of mind, and moral dilemmas). Additionally, patients also performed tasks at home three times a week during the learning and memory module to promote the generalization of the use of learning strategies to daily life activities. Tasks performed at home included, for instance, writing a diary describing what they had done two days before, doing shopping without using a list or reading a piece of news to explain it in the next session.
Statistical analysis
Due to the use of multiple measures to assess the cognitive functions, composite scores were calculated for processing speed, verbal memory, verbal fluency, and executive functioning. Composite scores were calculated after all raw cognitive scores were converted into z-values based on the pooled MS group and the TMT-A scores were recoded (higher scores indicated a better performance). The Cronbach’s alpha coefficient was utilized to determine the internal consistency of each composite score. The normal distribution of the data was verified by the Shapiro–Wilk test. To examine the differences between the REHACOP and the control group in socio-demographic and clinical variables at baseline Chi-square test (χ2) and multivariate analysis of variance (MANOVA) or Mann–Whitney U-test were used for categorical and quantitative variables, respectively.
A multivariate analysis of covariance (MANCOVA) was used to analyze cognitive differences between groups at baseline while controlling for the potential effects of physical and mental fatigue. Finally, Group × Time interaction effects from a multivariate repeated measures analysis of covariance (repeated measures MANCOVA) determined cognitive rehabilitation efficacy. To analyze the influence of the presence of patients with and without cognitive impairment, which could benefit differently from the cognitive intervention, a dichotomous covariate indicating patientś cognitive status was introduced in the model (along with physical and mental fatigue scores). Patients’ cognitive status was established according to the SDMT performance.[Citation48] SDMT raw scores were converted into standardized scores based on forty healthy controls matched for age, gender, and education with the MS patients. Patients with a raw score below 1.5 SD regarding the mean of the healthy control group were considered as cognitively impaired, while those with a raw score above that threshold were considered as cognitively preserved. The repeated measures MANCOVA also controlled by default for between-group cognitive differences at baseline.[Citation49] The significance level was set at 0.05. Partial eta squared was obtained as an indicator of the effect size.
Results
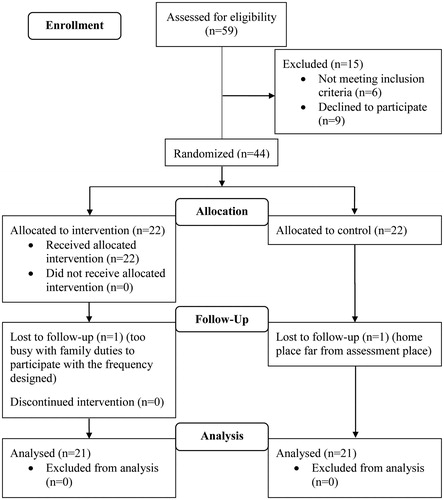
Forty-two patients completed their participation in the study. Only two patients (one from each condition) did not complete the study, resulting in an attrition rate of 3.85% (). Regarding the sociodemographic and clinical variables, differences between the REHACOP and control group were not found at baseline ().
All raw cognitive test scores are provided in . Good internal consistency was obtained for processing speed (α = 0.88), verbal memory (α = 0.86), verbal fluency (α = 0.85), and executive functioning (α = 0.77) composites scores. There were significant differences between the REHACOP and control group at baseline in attention, working memory, processing speed, and verbal memory, but not in verbal fluency or executive functioning (). The REHACOP group showed greater cognitive impairment than the control group in all assessed cognitive domains at baseline.
Table 2. Raw cognitive performance scores for the REHACOP and control group at baseline and follow-up.
Table 3. MANCOVA for cognitive performance in the REHACOP and control group at baseline.
Repeated measures MANCOVA Group × Time interactions revealed significant differences between the REHACOP and control group for processing speed, working memory, verbal memory, and executive functioning (). The REHACOP group significantly improved on all mentioned cognitive domains compared with the control group (). Effect sizes were large for processing speed and working memory. The remaining cognitive domains showed medium effect sizes. This pattern of improvement was also observed in attention and verbal fluency (), but the results were not significant.
Figure 2. Cognitive performance (Z scores) in the REHACOP and control group at baseline and follow-up.
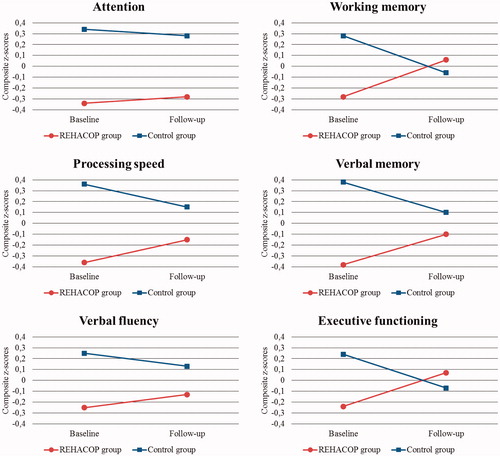
Table 4. Repeated measures MANCOVA for cognitive performance in the REHACOP and control group at baseline and follow-up.
Furthermore, to rule out the possible bias toward a favorable outcome for the experimental condition, an additional repeated measures MANCOVA was performed excluding the five patients who participated in private cognitive rehabilitation. Group × Time interactions remained the same, indicating that patients receiving only REHACOP exhibited processing speed (p = 0.014; np2 =0.18), working memory (p = 0.019; np2 =0.16), verbal memory (p = 0.017; np2 =0.17), and executive functioning (p = 0.040; np2 =0.13) improvements even after excluding patients receiving private cognitive rehabilitation.
Discussion
The purpose of this study was to examine the efficacy of an integrative cognitive rehabilitation programme in group format and look at individual outcomes. Significant cognitive improvements were detected for the treatment group in processing speed, working memory, verbal memory, and executive functioning after REHACOP implementation, with medium and large effect sizes. The results show that cognitive rehabilitation using an integrative approach, can be administered using a group format, significantly improving cognitive functioning in persons with MS. This is one of the first studies to demonstrate that group cognitive rehabilitation can be effective in persons with MS. In fact, to our knowledge, only one recent study has implemented group cognitive training including a neuropsychological assessment to examine the intervention effects.[Citation20]
The largest effect size after cognitive rehabilitation intervention was observed in processing speed. Three recent studies, have demonstrated improvements in persons with MS after RehaCom computerized cognitive rehabilitation programme implementation across attention, information processing (processing speed and working memory), and executive functioning domains.[Citation16–18] In another study, Fink et al. [Citation50] indirectly provided processing speed training and found enhanced processing speed in the treatment group. The present study also showed improved processing speed with treatment. Taken together, there is increasing evidence that behavioral approaches can significantly improve processing speed in persons with MS.
Several studies have examined the effects of cognitive rehabilitation on working memory. A clinical trial carried out by Vogt et al.[Citation51] specifically targeted to working memory cognitive training and reported a significant improvement in processing speed and working memory. Another intervention that was focused on verbal memory and working memory rehabilitation showed significant improvements in both cognitive domains.[Citation52] Moreover, the three studies that implemented the RehaCom computer programme reported an improvement in information processing.[Citation16–18] Thus, the present study found consistent findings with prior conclusions and showed that an integrated and group format cognitive rehabilitation programme can significantly improve working memory in persons with MS.
Verbal memory research has focused on the efficacy of memory training,[Citation19,Citation20,Citation52] as well as the effect of mnemonic strategies.[Citation14,Citation53,Citation54] Stuifbergen et al. [Citation19] implemented a cognitive rehabilitation programme for attention, memory and problem solving, and found significant improvements in learning and delayed verbal memory as well as an increased use of mnemonic strategies. Chiaravalloti et al. [Citation14] provided class I evidence for a learning and memory mnemonic strategy intervention. Moreover, two recent reviews have indicated that cognitive rehabilitation improves immediate and delayed verbal memory in MS.[Citation13,Citation55] The present results extend this conclusion using a group format for cognitive intervention.
Previous literature has shown conflicting results for the effect of cognitive rehabilitation in improving executive functioning in MS. The studies that implemented the RehaCom computer programme reported significant improvement.[Citation16–18] Other study that focused on memory, working memory, and executive functioning training, did not detect an improvement in inhibition or flexibility.[Citation20] In addition, Stuifbergen et al. [Citation19] showed no significant effects in any test of the Delis–Kaplan Executive Function System after cognitive rehabilitation. Differences across studies may be explained by executive functioning varying conceptualisations, training, and assessment methods. One potentially important point is that studies that support the efficacy of executive functioning rehabilitation also implemented integrative cognitive rehabilitation programmes. Therefore, it appears that interventions that use a bottom-up approach may be more effective in improving the targeted cognitive domain.
Improvements in attention or verbal fluency following treatment were not statistically significant. Our findings in attention were not consistent with previous research.[Citation16–18] Amato et al. [Citation56] trained several attention subtypes and reported a significant improvement in sustained attention. Another intervention study targeted attention improvement, also noted enhanced attention and executive functions.[Citation33] One possible explanation for this discrepancy may lie in the assessment tools. Previous studies have mainly used the Paced Auditory Serial Addition Test (PASAT) or the Stroop Color-Word Test, which also involve complex cognitive functions. Nevertheless, the present study used the BTA, which may have been influenced less by other cognitive domains.
As far as we know, no other study has specifically trained verbal fluency. Accordingly, most studies did not find significant enhancements in verbal fluency after cognitive rehabilitation.[Citation16,Citation19,Citation33,Citation56] However, a few studies found an improvement in phonological verbal fluency.[Citation18,Citation20] The lack of significant results in our study could be attributed to insufficient training using REHACOP because there were only two training sessions allocated for verbal fluency.
In addition to the previous methodologically rigorous studies that have demonstrated the efficacy of the REHACOP cognitive rehabilitation programme in schizophrenia [Citation24] and Parkinson’s disease,[Citation25] the present study provides preliminary findings about its efficacy in MS patients. These findings offer a new line of research and the subsequent possibility to utilize a new tool for the management of cognitive deficits in MS. Furthermore, the REHACOP might turn out to be a useful intervention to improve patientś psychological adjustment to the illness, general well-being and daily life activities, a focus for future research. It is worth noting that this acquires special relevance in middle age patients, as those included in this study, where social roles related to family life, occupational and leisure activities can be highly affected by cognitive impairment.
The findings of the present study should be interpreted in the context of methodological limitations. The present study was not blinded. A placebo control group was not employed, which might restrict the differentiation of the social effects, caused by the group treatment, from the cognitive rehabilitation effects. Another limitation was the fact that subject selection did not require current cognitive impairment. Although the inclusion of patients' with different cognitive status was controlled in repeated measures MANCOVA, cognitive rehabilitation studies need to be focused on those with impairment to truly examine any treatment effects. Afterward the randomization of the sample, significant differences in cognitive performance were found at baseline between the REHACOP and the control group. Even though repeated measures MANCOVA controls by default for these differences including a parameter of the between-groups mean differences at baseline in the model,[Citation49] the use of comparable groups at baseline would be desirable. A larger sample size is also important for future research in order to increase the representativeness of MS population. Despite these limitations, the present study still showed significant improvements in cognitive performance across a wide domain of cognitive functions, which likely speaks to the potential efficacy of the REHACOP intervention.
Further controlled research is required to confirm the results of the present study. A complementary long-term follow-up study will be performed to help establish the maintenance of the cognitive improvement 12 and 18 months following the intervention. The present study did not include an analysis of whether cognitive improvement had any effect on improving everyday functional activity. This is clearly needed in any future studies. Finally, future research should include magnetic resonance imaging to explore the presence of brain changes associated with cognitive rehabilitation, as several studies have previously revealed.[Citation15,Citation17,Citation18,Citation57]
Acknowledgements
The authors would like to thank ADEMBI and all the patients involved in the study.
Disclosure statement
N.O. and J.P. are coauthors and copyright holders of the REHACOP cognitive rehabilitation programme, published by Parima Digital, S.L. (Bilbao, Spain). J.D.L has received grant funding from Biogen IDEC. He also has served on an advisory board for Biogen IDEC, and a speaker for EMD Serono. O.R., A.R.A., M.M.B., A.G.G, N.C. and N.I.B. have no conflicts of interest to report.
Additional information
Funding
References
- Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–1151.
- Kalmar JH, Gaudino EA, Moore NB, et al. The relationship between cognitive deficits and everyday functional activities in multiple sclerosis. Neuropsychology. 2008;22:442–449.
- Ben Ari E, Johansson S, Ytterberg C, et al. How are cognitive impairment, fatigue and signs of depression related to participation in daily life among persons with multiple sclerosis? Disabil Rehabil. 2014;36:2012–2018.
- Strober L, Chiaravalloti N, Moore N, et al. Unemployment in multiple sclerosis (MS): utility of the MS functional composite and cognitive testing. Mult Scler. 2014;20:112–115.
- Hakim E, Bakheit A, Bryant T, et al. The social impact of multiple sclerosis – a study of 305 patients and their relatives. Disabil Rehabil. 2000;22:288–293.
- Ford HL, Gerry E, Johnson MH, et al. Health status and quality of life of people with multiple sclerosis. Disabil Rehabil. 2001;23:516–521.
- Benito-León J, Manuel Morales J, Rivera-Navarro J, et al. A review about the impact of multiple sclerosis on health-related quality of life. Disabil Rehabil. 2003;25:1291–1303.
- Fernández O, Baumstarck-Barrau K, Simeoni MC, MusiQoL study group, et al. Patient characteristics and determinants of quality of life in an international population with multiple sclerosis: assessment using the MusiQoL and SF-36 questionnaires. Mult Scler. 2011;17:1238–1249.
- Ruet A, Deloire M, Hamel D, et al. Cognitive impairment, health-related quality of life and vocational status at early stages of multiple sclerosis: a 7-year longitudinal study. J Neurol. 2013;260:776–784.
- Amato MP, Langdon D, Montalban X, et al. Treatment of cognitive impairment in multiple sclerosis: position paper. J Neurol. 2013;260:1452–1468.
- Thomas PW, Thomas S, Hillier C, et al. Psychological interventions for multiple sclerosis. Cochrane Database Syst Rev. 2006;1:CD004431.
- O’Brien AR, Chiaravalloti N, Goverover Y, et al. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: a review of the literature. Arch Phys Med Rehabil. 2008;89:761–769.
- Rosti-Otajärvi EM, Hämäläinen PI. Neuropsychological rehabilitation for multiple sclerosis. Cochrane Database Syst Rev. 2014;2:CD009131.
- Chiaravalloti ND, Moore NB, Nikelshpur OM, et al. An RCT to treat learning impairment in multiple sclerosis: the MEMREHAB trial. Neurology. 2013;81:2066–2072.
- Leavitt VM, Wylie GR, Girgis PA, et al. Increased functional connectivity within memory networks following memory rehabilitation in multiple sclerosis. Brain Imaging Behav. 2014;8:394–402.
- Mattioli F, Stampatori C, Bellomi F, et al. Neuropsychological rehabilitation in adult multiple sclerosis. J Neurol Sci. 2010;31:271–274.
- Filippi M, Riccitelli G, Mattioli F, et al. Multiple sclerosis: effects of cognitive rehabilitation on structural and functional MR imaging measures – an explorative study. Radiology. 2012;262:932–940.
- Parisi L, Rocca MA, Mattioli F, et al. Changes of brain resting state functional connectivity predict the persistence of cognitive rehabilitation effects in patients with multiple sclerosis. Mult Scler. 2013;20:686–694.
- Stuifbergen AK, Becker H, Perez F, et al. A randomized controlled trial of a cognitive rehabilitation intervention for persons with multiple sclerosis. Clin Rehabil. 2012;26:882–893.
- Brissart H, Leroy M, Morele E, et al. Cognitive rehabilitation in multiple sclerosis. Neurocase. 2013;19:553–565.
- Carr SE, das Nair R, Schwartz AF, et al. Group memory rehabilitation for people with multiple sclerosis: a feasibility randomized controlled trial. Clin Rehabil. 2014;28:552–561.
- Graziano F, Calandri E, Borghi M, et al. The effects of a group-based cognitive behavioral therapy on people with multiple sclerosis: a randomized controlled trial. Clin Rehabil. 2014;28:264–274.
- Ojeda N, Peña J. REHACOP: programa de rehabilitación neuropsicológica en psicosis. 1st ed. Bilbao: Parima Digital, S.L.; 2012.
- Sánchez P, Peña J, Bengoetxea E, et al. Improvements in negative symptoms and functional outcome after a new generation cognitive remediation program: a randomized controlled trial. Schizophr Bull. 2014;40:707–715.
- Pena J, Ibarretxe-Bilbao N, Garcia-Gorostiaga I, et al. Improving functional disability and cognition in Parkinson disease: randomized controlled trial. Neurology. 2014;83:2167–2174.
- Tamminga C, Holcomb H. Phenotype of schizophrenia: a review and formulation. Mol Psychiatry. 2005;10:27–39.
- Alves G, Forsaa EB, Pedersen KF, et al. Epidemiology of Parkinson’s disease. J Neurol. 2008;255:18–32.
- Kamm CP, Uitdehaag BM, Polman CH. Multiple sclerosis: current knowledge and future outlook. Eur Neurol. 2014;72:132–141.
- Bowie CR, Harvey PD. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr Dis Treat. 2006;2:531–536.
- Palavra NC, Naismith SL, Lewis SJ. Mild cognitive impairment in Parkinson’s disease: a review of current concepts. Neurol Res Int. 2013;2013:1–8.
- McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127.
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198.
- Cerasa A, Gioia MC, Valentino P, et al. Computer-assisted cognitive rehabilitation of attention deficits for multiple sclerosis: a randomized trial with fMRI correlates. Neurorehabil Neural Repair. 2013;27:284–295.
- Faul F, Erdfelder E, Lang A, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191.
- Schretlen DJ. Brief test of attention professional manual. Odesa (FL): Psychological Assessment Resources; 1997.
- Smith A. Test de símbolos y dígitos. Madrid: TEA Ediciones; 2002.
- Reitan R, Wolfson D. The Halstead-Reitan neuropsychological test battery. Tucson: Neuropsychology Press; 1985.
- Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Dev Psychol. 1991;27:763–776.
- Weschler D. Escala de inteligencia de Weschler para adultos-III. Madrid: TEA Ediciones; 2002.
- Brandt J, Benedict RH. Hopkins verbal learning test-revised: professional manual. Lutz (FL): Psychological Assessment Resources; 2001.
- Schretlen DJ, Vannorsdall TD. Calibrated ideational fluency assessment (CIFA) professional manual. Lutz (FL): Psychological Assessment Resources; 2010.
- Golden CJ. STROOP: test de colores y palabras. Madrid: TEA Ediciones; 2001.
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452.
- Gomar JJ, Ortiz-Gil J, McKenna PJ, et al. Validation of the word accentuation test (TAP) as a means of estimating premorbid IQ in Spanish speakers. Schizophr Res. 2011;128:175–176.
- Rami L, Valls-Pedret C, Bartrés-Faz D, et al. Cuestionario de reserva cognitiva. Valores obtenidos en población anciana sana y con enfermedad de Alzheimer. Rev Neurol. 2011;52:195–201.
- Martínez J, Onís M, Dueñas R, et al. Versión española del cuestionario de Yesavage abreviado (GDS) para el despistaje de depresión en mayores de 65 años: adaptación y validación. Medifam. 2002;12:620–630.
- Krupp LB, LaRocca NG, Muir-Nash J, et al. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123.
- Deloire MS, Bonnet MC, Salort E, et al. How to detect cognitive dysfunction at early stages of multiple sclerosis? Mult Scler. 2006;12:445–452.
- Van Breukelen GJ. ANCOVA versus change from baseline had more power in randomized studies and more bias in nonrandomized studies. J Clin Epidemiol. 2006;59:920–925.
- Fink F, Rischkau E, Butt M, et al. Efficacy of an executive function intervention programme in MS: a placebo-controlled and pseudo-randomized trial. Mult Scler. 2010;16:1148–1151.
- Vogt A, Kappos L, Calabrese P, et al. Working memory training in patients with multiple sclerosis – comparison of two different training schedules. Restor Neurol Neurosci. 2009;27:225–235.
- Hildebrandt H, Lanz M, Hahn H, et al. Cognitive training in MS: effects and relation to brain atrophy. Restor Neurol Neurosci. 2007;25:33–43.
- Chiaravalloti ND, DeLuca J, Moore NB, et al. Treating learning impairments improves memory performance in multiple sclerosis: a randomized clinical trial. Mult Scler. 2005;11:58–68.
- Chiaravalloti ND, DeLuca J. Self-generation as a means of maximizing learning in multiple sclerosis: an application of the generation effect. Arch Phys Med Rehabil. 2002;83:1070–1079.
- das Nair R, Martin KJ, Lincoln NB. Memory rehabilitation for people withmultiple sclerosis. Cochrane Database Syst Rev. 2016;3:CD008754.
- Amato MP, Goretti B, Viterbo RG, et al. Computer-assisted rehabilitation of attention in patients with multiple sclerosis: results of a randomized, double-blind trial. Mult Scler. 2014;20:91–98.
- Chiaravalloti ND, Wylie G, Leavitt V, et al. Increased cerebral activation after behavioral treatment for memory deficits in MS. J Neurol. 2012;259:1337–1346.