Abstract
Purpose
The purpose was to develop a questionnaire instrument to measure difficulties in activities and participation, and impact of environmental factors in chemical intolerance, based on the International Classification of Functioning, Disability and Health, and to assess its validity and reliability.
Method
Development in three steps: (1) choosing items of relevance for chemical intolerance with an expert group, (2) conducting interviews with persons with chemical intolerance, using sampling to redundancy, (3) conducting a survey with 112 respondents at a first assessment and 91 at a second assessment for test-retest.
Results
The final version of the instrument consists of 57 items divided in three parts, which showed good internal consistency in each part, Cronbach alpha: 0.73–0.87. It had good content validity, readability and face validity. Test-retest showed good to very good (≥0.61) Kappa agreement for 37 items, and moderate (0.41–0.60) for 17 items. Three items had poor or fair (<0.41) Kappa agreement.
Conclusion
The instrument was found to be valid and reliable. It can be used as a clinical tool to help persons with chemical intolerance to receive the best suited help and support for each individual, identify key points in rehabilitation, measure rehabilitation outcome and establish priority for treatment.
The questionnaire instrument based on the International Classification of Functioning, Disability and Health which was developed and evaluated in this study, can be used to measure difficulties in activities and participation, and impact of environmental factors in chemical intolerance.
Persons with chemical intolerance report lack of support from healthcare and society. Using this questionnaire instrument can help forming the best suited help and support for each individual based on his/her preconditions.
This questionnaire instrument can be used to identify key points in rehabilitation and measure rehabilitation outcome.
IMPLICATIONS FOR REHABILITATION
Introduction
Chemical intolerance is a term used to describe hypersensitivity to volatiles of chemical substances in doses that are not known to cause toxic effects. For those who have chemical intolerance, exposure to these chemicals cause symptoms such as headache, respiratory problems, difficulty concentrating, excessive fatigue, and gastrointestinal symptoms. Chemicals associated with symptoms are common everyday products, e.g. perfumes, detergents, flame retardants and ink [Citation1]. Chemical intolerance is an overall term for these problems and comprises, for example, the conditions: multiple chemical sensitivity [Citation2,Citation3] and sensory hyperreactivity [Citation4,Citation5]. Chemical intolerance is a frequently reported problem with a prevalence ranging from 6% to 33% depending on definition [Citation4,Citation6–12]. Chemical intolerance is overrepresented among women [Citation6–8,Citation10,Citation11]. There is empirical support for several underlying mechanisms, such as neurogenic inflammation, classical conditioning, nocebo effect and neural sensitization, but other theories has also been suggested such as immune mechanisms and genetics [Citation1].
Qualitative studies show that chemical intolerance may lead to difficulties with work, finances, housing situation, social relations and accessibility to the community such as using public transport, going to the cinema/theater/restaurant, visiting shops, utilizing health care and performing recreational activities [Citation6,Citation13–18]. It may also affect an individual’s identity [Citation19] and may lead to depression [Citation15]. Furthermore, individuals with chemical intolerance report lack of support and understanding, and malpractice from medical personnel [Citation14–16,Citation20,Citation21]. General practitioners are commonly at a loss about how to help patients with chemical intolerance [Citation22]. Persons with chemical intolerance also report poor support from society [Citation13–15,Citation17]. Hence prior research suggests that chemical intolerance can be a disabling condition.
The International Classification of Functioning, Disability and Health (ICF) was published in 2001 as a complement to the International Classification of Diseases (ICD), and a Swedish version of ICF was published in 2003 by the Swedish National Board of Health and Welfare [Citation23]. ICF is based on the biopsychosocial model of health that was introduced by Engel in 1977 [Citation24]. According to the ICF terminology, functioning and disability is viewed as the outcome of interactions between a person’s health condition and contextual factors. ICF is a tool to assess the individual’s entire life situation, how well different life activities work and how involved the individual is in society. ICF contains the areas: body functions, body structures, activities and participation, and environmental factors. There are several chapters within these areas and for each chapter there are several items for coding. It contains in total 1424 codes for classification [Citation23]. Core sets of ICF [Citation25–28] and questionnaires instruments based on ICF [Citation29–31] have been developed for specific health conditions.
To the best of our knowledge, there is no published core set or questionnaire instrument based on ICF for chemical intolerance. The purpose of this study was therefore to develop an instrument for measuring difficulties in activities and participation, and impact of environmental factors in chemical intolerance, based on ICF, and to evaluate its validity and reliability. The ICF-areas Activities and participation, and Environmental factors were regarded as the most important for chemical intolerance, and not covered by other questionnaire instruments. The questionnaire instrument was given the name APECI-ICF which stands for Activities, Participation and Environmental factors for chemical intolerance measured with ICF. According to the ICF-manual, four different methods can be used to distinguish between activities and participation [Citation23]. We chose the method that regards each item as both activities and participation, and the items were therefore prefixed with the letter d according to how to use the prefix [Citation23]. Each ICF item in the area Activities and participation can be coded regarding both performance and capacity. Performance refers to what an individual does in his/her current environment, whereas capacity refers to task execution in a standardized environment [Citation23]. We considered performance as the most appropriate for this instrument for its purpose of studying persons in their current environment.
Method
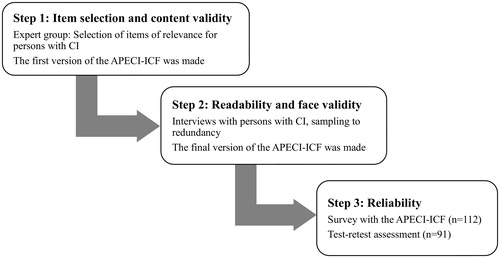
The development of the APECI-ICF was inspired by Streiner and Norman [Citation32], and was carried out in three steps, as showed in .
Step 1: item selection and content validity
As a first step, an expert group was established, that consisted of the present authors and eight recruited experts. The experts had considerable knowledge of chemical intolerance from different perspectives, and they had different opinions about the underlying mechanisms of chemical intolerance. The expert group consisted of a nurse, four physicians (an ear nose and throat specialist, a pulmonary and allergy specialist, an occupational and environmental health specialists, and a general practitioner), an occupational hygienist, two researchers in psychology, a researcher in public health, and three representatives from patient organizations for chemical intolerance.
The experts were divided into two groups to enhance discussions. The experts participated in person or by weblink. The first author led the discussion in both expert group meetings. All ICF items in the areas Activities and participation and Environmental factors were discussed regarding relevance to chemical intolerance in both expert groups. The items were determined as having “high”, “medium” or “low” relevance to chemical intolerance.
The authors summarized the results from the two expert group meetings and made the first draft of the APECI–ICF based on these results. We used the original response alternatives from the ICF which constitute an ordinal scale with five alternatives: No, Mild, Moderate, Severe/Substantial, and Complete difficulties/barriers/facilitators. We also included the original ICF response alternative: Not applicable (e.g. when asked about relationships with children, but the respondent does not have children). The first draft was then sent to all experts for comments and revision suggestions, resulting in a pilot version of the APECI-ICF.
Step 2: readability and face validity
Next, the pilot of the APECI-ICF was tested on a sample with chemical intolerance who were recruited trough advertisement in two national support groups for chemical intolerance on Facebook: “In the air - be aware” and “MCS Sweden”. In total, 45 persons reported interest to participate in the study. The inclusion criteria were: having multiple chemical sensitivity or sensory hyperreactivity (reporting having had the condition verified by a physician) and having had it for 1 year or more, being 18 years or older, living in Sweden, and being fluent in Swedish. Among those who fulfilled the inclusion criteria, participants were chosen in order to achieve variation in age, sex, home district, occupational condition and diagnosis of multiple chemical sensitivity or sensory hyperereactivity. See for characteristics of the participants.
Table 1. Characteristics of the participants with chemical intolerance included in step 2 and 3.
The participants were sent (by post) an information letter, a background questionnaire (age, education, etc), the APECI-ICF, the Nevo Face Validity Scale [Citation33], and the Chemical Sensitivity Scale for Sensory Hyperreactivity (CCS-SHR) [Citation5]. The CSS-SHR was used to quantify degree of affective reactions to and behavioral disruptions by odorous and pungent exposure. Based on the scores for the present sample (mean = 50.5; n = 19) and on normative data (mean = 29.7, SD = 8.73) [Citation34], degree of affective reactions/behavioral disruptions correspond, in average, to the 99th percentile of the general population.
The participants were contacted by telephone to book the interview and to inform them that they were going to receive the questionnaires by post and that they should not fill in the APECI-ICF and the Nevo Face Validity Scale until during the interview. The interviews were carried out by telephone, and both the interviewer and the participant had the APECI-ICF in front of them. To evaluate its readability, the questions/items were responded to one at a time, and the participant was instructed to think out loud about the answer. If needed, the interviewer asked questions such as “What led to your answer?” This was done in order to determine whether the participant had interpreted the question as intended. The interviewer wrote down all comments, suggestions and problems that were revealed during responding to the items. The interviewer also asked the participant whether the instructions were understandable and whether the response alternatives were easy to understand and choose from.
To evaluate face validity of the APECI-ICF, the participant responded to the five level Nevo Face Validity Scale with which the suitability of a questionnaire or test for its intended use is rated. Its response alternatives range from “The test is irrelevant and therefore unsuitable for the given purpose” to “The test is extremely suitable for the given purpose” [Citation33]. Furthermore, the participant was asked whether there were any items in the APECI-ICF that he/her regarded as being more important than others or irrelevant, and whether there were any questions missing in the APECI-ICF.
The interviews were carried out with participants, one at a time, until no new problems were identified, so-called sampling to redundancy [Citation32]. After one round of interviews the project group revised the instrument according to the results from the first round. A second round of interviews was then performed with new participants and with sampling to redundancy and then the APECI-ICF was revised again based on the result from the second round. Thereafter a third round of interviews took place with new participants and sampling to redundancy, resulting in the final version of the APECI-ICF.
Step 3: reliability
In this last step, the final version of the APECI-ICF was used in a survey to evaluate its reliability by conducting test-retest analysis and testing for internal consistency. Participants were recruited through advertisement in the two largest daily newspaper in southern Sweden, Metro and Dagens Nyheter, and in the largest daily newspaper for northern Sweden, Västerbotten-Kuriren. Advertisement were also made on the national Facebook page for the Swedish Asthma and Allergy Association, and in a national Facebook group for persons with chemical intolerance, called “In the air be aware”. The inclusion criteria were having self-reported chemical intolerance, being 18 years or older and living in Sweden.
There were 122 persons who reported interest in participating in the web survey, and were sent an email with information and linkage to the survey. In addition, six persons wanted to participate by using printed questionnaires, and were sent these by post with a prepaid return envelope. All participants received the APECI-ICF, a questionnaire with background variables and the CCS-SHR [Citation5]. Based on the CSS-SHR scores for the present sample (mean = 48.5; n = 91) and on normative data (mean = 29.7, SD = 8.73) [Citation34], degree of affective reactions/behavioral disruptions correspond, in average, to the 98th percentile of the general population. After 2 weeks, the APECI-ICF was again sent to the participants. Of those who participated by web survey, 106 completed the first and 86 also the second questionnaire (test retest). Among those who participated by post, all six completed both the first and second questionnaire. In total, 91 person completed both the first and the second questionnaire. The samples’ characteristics are given in .
The data were analyzed with SPSS Statistics 23. The response alternative “Not applicable” was not included in the test-retest analysis since it is not a response alternative within the ordinal scale 0–4. Test- retest reliability was analyzed using weighted Kappa (quadratic) since it is the most useful measure for agreement when the data is ordinal [Citation35]. Quadratic weighted Kappa penalizes disagreement of two or more categories much more severely than one category disagreement, and was considered suitable for the APECI-ICF. Kappa was interpreted according to Altman 1991 [Citation36]: < 0.20 = Poor; 0.21–0.40 = Fair; 0.41–0.60 = Moderate; 0.61–0.80 = Good; 0.81–1 = Very good.
Kappa agreement depends on the prevalence of responses within the different response alternatives, where high frequency of a certain alternative and few or none of other alternatives lead to low Kappa agreement even when the percent agreement is high [Citation37]. The data from our study was distributed in this asymmetrical way, motivating calculation of percent agreement. We also calculated “> one category disagreement” which refers to the percentage of participants who at the second occasion (test-retest) gave a response alternative that was more than one category different from their alternative at the first occasion.
Quadratic weighted Kappa can give a falsely lower Kappa agreement when there are very few cases that have more than one category disagreement because too much weight is given to one individual case. This can be controlled for with a sensibility analysis, i.e. removing cases that had three category disagreement, and then running the analysis again for any possible difference. A sensibility analysis was therefore performed for those two items that had low quadratic Kappa agreement but higher linear Kappa agreement (when tested), high percent agreement and very few cases with “> one category of disagreement”.
Internal consistency for each of the three subscales were calculated using Cronbach alpha. The response alternative, not applicable, was included in the analysis because when excluded, it was not possible to calculate Cronbach alpha since it resulted in too much missing data. Of all possible cases and responses, 14% were not applicable, and 0.3% were missing data.
Ethical considerations
The study was conducted in accordance with the Helsinki Declaration and approved by the Regional Ethics Committee in Umeå (Dnr: 2014/61-32Ö). All participants gave their informed consent to participate.
Results
Step 1: item selection and content validity
The expert group regarded 67 items to be of high relevance for CI, and were therefore chosen for the first draft of the instrument. There were 40 items in the area Activities and participation: Seven items in the chapter Learning and applying knowledge, zero in General tasks and demands, zero in Communication, four in Mobility, two in Self-care, four in Domestic life, twelve in Interpersonal interactions and relationships, nine in Major life areas and two in Community, social and civic life. There were 27 items in the area Environmental factors: Seven in the chapter Products and technique, four in Natural environment and human-made changes to environment, seven in Support and relationships, eight in Attitudes, and two in Services, systems and policies. The items in the area Environmental factors can be regarded both as barriers and facilitator. To be able to specify and therefore make the APECI-ICF easier to understand as a self-report instrument, the area Environmental factors was simplified by sorting its items into possible barriers and possible facilitators, instead of having each item as both a barrier and facilitator. Of the chosen items in the area Environmental factors, 13 items were regarded as possible barriers and 14 as possible facilitators for persons with chemical intolerance.
Step 2: readability and face validity
For the first round of interviews, 11 participants were needed to reach redundancy. The result showed that some instructions, especially in the part with possible facilitators, needed to be clarified. Some items were regarded as difficult to understand and were therefore revised, and for some of these a note on the side was provided with a short explanation. The participants reported that there were too many and to similar items about social relationships, so those items were revised and reduced. In some other areas there were some items that were regarded as too similar, and were therefore removed. The instrument was shortened according to these results, and in total, nine items were removed. In the second round of interviews with sampling to redundancy five participants were needed. The result showed no major problems, but some instructions needed to be clarified, some items were altered slightly, and two items were removed and one was added. In the third round no new problems were revealed in the three first interviews, so therefore no further interviews were needed and the final version of the APECI-ICF was established.
The mean value on the Nevo Face Validity Scale was 4.4 for all 19 participants, of which 11 filled in the first version, five the second, and three the final version. The final version of the APECI-ICF consisted of 57 items; 37 in Activities and participation, 9 in Environmental factors that may be barriers and 11 in Environmental factors that may be facilitators. The final version is presented as Supplementary Material, translated to English (instructions not included).
Step 3: reliability
In the area Activities and participation, 3 items had very good Kappa agreement and 24 had good Kappa agreement, as seen in . Nine items had moderate Kappa agreement of which, five had high or quite high percent agreement, low “> one category disagreement” and an interquartile range of 0 or 0.5 as seen in column 3–4 in . One item, Part-time employment had poor Kappa agreement (0.18), but high percent agreement (86.7%) and quite low percent (4.4%) “> one category disagreement”. After the sensibility analysis the Kappa agreement for Part time employment was 0.57.
Table 2. Test-retest result, sorted by ICF chapter, for each item in the area Activities and participation: Kappa agreement, percent agreement, “>1 category disagreement”**, and interquartile range and range for the first and second measurement (when differing from the first).
In the area Environmental factors, one item had very good Kappa agreement and nine items had good Kappa agreement, as shown in Eight items had moderate agreement, of which six had high percent agreement and low “<one category of disagreement” and an interquartile range of 0, as seen in column 3–4 in . Two items had fair Kappa agreement: Support from health care professionals, which had a percent agreement of 79.1% and 7.7% “> one category disagreement”, and Housing services which had a percent agreement of 84.4% and 3.2% “> one category disagreement”. Both items had an interquartile range of 0. After the sensibility analysis, the item Housing services had a Kappa agreement of 0.49 instead of 0.34.
Table 3. Test-retest result, sorted by ICF chapter, for each item in the area Environmental factors: Kappa agreement, percent agreement, “>1 category disagreement”**, and interquartile range and range for the first and second measurement (when differing from the first).
Regarding internal consistency, Cronbach alpha was 0.87 for the part Activities and participation. For the part Environmental factors that may be barriers it was 0.73, and for the part Environmental factors that may be facilitators it was 0.83.
Discussion
In this study, the APECI-ICF, an instrument for measuring difficulties in activities and participation, and impact of environmental factors in chemical intolerance was developed and evaluated with respect to validity and reliability. The final version of the instrument consist of 57 items: 37 in Activities and participation, 9 in Environmental factors that may be barriers and 11 in Environmental factors that may be facilitators. The APECI-ICF was found to have good content validity according to experts with respect to various perspectives of chemical intolerance, and good readability and face validity assessed with a sample with chemical intolerance. This sample found it to be understandable, easy to respond to and very relevant with a mean score of 4.4 on the Nevo Face Validity Scale. Good reliability was found in terms of internal consistency with Cronbach alpha values of 0.73–0.87 for the three parts of the APECI-ICF and in terms of stability (test-retest). The test-retest result showed good to very good (≥0.61) Kappa agreement for 37 items, and moderate Kappa agreement (0.41–0.60) for 17 items. Only 3 items had poor or fair (<0.41) Kappa agreement.
Several of the items in our study had low variance in terms of range and interquartile range, as seen in column 3–5 in and , which can have led to a falsely lower Kappa agreement [Citation37]. Among the 17 items with moderate Kappa agreement it is likely that 11 had a falsely lower Kappa agreement since they had high (>80%) or quite high (>70%) percent agreement and low (0–3%) “> one category disagreement” as well as low variance with an interquartile range of 0–0.5. On the same grounds, it is likely that two of those three items with low or fair Kappa agreement had a falsely lower Kappa agreement. Their Kappa agreement also increased considerably after the sensibility analysis. The one item left with a truly fair Kappa agreement (0.34) was Support from health care professionals. For this item it is possible that responses within a person vary considerably due to having met several health care professionals that vary in support. Therefore the response depends on which health care professional the person refers to at the time.
Our study showed Kappa agreement of 0.45–0.94 (of which one item was sensibility adjusted) on test-retest for the 37 items of the Activities and participation part, and Cronbach alpha 0.87. The present Kappa agreement are comparable with and somewhat higher than those reported by Post et al. [Citation30] of 0.44–0.72 for an ICF-instrument regarding Activities and participation, with 33 items. Cronbach alpha for that instrument was 0.96 (31), which is a bit higher than that for the APECI-ICF. Rogers et al. developed a self-report version of the ICF brief core set for head and neck cancer which showed Kappa agreements on test-retest of 0.68–0.92 and “> one category disagreement” of 0–5.3% on its six items on Activities and participation [Citation31]. Our study showed “> one category disagreement” of 0–16.1% on the 37 items for Activities and participation. With four items on Environmental factors Rogers et al. showed Kappa agreement of 0.52–0.80 and “>one category disagreement” of 0–15% [Citation31]. Our study showed Kappa agreements of 0.34–0.83 and “> one category disagreements” of 0–7.8% on the 20 items on Environmental factors. However, according to Jakobsson and Westergren it is difficult to compare Kappa agreement between studies since it depends both on the number of response alternatives for the variables and the distribution across the response alternatives [Citation35]. These comparisons should therefore be interpreted with caution.
The drop-out rate was quite low in this study. In step two, with pilot testing on persons with chemical intolerance, there was no drop-out, and in step three with the survey, 128 persons were sent the questionnaire, of which 112 (88%) participated, and of those, 91 (81%) participated also in the retest survey. The drop-out rate was probably low since we advertised for participants. Some drop-outs were due to the email from the web survey system being interpreted as junk mail in some persons, email system. There was an overrepresentation of women in the study, which was expected since the prevalence of chemical intolerance is higher in women than in men. The use of advertisement to recruit participants may have led to getting more persons that were severely ill since it is possible that those persons were more likely to report interest in participating in the study.
Persons with chemical intolerance often receive limited help and support from healthcare and society. The APECI-ICF can be used to measure difficulties in activities and participation, and impact of environmental factors in chemical intolerance, and therefore make it possible to receive the best suited help and support for each individual based on his/her preconditions, from e.g. health care, the social insurance office and employment office. Several persons with chemical intolerance end up in a downward spiral with decreasing participation in the society and poor health and wellbeing. The APECI-ICF can be used to identify problem areas and thereby enhance rehabilitation at an early point in time. The part facilitators can be used to identify improvement potential and possible solutions to the problems and to find tools in the rehabilitation process. The APECI-ICF can also be used to measure rehabilitation outcome. Limited access to clinical treatment for chemical intolerance calls for instruments for quantifying disability in order to establish priority for treatment. The APECI-ICF may play an important role in this respect.
Conclusions
The APECI-ICF that was developed in this study was found to be valid and reliable in terms of good content validity, face validity, readability, internal consistency and stability. It can be used as a clinical tool to measure difficulties in activities and participation, and impact of environmental factors in persons with chemical intolerance and thereby help these to receive support from the society as well as identifying key points in rehabilitation, measure rehabilitation outcome, and establish priority for treatment.
Supplemental Material
Download PDF (100.9 KB)Acknowledgements
We are grateful to all the experts: Berndt Karlsson, Eva Millqvist, Mats Bende, Linus Andersson, Ewa Ternesten-Hasseus, Solveig Enberg, Lisa Billö and Sven-Olof Billö, for valuable help in developing the APECI-ICF.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Dantoft TM, Andersson L, Nordin S, Skovbjerg S. Chemical intolerance. CRR. 2015;11(2):167–184.
- Nethercott JR, Lee Davidoff L, Curbow B, et al. Multiple chemical sensitivities syndrome: toward a working case definition. Arch Environ Health. 1993;48(1):19–26.
- Bartha L. Multiple chemical sensitivity: a 1999 consensus. Arch Environ Health. 1999;54:147–149.
- Johansson Å, Millqvist E, Nordin S, et al. Relationship between self-reported odor intolerance and sensitivity to inhaled capsaicin: proposed definition of airway sensory hyperreactivity and estimation of its prevalence. Chest. 2006;129(6):1623–1628.
- Nordin S, Millqvist E, LöWhagen O, et al. A short chemical sensitivity scale for assessment of airway sensory hyperreactivity. Int Arch Occup Environ Health. 2004;77(4):249–254.
- Berg ND, Linneberg A, Dirksen A, et al. Prevalence of self-reported symptoms and consequences related to inhalation of airborne chemicals in a Danish general population. Int Arch Occup Environ Health. 2008;81(7):881–887.
- Caress SM, Steinemann AC. A review of a two-phase population study of multiple chemical sensitivities. Environ Health Perspect. 2003;111(12):1490.
- Johansson A, Bramerson A, Millqvist E, et al. Prevalence and risk factors for self-reported odour intolerance: the Skövde population-based study. Int Arch Occup Environ Health. 2005;78(7):559–564.
- Hausteiner C, Bornschein S, Hansen J, et al. Self-reported chemical sensitivity in Germany: a population-based survey. Int J Hyg Environ Health.. 2005;208(4):271–278.
- Hojo S, Ishikawa S, Kumano H, et al. Clinical characteristics of physician-diagnosed patients with multiple chemical sensitivity in Japan. Int J Hyg Environ Health. 2008;211(5-6):682–689.
- Kreutzer R, Neutra RR, Lashuay N. Prevalence of people reporting sensitivities to chemicals in a population based survey. Am J Epidemiol. 1999;150(1):1–12.
- Karvala K, Sainio M, Palmquist E, et al. Prevalence of various environmental intolerances in a Swedish and Finnish general population. Environ Res. 2018;161:220–228.
- Söderholm A, Söderberg A, Nordin S. The experience of living with sensory hyperreactivity—accessibility, financial security, and social relationships. Health Care Women Int. 2011;32(8):686–707.
- Lipson JG. Multiple chemical sensitivities: stigma and social experiences. Med Anthropol Q. 2004;18(2):200–213.
- Gibson DPR, Cheavens J, Warren ML. Chemical sensitivity/chemical injury and life disruption. Women Ther. 1996;19(2):63–79.
- Skovbjerg S, Brorson S, Rasmussen A, et al. Impact of self-reported multiple chemical sensitivity on everyday life: a qualitative study. Scand J Public Health. 2009;37(6):621–626.
- Gibson PR. Of the world but not in it: barriers to community access and education for persons with environmental sensitivities. Health Care Women Int. 2009;31(1):3–16.
- Gibson PR, Sledd LG, McEnroe WH, et al. Isolation and lack of access in multiple chemical sensitivity: a qualitative study. Nurs Health Sci. 2011;13(3):232–237.
- Gibson PR, Placek E, Lane J, et al. Disability-induced identity changes in persons with multiple chemical sensitivity. Qual Health Res. 2005;15(4):502–524.
- Larsson C, Mårtensson L. Experiences of problems in individuals with hypersensitivity to odours and chemicals. J Clin Nurs. 2009;18(5):737–744.
- Gibson PR, Leaf B, Komisarcik V. Unmet medical care needs in persons with multiple chemical sensitivity: a grounded theory of contested illness. JNEP. 2016;6(5):75.
- Skovbjerg S, Johansen JD, Rasmussen A, et al. General practitioners’ experiences with provision of healthcare to patients with self-reported multiple chemical sensitivity. Scand J Prim Health Care. 2009;27(3):148–152.
- Socialstyrelsen. Klassifikation av funktionstillstånd, funktionshinder och hälsa. Stockholm: Socialstyrelsen (Swedish National Board of Health and Welfare); 2003.
- Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129–136.
- Stier-Jarmer M, Grill E, Ewert T, et al. ICF Core Set for patients with neurological conditions in early post-acute rehabilitation facilities. Disabil Rehabil. 2005;27(7–8):389–395.
- Stucki A, Stoll T, Cieza A, et al. ICF Core Sets for obstructive pulmonary diseases. J Rehabil Med. 2004;36(44 Suppl):114–120.
- Cieza A, Chatterji S, Andersen C, et al. ICF Core Sets for depression. J Rehabil Med. 2004;36(44 Suppl):128–134.
- Cieza A, Stucki G, Weigl M, et al. ICF Core Sets for low back pain. J Rehabil Med. 2004;36(44 Suppl):69–74.
- Devoogdt N, Van Kampen M, Geraerts I, et al. Lymphoedema Functioning, Disability and Health questionnaire (Lymph-ICF): reliability and validity. Phys Ther. 2011;91(6):944–957.
- Post MW, de Witte LP, Reichrath E, et al. Development and validation of IMPACT-S, an ICF-based questionnaire to measure activities and participation. J Rehabil Med. 2008;40(8):620–627.
- Rogers S, Forgie S, Lowe D, et al. Development of the International Classification of Functioning, Disability and Health as a brief head and neck cancer patient questionnaire. Int J Oral Maxillofac Surg. 2010;39(10):975–982.
- Streiner DL, Norman GR. Health measurement scales: a practical guide to their development and use. Oxford (UK): Oxford University Press; 2003.
- Nevo B. Face validity revisited. J Educ Meas. 1985;22(4):287–293.
- Nordin S, Palmquist E, Bende M, et al. Normative data for the chemical sensitivity scale for sensory hyperreactivity: the Västerbotten environmental health study. Int Arch Occup Environ Health.. 2013;86(7):749–753.
- Jakobsson U, Westergren A. Statistical methods for assessing agreement for ordinal data. Scand J Caring Sci. 2005;19(4):427–431.
- Altman DG. Practical statistics for medical research. London (UK): Chapman & Hall; 1991. p. 404.
- Byrt T, Bishop J, Carlin JB. Bias, prevalence and kappa. J Clin Epidemiol. 1993;46(5):423–429.