Abstract
Aim
This meta-analysis aimed to determine the effect of aerobic training, compared to non-aerobic interventions, on vascular and metabolic risk factors for recurrent stroke.
Method
This study was conducted using the Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines (PRISMA). Searches were performed in PubMed, Embase, Cochrane library and Cinahl up to May 8th 2019. Randomized clinical trials evaluating the effect of solely aerobic training on vascular and metabolic risk factors for recurrent stroke were included in a meta-analysis if relevant outcomes were reported in at least two articles.
Results
Our search resulted in a total of 7381 hits. Eleven outcomes out of nine articles were included in the meta-analysis. A significant positive effect of aerobic training was found on systolic blood pressure (−3.59 mmHg, 95% CI −6.14 to −1.05) and fasting glucose (−0.12 mmol/l, 95% CI −0.23 to −0.02). The effect on systolic blood pressure further improved when only high-quality studies were included (−4.95 mmHg, 95% CI −8.24 to −1.66).
Conclusion
Aerobic training results in a significant positive effect on systolic blood pressure and fasting glucose after stroke when compared to non-aerobic usual care or non-aerobic exercise.
Aerobic training has a positive effect on two of the most important vascular risk factors for recurrent stroke (i.e., systolic blood pressure and fasting glucose).
The effect of solely aerobic training seems to be comparable to the effect of combined strength exercise and aerobic training for systolic blood pressure and fasting glucose.
Since aerobic training has a significant effect on risk factors for recurrent stroke, implementation of aerobic training in daily life is important to reduce long term stroke risk.
Previous research has showed that other metabolic risk factors can be altered by other interventions (e.g., strength exercise or lifestyle coaching), therefore, post-stroke prevention programs should be tailored in order to target specific risk-factors for individual patients.
Implications for rehabilitation
Introduction
Stroke is one of the leading causes of death and disability with 79.6 million people worldwide living with stroke and 13.7 million new cases in 2016 [Citation1–4]. Previous stroke or transient ischemic attack (TIA) is a strong risk factor in itself for recurrent stroke [Citation5]. Recurrent stroke often results in an accumulation of physical and cognitive disabilities and further loss of quality of life [Citation6,Citation7]. Therefore, secondary prevention after stroke is essential.
There are many factors contributing to the risk of recurrent stroke, of which some are modifiable. Hypertension is the strongest attributable modifiable risk factor for stroke [Citation8]. A reduction of 5.1 mmHg in systolic blood pressure (SBP) is associated with a 22% reduction in the odds of having a recurrent stroke, and a reduction of 10 mmHg is associated with a risk reduction of 31% [Citation9,Citation10]. However, only 10.6% of American adults with a history of stroke or myocardial infarction achieve control of their vascular risk [Citation5]. Therefore, the risk for recurrent stroke remains high and an urgent need exists to improve the quality of secondary prevention after stroke [Citation11].
Several systematic reviews have addressed exercise as secondary prevention after stroke. These reviews all included studies in which the intervention existed of a combination of aerobic training with strength exercise and/or lifestyle interventions [Citation12–15]. D’Isabella et al. found that combined aerobic training and strength exercise has a risk reducing effect on several vascular risk factors after stroke [Citation15]. However, the effects of isolated parts of these multi-domain interventions is not clear. Additional evidence regarding the effectiveness of isolated interventions could help to optimize prevention programs. In their review, D’Isabella et al. concluded that additional research is needed to differentiate the effectiveness of exercise type and dose-response relationship of included interventions [Citation15].
There is some evidence available regarding the effectiveness of solely aerobic training as secondary prevention. Aerobic training can be any physical training that depends primarily on the aerobic energy-generating process, on the condition that it is of sufficient intensity to maintain or improve physical fitness [Citation16]. Aerobic training is usually performed at target heartrates between 50–85% of heart rate reserve for at least 20 min per session [Citation17,Citation18]. Aerobic training is proofed to have a positive effect on blood pressure in hypertensive patients without comorbidities [Citation19]. Furthermore, several clinical trials have analyzed the effect of solely aerobic training on risk factors after stroke [Citation20–23]. The findings of these trials suggest that aerobic training could have a risk reducing effect on several vascular or metabolic risk factors (e.g., blood pressure, heart rate, insulin or lipid profiles). To date, however, there has been no systematic review of this evidence and it remains uncertain which risk factors can be targeted by aerobic training and to which extend. Therefore, this systematic review aims to determine the effect of solely aerobic training on vascular and metabolic risk factors for recurrent stroke.
Methods
In- and exclusion criteria
Studies were eligible for this review if the following inclusion criteria were met: (1) Randomized controlled trial (RCT) study design; (2) participants’ age ≥18 years; (3) clinical diagnosis of stroke or TIA; (4) interventions consisted of aerobic training; (5) comparison with usual care without aerobic training or other non-aerobic training (i.e., training without prolonged periods in which maximum heart rate exceeds 50% of heart rate reserve); (6) evaluating the effect of aerobic training (compared to non-aerobic interventions) on vascular or metabolic risk factors for stroke and (7) full-text article available in Dutch or English. Studies were excluded in case of (1) a combination of therapies within the intervention group and (2) when the reported outcomes were not reported in any other included article (and consequently pooling for meta-analysis was not possible). As primary outcomes we analyzed systolic blood pressure, diastolic blood pressure (DBP) and resting heart rate (RHR). As secondary outcomes we planned to include all other vascular and metabolic risk factors for recurrent stroke that were identified by our search.
Literature search
The reporting of this systematic review was conducted in accordance with the PRISMA guidelines for systematic reviews [Citation24]. Searches were performed in PubMed (1966 to present), Embase (1947 to present), Cochrane library (1993 to present) and Cinahl (1982 to present) up to May 8th 2019. The search strategy consisted of four components: 1) stroke; 2) aerobic training; 3) secondary prevention and 4) vascular/metabolic risk factors. For the stroke component, the search string as described by Veerbeek et al. [Citation25] was used, which was extended with a search string for TIA. Our search aimed to identify all relevant vascular and metabolic risk factors for recurrent stroke. Known relevant vascular and metabolic risk factors (e.g., blood pressure, hemodynamics, insulin or lipid profiles) were included in the search string and possible relevant studies were screened to identify additional relevant risk factors. The search strategy was formulated in PubMed and adapted for use in other databases (see supplementary material). Reference lists of included studies and relevant systematic reviews were screened to check for additional relevant publications by one researcher (RB).
Study selection procedure
Study selection was performed in two steps by two independent reviewers (RB and RW). After all duplicates were identified and removed, remaining studies were first screened by title and abstract. After that, relevant full-text articles were obtained and screened for eligibility. Disagreements about the eligibility of a study were resolved by consensus. In case of doubt of eligibility of studies, the authors of the study were contacted to obtain additional information.
Methodological quality
The methodological quality of included studies was rated independently by two reviewers (RB and CO) using the PEDro scale [Citation26]. Afterward, PEDro scores were compared, and any disagreements were solved by consensus. The PEDro scale consists of 11 items to rate internal- and external validity. Each item is given a score of 0 or 1. The total count of all item scores, except for item 1, gives an overall score between 0 and 10. A score of ≤3 is considered as poor quality, 4–5 is considered as fair quality, 6–10 is considered as high quality [Citation26].
Data extraction
One reviewer (RB) extracted the following information from the included publications: author, publication year, sample size, diagnosis and time since onset. Descriptive data regarding frequency, intensity, time and type (FITT principle) [Citation27] of the intervention were extracted to the intervention characteristics table. Furthermore, all identified relevant outcomes were extracted to a data table. Mean change scores and standard deviations of variables that were included in the meta-analysis was then extracted to RevMan 5 (Review Manager version 5.3) [Citation28] by one researcher (RB). If mean change scores were not reported, study authors were contacted to obtain additional information. When mean change scores could not be obtained, the missing data was calculated from post-intervention data, according to the recommended methods described in the Cochrane handbook version 5.1 [Citation29].
Meta-analysis
Relevant outcomes were pooled for meta-analysis when at least two studies reported an outcome variable. Data regarding sample size, mean change from baseline scores and the standard deviation were extracted to Review Manager version 5.3 [Citation28]. In case this data was not presented in studies, study authors were contacted to ask whether data was available. All meta-analysis were performed using an inverse variance model, random effects and mean difference analysis (95% confidence interval). Forest plots were used to estimate the effect size. Sensitivity analyses were performed using only high-quality studies (PEDro scores ≥6) and studies with a shorter intervention period (6–12 weeks). Measures for heterogeneity (I2) were reported for each outcome measure, and funnel plots were visually inspected to assess for publication bias. I2 was considered to be low (≤25%), moderate (26–75%) or high (>75%) [Citation30].
Results
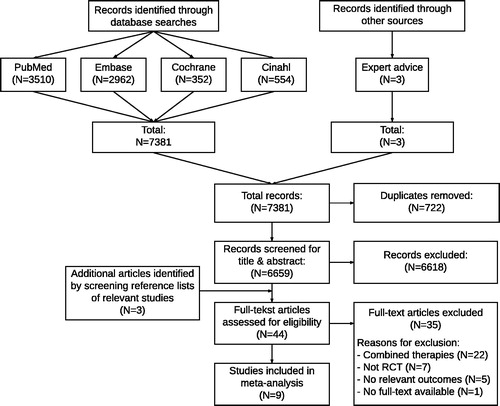
The search strategy resulted in a total of 7381 hits, of which 6659 remained after removing duplicates. Three additional articles were included by hand searching reference lists of relevant studies. After screening for title and abstract 44 articles remained. After full-text screening, a total of eleven articles were eligible for inclusion. Meta-analyses were performed on an outcome that was reported by at least two articles. The outcomes of two articles [Citation31,Citation32] were only reported once and therefore could not be pooled in a meta-analysis. Hence, nine articles were included in the meta-analysis () [Citation20–23,Citation33–37]. Reasons for exclusion were: combined therapies (N = 22); no RCT study design (N = 7); no relevant outcome measures (N = 5) and no full-text article available (N = 1).
Methodological quality
The methodological quality of included studies ranged from four to eight out of ten on the PEDro scale (). None of the studies were rated as poor methodological quality, four studies were rated as fair methodological quality [Citation20,Citation21,Citation36,Citation37], and five studies were rated as high methodological quality [Citation22,Citation23,Citation33–35]. Most common causes for risk of bias in individual studies were lack of concealed allocation, lack of blinding of subjects, therapists or assessors, relatively large amounts of missing data within key outcomes and no reported intention-to-treat analysis.
Table 1. PEDro assessments.
Data extraction
Detailed study characteristics and intervention characteristics are presented in and Citation3. All included studies were randomized clinical trials. Sample size ranged from 20 to 128 participants with a total included sample of 527 participants. Time after stroke or TIA at inclusion differed from less than one week up to and over one year. Seven studies aimed to exercise at 50–90% of heart rate reserve. One study aimed to exercise at maximum attainable level [Citation20] and one study aimed to exercise 10 to 15 on the Borg scale of perceived exertion [Citation37]. Duration of the intervention differed from six weeks to six months. In the majority of the studies (n = 7) participants exercised three times a week, in one study two times and in one study five times a week (). All studies reported pre- and post-intervention mean and standard deviation scores. Three studies also reported mean and standard deviation of change from baseline.
Table 2. Study characteristics.
Table 3. Intervention characteristics.
Meta-analysis
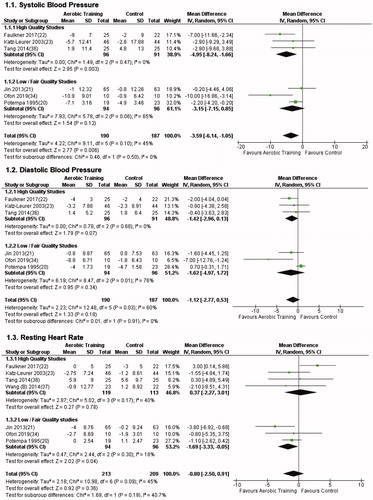
In total, nine studies (N = 527) [Citation20–23,Citation33–37] were included in the meta-analysis. We identified a total of 44 vascular or metabolic outcome variables that were reported in the eligible studies. Eleven of these outcomes were reported by at least two studies and were therefore included in the meta-analysis (). The primary outcomes SBP and DBP were reported in six studies, the primary outcome RHR was reported in seven studies.
Table 4. Summary of meta-analysis.
The meta-analysis can be found in and (forest plots of secondary outcomes as Supplementary Material). The meta-analysis of our primary outcomes showed a significant positive effect of aerobic training on SBP (−3.59 mmHg, 95% CI −6.14 to −1.05). The meta-analyses of secondary outcomes only yielded a significant result in fasting glucose (−0.12 mmol/l, 95% CI −0.23 to −0.02). The results for SBP further improved (−4.95 mmHg 95%, CI −8.24 to −1.66) when only high-quality studies were included (PEDro ≥6). Furthermore, the results for SBP also remained significant when only trials of shorter duration (≤12 weeks) were included (−3.79 mmHg, 95% CI −6.75 to −0.83). However, the results for fasting glucose were non-significant when we only included only high-quality studies (−0.09 mmol/l, 95% CI −0.26 to 0.08) and also when we only included trials of shorter duration (−0.13 mmol/l, 95% CI −0.28 to 0.02). The meta-analysis of all other outcomes were non-significant.
Low heterogeneity was observed within the outcome fasting glucose (I2=3%), as well as HDL cholesterol (I2=0%), LDL cholesterol (I2=0%) and peripheral pulse pressure (I2=0%). Moderate to high heterogeneity was observed in all other outcomes (range I2: 35–95%). Visual inspection of funnel plots did not result in any indication for publication bias.
Discussion
In this study, we explored the effect of aerobic training on vascular and metabolic risk factors for recurrent stroke. Our results show a statistically significant risk-reducing effect of aerobic training on SBP and fasting glucose compared to (non-aerobic) usual care or non-aerobic exercise. The effects of aerobic training on all other vascular and metabolic risk factors were non-significant.
Our study is the first review to evaluate the effect of solely aerobic training on vascular and metabolic risk factors for recurrent stroke. To get a broad picture of available evidence, we decided to include all vascular and metabolic risk factors which were identified by our search. This resulted in a total of 44 reported outcomes. Eleven outcomes were reported in at least two articles and therefore could be included in the meta-analysis. Our analysis yielded a significant effect of aerobic training on SBP and fasting glucose, which is particularly relevant since high blood pressure is one of the strongest risk factors for both first and recurrent stroke [Citation38,Citation39]. SBP seems to add more to the risk for stroke than DBP [Citation40]. A systolic blood pressures reduction of 5.1 mmHg was associated with a 22% reduction in the odds of having a recurrent stroke [Citation10]. In our review, four out of six studies found a reduction in systolic blood pressure >5.1 mmHg. Furthermore, fasting glucose is also an independent risk factor for first-ever stroke [Citation41] and for vascular changes like arterial stiffness [Citation42], although it seems to contribute more specifically to the risk of ischemic stroke and less to intracerebral hemorrhagic stroke [Citation43]. The fact that we found a significant effect on both SBP and fasting glucose shows that aerobic training is an important aspect of secondary prevention after stroke.
Three previous reviews have addressed the topic of exercise as a secondary prevention strategy after stroke. The review of Saunders et al. reported no significant effect of aerobic training on SBP and DBP. However, they included a combination of exercise and lifestyle interventions, and some relevant studies were not included in their review. Furthermore, they did not include any other vascular or metabolic risk factors besides SBP and DBP [Citation13]. The review of Wang et al. [Citation12] reported a significant result of combined aerobic training and resistance exercises on both systolic blood pressure (−4.30 mmHg, 95% CI −6.77 to −1.83) and diastolic blood pressure (−2.58 mmHg, 95% CI −4.71 to −0.46). The review of D’Isabella et al. did find significant effects of exercise training both with and without lifestyle coaching. The significant results of combined aerobic training and strength exercise on SBP (−2.51 mmHg, 95% CI −4.72 to −0.30) and fasting glucose (−0.15 mmol/l, 95% CI −0.28 to −0.02) in their meta-analysis are comparable to our results with only aerobic training. Therefore, our results seem to indicate that aerobic training is a valuable risk-reducing intervention after stroke, even without the addition of strength exercises or lifestyle coaching. In their analysis of combined exercise, D’Isabella et al. also reported a significant effect on HDL cholesterol (0.13 mmol/l, 95% CI 0.05 to 0.21), which was non-significant in our analysis of solely aerobic training (0.02 mmol/l, 95% CI −0.05 to 0.08). The significant effect that they found might have been caused by the addition of strength exercise in their analysis. Fasting insulin and HDL cholesterol were also significant within the analysis of combined exercise therapy and lifestyle coaching in their review. This indicates that some of the risk factors that were non-significant in our analyses are probably more receptive to other lifestyle factors like nutrition or strength exercise [Citation44]. A tailored approach of secondary prevention, personalized to the specific risk factors of the individual patient, might improve the effectiveness of secondary prevention programs.
Other outcomes which were analyzed in our review yielded no significant results. This could have been caused by a small number of included studies and participants or by the moderate to high heterogeneity that was present in most outcomes (). However, DBP, resting heart rate, total cholesterol, LDL cholesterol, and triglycerides were also non-significant in the study of D’Isabella et al.
Current secondary prevention programs are usually facility based, partly because of the need for professional guidance and materials used for strength exercise. However, previous studies have identified the costs of the program as well as availability and accessibility of the facility as two of the main barriers to engage in physical activity after stroke [Citation45,Citation46]. Furthermore, patients often stop exercising when the prevention program is completed, thereby reversing the progress that they have made during the program. An advantage of aerobic training is its potential to be executed in the home environment, therefore making it easier for patients to self-manage their risk factors. Patients can be incited to accomplish a lasting change in lifestyle and reduce long-term risk for recurrent stroke. Hence, aerobic training seems to contribute significantly to the effectiveness of secondary prevention programs after stroke.
The studies included in our review used relatively small study samples. Between studies, there was considerable diversity in stroke severity, time since stroke onset and duration of the intervention. While all intervention groups did execute training within the aerobic range of 50 to 85% of heart rate reserve, There was a considerable difference in the training intensity (). The training programs of the included studies were comparable in frequency, time and type of training. All included studies included a reasonable description of the FITT principle of the intervention (). Additional sensitivity analyses were performed to compensate for differences in the duration of the intervention and methodological quality of the studies. However, due to the limited number of included studies it was not possible to perform a sensitivity analysis for training intensity. The impact of training intensity on the effectiveness of aerobic training as secondary prevention after stroke is an interesting and relevant topic for future research. Sensitivity analyses with only high-quality studies (PEDro score ≥ 6) did lower I2 in most cases, and caused an increase in effect within the outcome SBP (mean differences from −3.59 to −4.95). Three out of nine included studies reported standard deviation of change from baseline. The calculation of change scores of other included studies from pre- and post-intervention standard deviation scores was performed according to the methods described in the Cochrane handbook [Citation29]. To optimize the possibility for study data to be included in meta-analyses, authors should consider to report both pre- and post-intervention data and change from baseline data.
Conclusion
Aerobic training results in a significant risk reducing effect on SBP and fasting glucose after stroke when compared to (non-aerobic) usual care or other non-aerobic exercise. Future research should examine optimal dose-response relationships and long term effects of aerobic training, as well as the optimum combination of therapies to target specific risk factors for stroke, in order to enable secondary prevention programs to be tailored to the needs of individual patients.
Supplementary_material.pdf
Download PDF (1.1 MB)Disclosure statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors declare no conflicts of interest.
References
- Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study. Lancet. 2017;390(10100):1211–1259.
- Ministry of health welfare and, S. Prevalence and incidence of stroke in the Netherlands [Internet]. 2017 [cited 2017 Dec 10]. Available from: https://www.volksgezondheidenzorg.info/onderwerp/beroerte/cijfers-context/hu1idige-situatie#!node-prevalentie-en-nieuwe-gevallen-van-beroerte
- Centraal bureau voor de statistiek. Cause of death statistics [Internet]. 2017 [cited 2017 Dec 10]. Available from: https://bronnen.zorggegevens.nl/Bron?naam=Doodsoorzakenstatistiek
- World Health Organisation (WHO). Top ten causes of death [Internet]. Fact sheet N°310. 2015 [cited 2017 Dec 17]. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/
- Muntner P, DeSalvo KB, Wildman RP, et al. Trends in the prevalence, awareness, treatment, and control of cardiovascular disease risk factors among noninstitutionalized patients with a history of myocardial infarction and stroke. Am J Epidemiol. 2006;163(10):913–920.
- Lai S, Studenski S, Duncan PW, et al. Persisting consequences of stroke measured by the Stroke Impact Scale. Stroke. 2002;33(7):1840–1845.
- Loeb C, Gandolfo C, Croce R, et al. Dementia associated with lacunar infarction. Stroke. 1992;23(9):1225–1229.
- O’Donnell MJO, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388(10046):761–775.
- Lawes CMM, Bennett DA, Feigin VL, et al. Blood pressure and stroke, an overview of published reviews. Stroke. 2004;35(3):776–785.
- Wang Z, Chen Z, Richart T, et al. Blood pressure lowering for the prevention of stroke recurrence. Int J Cardiol. 2011;152:S28.
- Cheng E, Chen A, Vassar S, et al. Comparison of secondary prevention care after myocardial infarction and stroke. Cerebrovasc Dis. 2006;21(4):235–241.
- Wang C, Redgrave J, Shafizadeh M, et al. Aerobic exercise interventions reduce blood pressure in patients after stroke or transient ischaemic attack: a systematic review and meta-analysis. Br J Sports Med. 2018;9:1–12.
- Saunders DH, Sanderson M, Hayes S, et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2016;(3):CD003316.
- MacKay-Lyons M, Thornton M, Ruggles T, et al. Non-pharmacological interventions for preventing secondary vascular events after stroke or transient ischemic attack (Review). Cochrane Collab. 2013;3:1465–1858.
- D’Isabella NT, Shkredova DA, Richardson JA, et al. Effects of exercise on cardiovascular risk factors following stroke or transient ischemic attack: a systematic review and meta-analysis. Clin Rehabil [Rehabil]. 2017;31(12):1561–1572.
- Mackay-Lyons M, Billinger SA, Eng J, et al. Aerobic Exercise Recommendations to Optimize Best practices In Care after Stroke: AEROBICS 2019 Update, Physical Therapy, pzz153.
- Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37(1):153–156.
- De Morree JJ, Jongert MWA, van der Poel G. Inspanningsfysiologie, oefentherapie en training. 1st ed. Houten, The Netherlands: Bohn Stafleu van Loghum; 2007. p. 150–159.
- Semlitsch T, Jeitler K, Hemkens LG, et al. Increasing physical activity for the treatment of hypertension: a systematic review and meta-analysis. Sports Med. 2013;43(10):1009–1023.
- Potempa K, Lopez M, Braun L, et al. Physiological outcomes of aerobic exercise training in hemiparetic stroke patients. Stroke. 1995;26(1):101–105.
- Jin H, Jiang Y, Wei Q, et al. Effects of aerobic cycling training on cardiovascular fitness and heart rate recovery in patients with chronic stroke. NeuroRehabilitation. 2013;32(2):327–335.
- Faulkner J, Tzeng Y, Lambrick D, et al. A randomized controlled trial to assess the central hemodynamic response to exercise in patients with transient ischaemic attack and minor stroke. J Hum Hypertens. 2017;31(3):172–177.
- Katz-Leurer M, Shochina M, Carmeli E, et al. The influence of early aerobic training on the functional capacity in patients with cerebrovascular accident at the subacute stage. Arch Phys Med Rehabil. 2003;84(11):1609–1614.
- Moher D, Liberati A, Tetzlaff J, et al. Reprint--preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009;89(9):873–880.
- Veerbeek JM, Van Wegen E, Van Peppen R, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One. 2014;9(2):e87987.
- Maher CG, Sherrington C, Herbert RD, et al. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–721.
- Barisic A, Leatherdale ST, Kreiger N. Importance of Frequency, Intensity, Time and Type (FITT) in physical activity assessment for epidemiological research. Can J Public Health. 2011;102(3):174–175.
- Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. Available from: https://community.cochrane.org/help/tools-and-software
- Higgins JPT, Deeks JJA, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) [Internet]. The Cochrane Collaboration; 2011 [cited 2019 Nov 12]. Available from: www.handbook.cochrane.org
- Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560.
- Ivey FM, Hafer-Macko CE, Ryan AS, et al. Impaired leg vasodilatory function after stroke, adaptations with treadmill exercise training. Stroke. 2010;41(12):2913–2917.
- Ivey FM, Ryan AS, Hafer-Macko CE, et al. Improved cerebral vasomotor reactivity after exercise training in hemiparetic stroke survivors. Stroke. 2011;42(7):1994–2000.
- Wang Z, Wang L, Fan H, et al. Adapted low intensity ergometer aerobic training for early and severely impaired stroke survivors: a pilot randomized controlled trial to explore its feasibility and efficacy. J Phys Ther Sci. 2014;26(9):1449–1454.
- Tang A, Eng J, Krassioukov A, et al. Exercise-induced changes in cardiovascular function after stroke: a randomized controlled trial. Int J Stroke. 2014;9(7):883–889.
- Wang Z, Wang L, Fan H, et al. Effect of low-intensity ergometer aerobic training on glucose tolerance in severely impaired nondiabetic stroke patients. J Stroke Cerebrovasc Dis. 2014;23(3):e187–93.
- Ivey FM, Ryan AS, Hafer-Macko CE, et al. Treadmill aerobic training improves glucose tolerance and indices of insulin sensitivity in disabled stroke survivors. Stroke. 2007;38(10):2752–2759.
- Ofori EK, Frimpong E, Ademeluyi A, et al. Ergometer cycling improves the ambulatory function and cardiovascular fitness of stroke patients – a randomized controlled trial. J Phys Ther Sci. 2019;31:211–216.
- Feigin V, Roth G, Naghavi M, et al. Global burden of stroke and risk factors in 188 countries, during 1990– 2013: a systematic analysis for the global burden of disease study 2013. Lancet Neurol. 2016;15(9):913–924.
- Kernan W, Ovbiagele B, Black H, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236.
- Inoue R, Ohkubo T, Kikuya M, et al. Stroke risk in systolic and combined systolic and diastolic hypertension determined using ambulatory blood pressure. Am J Hypertens. 2007;20(10):1125–1131.
- Lee G, Kim SM, Choi S, et al. The effect of change in fasting glucose on the risk of myocardial infarction, stroke, and all-cause mortality: a nationwide cohort study. Cardiovasc Diabetol. 2018;17(1):51.
- Liu Z, Wu K, Dai X, et al. Grading effect of abnormal glucose status on arterial stiffness and a new threshold of 2-h post-load glucose based on a Chinese community study. J Diabetes Investig. 2018;9(3):616–614.
- Sung J, Song Y-M, Ebrahim S, et al. Fasting blood glucose and the risk of stroke and myocardial infarction. Circulation. 2009;119(6):812–819.
- Fung TT, Stampfer MJ, Manson JE, et al. Prospective study of major dietary patterns and stroke risk in women. Stroke. 2004;35(9):2014–2019.
- Nicholson S, Sniehotta FF, Van Wijck F, et al. A systematic review of perceived barriers and motivators to physical activity after stroke. Int J Stroke. 2013;8(5):357–364.
- Rimmer J, Wang E, Snith D. Barriers associated with exercise and community access for individuals with stroke. J Rehabil Res Dev. 2008;45(2):315–322.