?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
The ability to adapt walking is important for safe ambulation. Assessments of impairments in walking adaptability with the Interactive Walkway may aid in the development of individualized therapy strategies of stroke patients. The Interactive Walkway is an overground walkway with Kinect v2 sensors for a markerless registration of full-body kinematics, which can be augmented with (gait-dependent) visual context to assess walking adaptability. This study aims to evaluate the potential of the Interactive Walkway as a new technology for assessing walking adaptability in stroke patients. Materials and methods: 30 stroke patients and 30 controls performed clinical tests, quantitative gait assessments and various walking-adaptability tasks on the Interactive Walkway. Outcome measures were compared between stroke patients and controls to examine known-groups validity. Pearson’s correlation coefficients were calculated to assess the relationship between walking-adaptability outcomes and commonly used clinical test scores of walking ability and spatiotemporal gait parameters of unconstrained walking. Results: Good known-groups validity for walking-adaptability outcomes was demonstrated. In addition, the vast majority of walking-adaptability outcomes did not or only moderately correlate with clinical test scores of walking ability and unconstrained walking parameters. Conclusion: Interactive Walkway walking-adaptability outcomes have good known-groups validity and complement standard clinical tests and spatiotemporal gait parameters.
The Interactive Walkway allows for a comprehensive walking-adaptability assessment.
Good known-groups validity for walking-adaptability tasks was demonstrated and walking-adaptability tasks complemented clinical tests and gait parameters.
The Interactive Walkway has potential for monitoring recovery of walking after stroke.
Assessments of walking adaptability may contribute to individualized interventions.
IMPLICATIONS FOR REHABILITATION
Introduction
Walking adaptability is essential for safe and independent ambulation [Citation1]. It is defined as the ability to adapt walking to meet behavioral task goals and demands of the environment [Citation1] and includes, among others, the ability to avoid obstacles, make sudden stops, place feet accurately in a cluttered environment and walk while performing a dual task [Citation1]. Laboratory studies showed that stroke patients generally have a reduced ability to adapt walking to environmental circumstances [Citation2–5]. This reduced walking adaptability makes these patients more susceptible to walking-related falls due to trips, slips or misplaced steps [Citation6–8]. Assessing walking adaptability thus seems essential to better understand and treat walking limitations. Unfortunately, there is no comprehensive clinical test of walking adaptability [Citation1] and laboratory studies have thus far typically focused on specific aspects of walking adaptability, mainly obstacle avoidance [Citation2–5,Citation9,Citation10].
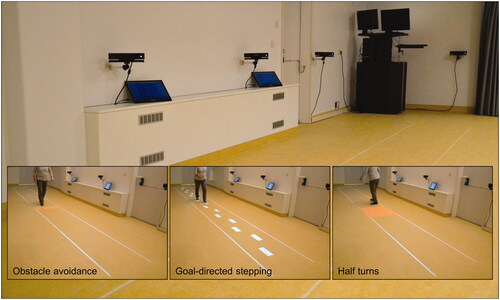
The Interactive Walkway () may help fill this void. It is an overground walkway equipped with multiple Kinect v2 sensors for markerless 3D full-body motion registration [Citation11], from which spatiotemporal gait parameters can be derived. The Interactive Walkway is augmented with projected (gait-dependent) visual context, such as suddenly appearing obstacles and stop cues (based on real-time processed gait data), to assess walking adaptability [Citation12]. Furthermore, attention-demanding secondary tasks, such as serial-3 subtractions [Citation10] or an auditory Stroop task [Citation3,Citation9], can be added to assess dual-task walking.
The aim of this study is to evaluate the potential of the Interactive Walkway as a new technology for assessing walking adaptability in stroke patients. To this end, we will (1) evaluate the known-groups validity of Interactive Walkway outcome measures by comparing them between stroke patients and controls, and (2) relate these outcome measures to commonly used clinical test scores for walking ability and spatiotemporal gait parameters of unconstrained walking; considering the Interactive Walkway’s strong focus on walking adaptability rather than general walking ability, we expected no or only moderate correlations.
Materials and methods
Subjects
Stroke patients were recruited from the outpatient clinic of the Leiden University Medical Center and from a list of patients who were discharged from the Rijnlands Rehabilitation Center. Controls were recruited via advertisement. In- and exclusion criteria are presented in , and differed between groups. Data was collected within the Technology in Motion project (protocol registered as NL54281.058.15; http://www.ccmo.nl/nl/ccmo-register). All subjects gave written informed consent, and the study was approved by the local medical ethics committee (P15.232).
Table 1. In- and exclusion criteria for stroke patients and controls.
Clinical tests for assessing walking ability
Two gait tests were included: the Timed-Up-and-Go test [Citation14,Citation15] and the 10-m walking test at comfortable and maximum walking speed [Citation15,Citation16]. Longer completion times indicate a poorer walking ability. The Tinetti Balance Assessment [Citation17,Citation18] has two sections that evaluate gait and balance performance, of which the combined score was used in this study (possible range 0–28; higher scores indicate better performance). Two balance tests were administered (with higher scores indicating a better balance): the 7-item Berg Balance Scale [Citation19], to measure static and dynamic balance during specific movement tasks (possible range 0–14), and the Functional Reach Test [Citation20,Citation21], to determine the maximal distance one can reach forward from a standing position.
The Interactive Walkway to quantify unconstrained walking and to assess walking adaptability
Unconstrained walking and walking adaptability were assessed on the Interactive Walkway (; Supplementary file S1). The Interactive Walkway comprised four spatially and temporally integrated Kinect v2 sensors with optimized inter-sensor distances [Citation22], providing markerless 3D full-body kinematics of various body points (e.g., ankles, spine base and spine shoulder). The Interactive Walkway was further equipped with a projector (EPSON EB-585W, ultra-short-throw 3LCD projector) to augment the entire 8-m walkway with visual context for the walking-adaptability tasks. The coordinate systems of the sensors and projector were spatially aligned to a common coordinate system using a spatial calibration grid. Interactive Walkway data was sampled at 30 Hz using custom-written software utilizing the Kinect-for-Windows Software Development Kit (SDK 2.0) and recently validated for unconstrained walking and walking adaptability assessments [Citation11,Citation12].
Subjects performed unconstrained walking and various walking-adaptability tasks on the Interactive Walkway (; see for more details and Supplementary video S1 for a video of the tasks). Unconstrained walking was assessed with an 8-m walking test. Walking adaptability was assessed with the following tasks: obstacle avoidance, sudden stops-and-starts, goal-directed stepping (with symmetric and irregular stepping stones), narrow walkway, speed adjustments (speeding up and slowing down), slalom, turning (half and full turns in both directions) and dual-task walking (plain and augmented). The Interactive Walkway assessment comprised a total of 35 trials. details the number of trials per task and their difficulty level. Dual-task walking was assessed by adding an auditory Stroop task [Citation23] in which the words high and low (in Dutch) were pronounced at a high or low pitch (i.e., congruent and incongruent stimuli) to both the plain 8-m walking test and the augmented obstacle-avoidance task, respectively. The subject had to respond with the pitch of the spoken word. All Interactive Walkway tasks were performed at a self-selected walking speed.
Figure 2. Schematics of unconstrained walking and walking adaptability tasks on the Interactive Walkway. The available response distance of the suddenly appearing obstacles and cues varied over subjects depending on their own gait characteristics.
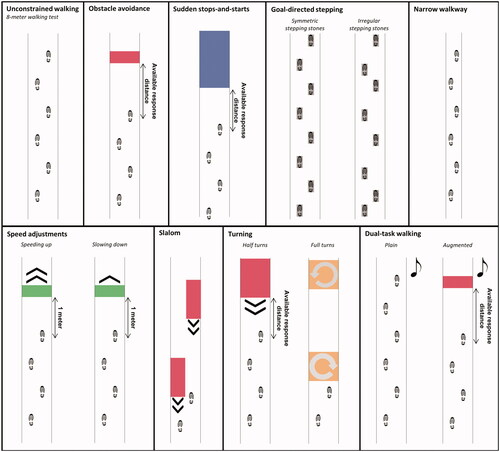
Table 2. Interactive Walkway tasks and outcome measures.
Procedure
Half of the subjects started with the block of clinical tests, the other half with the Interactive Walkway assessment. For the Interactive Walkway, every participant had a different order of the tests to prevent systematic order effects. However, the 8MWT was always performed first, which enabled us to adjust the settings of the walking-adaptability tasks to one’s own gait characteristics in an attempt to obtain a similar level of difficulty for each subject (see ). For example, available response times for suddenly appearing obstacles were controlled by self-selected walking speed during the 8-m walking test and available response distance (). Subsequently, the 8-m walking test was performed with the dual task (i.e., plain dual-task walking), preceded by a familiarization trial in which the auditory Stroop task was practiced while sitting. The remaining Interactive Walkway tasks were randomized in blocks (), with difficulty level randomized within the blocks and sufficient rest breaks in between trials to prevent fatigue. Stroke patients were permitted to use walking aids, including quad canes (n = 3), canes (n = 4), ankle foot orthoses (n = 11) and functional electrical stimulation (n = 1).
Data pre-processing and analysis
Data pre-processing followed Geerse et al. [Citation11,Citation12], as detailed in Supplementary file S1. The outcome measures of the Interactive Walkway tasks were calculated from specific body points’ time series (i.e., 3D time series of the ankles, spine base and spine shoulder), estimates of foot contact and foot off and step locations, as detailed in and Supplementary file S1. The average over trials per task per subject was calculated for all outcome measures.
Statistical analysis
The known-groups validity of clinical test scores, spatiotemporal gait parameters and Interactive Walkway walking-adaptability outcome measures was evaluated by comparing them between stroke patients and controls using independent-samples t-tests. We computed r ( to quantify the effect sizes, where values between 0.100 and 0.299 were regarded as small, between 0.300 and 0.499 as medium and above 0.500 as large effect sizes [Citation24].
Pearson’s correlation coefficients were determined only for stroke patients and calculated between Interactive Walkway walking-adaptability outcome measures and commonly used clinical test scores of walking ability and spatiotemporal gait parameters of unconstrained walking. Absolute correlations between 0–0.499, 0.500–0.699, 0.700–0.899 and 0.900–1.000 were regarded as low, moderate, high and very high, respectively [Citation25]. SPSS version 24 (IBM© SPSS©, Armonk, New York, United States) was used to perform the statistical analyses. Alpha was set at 0.05. No adjustment for multiple comparisons was made due to the exploratory nature of this study.
Results
In total, 30 stroke patients and 30 age- and sex-matched controls (mean ± std: 62.5 ± 10.1 vs. 62.9 ± 10.3 years, respectively; 18 males and 12 females in each groups) were included in this study. Stroke patients were 7.9 ± 7.3 years post-stroke, had a Fugl-Meyer Assessment lower extremity score of 19.7 ± 7.4 (possible range 0–34; higher scores indicate better motor function) and a Montreal Cognitive Assessment score of 24.9 ± 2.9 (possible range 0–30; higher scores indicate better cognitive abilities), which was not assessed in five stroke patients due to (severe) aphasia. Controls had a significantly higher Montreal Cognitive Assessment score of 27.7 ± 1.4 (p < 0.001).
In total, 91 trials (4.2% of all trials; 5.0–13.3% of trials per task) were not performed (63 trials; four patients were unable to complete all trials due to a reduced fitness level) or were not recorded correctly (28 trials; due to experimentation errors or one or more Kinect sensors failing to recognize stroke or control subjects).
Known-groups validity
Stroke patients performed significantly worse on all clinical tests compared to controls (p ≤ 0.001; ). This was also seen for the spatiotemporal gait parameters: all outcome measures showed values associated with lower walking speeds, wider step widths and less symmetric steps for stroke patients (p < 0.001; ). Furthermore, stroke patients performed significantly worse than controls on all Interactive Walkway walking-adaptability outcome measures, except stepping accuracy on irregular stepping stones, normalized walking speed of speeding up trials, turning time of half turns and normalized success rate during augmented dual-task walking ().
Table 3. Means, standard deviations and between-groups statistics of outcome measures of clinical tests, unconstrained walking and walking adaptability tasks on the Interactive Walkway for stroke patients and controls.
Correlations
For the vast majority of walking-adaptability outcome measures we found no-to-moderate correlations with clinical test scores and unconstrained-walking parameters. That is, of the 156 possible correlations between clinical test scores and Interactive Walkway walking-adaptability outcome measures (left block in ), 56 (35.9%) were significant, out of which 2 (1.3%) were very high, 4 (2.6%) were high, 31 (19.9%) were moderate and 19 (12.2%) were low. Of the 234 possible correlations between spatiotemporal gait parameters of unconstrained walking and Interactive Walkway walking-adaptability outcome measures (right block in ), 70 (29.9%) were significant, out of which 15 (6.4%) were high, 32 (13.7%) were moderate and 23 (9.8%) were low.
Figure 3. Overview of the correlation coefficients between commonly used clinical test scores [TUG, 10MWT-CWS, 10MWT-MWS, TBA, BBS, FRT] (x-axis), spatiotemporal gait parameters of unconstrained walking [UW1-9] (x-axis) and Interactive Walkway walking-adaptability outcome measures (OA1-3, SSS1-3, GDS1-4, NWW1-3, SA1-4, S1-2, T1-3, DT1-4; y-axis) in stroke patients. The order and abbreviations of the outcome measures on the axes is in agreement with .
![Figure 3. Overview of the correlation coefficients between commonly used clinical test scores [TUG, 10MWT-CWS, 10MWT-MWS, TBA, BBS, FRT] (x-axis), spatiotemporal gait parameters of unconstrained walking [UW1-9] (x-axis) and Interactive Walkway walking-adaptability outcome measures (OA1-3, SSS1-3, GDS1-4, NWW1-3, SA1-4, S1-2, T1-3, DT1-4; y-axis) in stroke patients. The order and abbreviations of the outcome measures on the axes is in agreement with Table 3.](/cms/asset/335ef445-5648-4eb2-9a0b-57b5da0c614a/idre_a_1731852_f0003_c.jpg)
Discussion
A stroke may result in impaired walking adaptability, affecting the ability to negotiate environmental challenges, which potentially contributes to the high fall risk seen in this population [Citation8]. Assessments of walking adaptability may guide gait rehabilitation programs or contribute to the design of future targeted and individualized interventions directed at improving safe community ambulation after stroke. However, currently available assessments of walking ability after stroke hardly take walking adaptability into account [Citation1]. We therefore evaluated the potential of the Interactive Walkway as a new technology for a quick, unobtrusive and comprehensive quantitative assessment of walking adaptability in stroke patients.
As a first step, we evaluated its known-groups validity. As expected, for almost all outcome measures stroke patients performed significantly worse than controls (). Group differences for spatiotemporal gait parameters of unconstrained walking, as measured with the Interactive Walkway, were as expected [Citation26–28] and in line with the results of an earlier study showing that the Kinect v2 sensor can measure spatiotemporal gait parameters with considerable accuracy in stroke patients [Citation29]. Also in accordance with the findings of previous studies, Interactive Walkway outcome measures of the various walking-adaptability tasks revealed that stroke patients have problems avoiding obstacles [Citation2,Citation4,Citation5], making sudden step adjustments [Citation30,Citation31], making full turns [Citation32] and combining walking with secondary tasks [Citation9,Citation28]. Besides, normalized walking speeds were significantly lower for stroke patients, indicating that they adjusted their walking speed more than controls when walking in complex environments. These results emphasize the importance of assessing walking adaptability in an overground setting, which allows stroke patients to lower their walking speed depending on their ability to meet environmental demands [Citation10]. In the current study, only stepping accuracy of the irregular stepping stones, normalized walking speed of speeding up trials, turning time of half turns and normalized success rate of augmented dual-task walking did not exhibit significant group differences. Nonetheless, medium and large effect sizes were found for all other Interactive Walkway outcome measures with differences occurring in the expected direction. Therefore, the results of this study suggest good known-groups validity for Interactive Walkway walking-adaptability tasks, similar to that of clinical tests of walking ability and spatiotemporal gait parameters quantified for unconstrained walking.
We assessed walking adaptability quite broadly and found that not all of the assessed tasks need to be included for a comprehensive assessment of walking adaptability. That is, Interactive Walkway tasks whose outcome measures do not exhibit group differences or are highly correlated with commonly used clinical test scores and/or uncontrained walking parameters can be excluded because they add little information. In this study, this concerned sudden starts, speed adjustments, full turns and augmented dual-task walking tasks. The vast majority of Interactive Walkway walking-adaptability tasks appeared to complement clinical test scores and unconstrained walking parameters, as evidenced by no-to-moderate correlations (). The various walking-adaptability tasks also seemed to assess different aspects of walking adaptability. That is, correlations among outcomes of the various walking-adaptability tasks were generally not significant (see Supplementary file S2), in contrast to clinical test scores and spatiotemporal gait parameters, which were highly interrelated and hence often somewhat redundant with one another. A comprehensive assessment of walking adaptability should thus include multiple complementary and discriminative Interactive Walkway tasks, such as obstacle avoidance, goal-directed stepping, narrow walkway and plain dual-task walking. A benefit of assessing walking adaptability comprehensively is that it may reveal specific walking limitations, which could then be targeted in individualized training programs [Citation33,Citation34]. Van Swigchem et al. [Citation4] found that even in mildly affected stroke patients walking adaptability may be reduced, possibly increasing their risk of falling. Training of walking adaptability, overground or on a treadmill, has shown to be effective in improving walking ability in stroke patients [Citation3,Citation8,Citation35,Citation36] and in reducing risk of falling [Citation8]. Interactive Walkway walking-adaptability assessments may assist in optimizing and patient-tailoring gait training programs by adjusting the training content and difficulty level to the specific needs and competences of the patient.
With this study, we examined the potential of walking-adaptability tasks on the Interactive Walkway for discriminating between stroke patient and controls and for providing information complementary to clinical test scores of walking ability and unconstrained walking parameters. Recent work found that walking-adaptability tasks also provide relevant information for the identification of fallers [Citation37]. Poor performance on the obstacle-avoidance and goal-directed stepping tasks were identified as risk factors for future falls [Citation37]. Identifying such walking-related fall-risk factors, which is possible with the Interactive Walkway, may further lead to more targeted, personalized and possibly more effective falls-prevention interventions.
One of the limitations of this study was that clinical tests, unconstrained walking and walking adaptability were only assessed in a single session. Future studies should examine their test-retest reliability to estimate minimal detectable change scores and responsiveness of the outcome measures that are essential for monitoring progress in gait rehabilitation. This can be done for a subset of tasks, namely those tasks that are deemed discriminative and complementary (as determined in the current study and in a recent related study with Parkinson’s disease patients [Citation38]) as well as tasks yielding potential risk factors for falls [Citation37]. A second limitation is that we noticed that the available response times were significantly lower for stroke patients on some walking-adaptability tasks, which were caused by a higher self-selected walking speed in those tasks than in the preceding unconstrained walking task. This could have negatively influenced the outcome measures on these tasks and as such have amplified group differences. In future studies the available response times should preferably be based on a real-time indication of walking speed, which is quite feasible with the Interactive Walkway. A third limitation could be that the Interactive Walkway only uses 2D projections to evoke step responses, which do not actually pose a physical risk for the patient. Although walking-adaptability tasks with 2D projections appeared effective, given the observed group differences with overall medium to large effect sizes, it may differ from interacting with real context. For example, Timmermans et al. [Citation10] recently observed that task prioritization differed in stroke patients negotiating physical and projected obstacles while concurrently performing an attention-demanding cognitive task: obstacle-avoidance performance was prioritized with physical obstacles, cognitive-task performance was prioritized with projected obstacles [Citation10].
Conclusion
We conclude that Interactive Walkway walking-adaptability assessments have good known-groups validity and provide information that is complementary to clinical test scores of walking ability and unconstrained walking parameters in stroke patients.
Supplementary_file_S2.pdf
Download PDF (452.1 KB)Supplementary_file_S1.pdf
Download PDF (114.8 KB)Supplementary_video_S1.mp4
Download MP4 Video (28.4 MB)Acknowledgements
We would like to acknowledge Bert Coolen for customizing the Interactive Walkway software to the specific purpose of this study. We would also like to thank Elma Ouwehand for her help with the measurements.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, DJG, upon reasonable request.
Additional information
Funding
References
- Balasubramanian CK, Clark DJ, Fox EJ. Walking adaptability after a stroke and its assessment in clinical settings. Stroke Res Treat. 2014;2014:591013.
- Den Otter AR, Geurts AC, de Haart M, et al. Step characteristics during obstacle avoidance in hemiplegic stroke. Exp Brain Res. 2005;161(2):180–192.
- Van Ooijen MW, Heeren A, Smulders K, et al. Improved gait adjustments after gait adaptability training are associated with reduced attentional demands in persons with stroke. Exp Brain Res. 2015;233(3):1007–1018.
- Van Swigchem R, van Duijnhoven HJ, den Boer J, et al. Deficits in motor response to avoid sudden obstacles during gait in functional walkers poststroke. Neurorehabil Neural Repair. 2013;27(3):230–239.
- Van Swigchem R, Roerdink M, Weerdesteyn V, et al. The capacity to restore steady gait after a step modification is reduced in people with poststroke foot drop using an ankle-foot orthosis. Phys Ther. 2014;94(5):654–663.
- Forster A, Young J. Incidence and consequences of falls due to stroke: a systematic inquiry. BMJ. 1995;311(6997):83–86.
- Hyndman D, Ashburn A, Stack E. Fall events among people with stroke living in the community: circumstances of falls and characteristics of fallers. Arch Phys Med Rehabil. 2002;83(2):165–170.
- Weerdesteyn V, de Niet M, van Duijnhoven HJ, et al. Falls in individuals with stroke. JRRD. 2008;45(8):1195–1213.
- Smulders K, van Swigchem R, de Swart BJ, et al. Community-dwelling people with chronic stroke need disproportionate attention while walking and negotiating obstacles. Gait Posture. 2012;36(1):127–132.
- Timmermans C, Roerdink M, Janssen TWJ, et al. Dual-task walking in challenging environments in people with stroke: cognitive-motor interference and task prioritization. Stroke Res Treat. 2018;2018:7928597.
- Geerse DJ, Coolen BH, Roerdink M. Kinematic validation of a multi-Kinect v2 instrumented 10-meter walkway for quantitative gait assessments. PLoS One. 2015;10(10):e0139913.
- Geerse DJ, Coolen BH, Roerdink M. Walking-adaptability assessments with the Interactive Walkway: between-systems agreement and sensitivity to task and subject variations. Gait Posture. 2017;54:194–201.
- Carson N, Leach L, Murphy KJ. A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores. Int J Geriatr Psychiatry. 2018;33(2):379–388.
- Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148.
- Flansbjer UB, Holmbäck AM, Downham D, et al. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. 2005;37(2):75–82.
- Collen FM, Wade DT, Bradshaw CM. Mobility after stroke: reliability of measures of impairment and disability. Int Disabil Stud. 1990;12(1):6–9.
- Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34(2):119–126.
- Canbek J, Fulk G, Nof L, et al. Test-retest reliability and construct validity of the tinetti performance-oriented mobility assessment in people with stroke. J Neurol Phys Ther. 2013;37(1):14–19.
- Chou CY, Chien CW, Hsueh IP, et al. Developing a short form of the Berg Balance Scale for people with stroke. Phys Ther. 2006;86(2):195–204.
- Duncan PW, Weiner DK, Chandler J, et al. Functional reach: a new clinical measure of balance. J Gerontol. 1990;45(6):M192–M197.
- Katz-Leurer M, Fisher I, Neeb M, et al. Reliability and validity of the modified functional reach test at the sub-acute stage post-stroke. Disabil Rehabil. 2009;31(3):243–248.
- Geerse D, Coolen B, Kolijn D, et al. Validation of foot placement locations from ankle data of a Kinect v2 sensor. Sensors-Basel. 2017;17(10):2301.
- Cohen G, Martin M. Hemisphere differences in an auditory Stroop test. Percept Psychophys. 1975;17(1):79–83.
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New York: Routledge; 1988.
- Mukaka MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24(3):69–71.
- Hak L, Houdijk H, van der Wurff P, et al. Stepping strategies used by post-stroke individuals to maintain margins of stability during walking. Clin Biomech. 2013;28(9–10):1041–1048.
- Phan PL, Blennerhassett JM, Lythgo N, et al. Over-ground walking on level and sloped surfaces in people with stroke compared to healthy matched adults. Disabil Rehabil. 2013;35(15):1302–1307.
- Yang YR, Chen YC, Lee CS, et al. Dual-task-related gait changes in individuals with stroke. Gait Posture. 2007;25(2):185–190.
- Latorre J, Llorens R, Colomer C, et al. Reliability and comparison of Kinect-based methods for estimating spatiotemporal gait parameters of healthy and post-stroke individuals. J Biomech. 2018;72:268–273.
- Roerdink M, Lamoth CJ, van Kordelaar J, et al. Rhythm perturbations in acoustically paced treadmill walking after stroke. Neurorehabil Neural Repair. 2009;23(7):668–678.
- Pelton TA, Johannsen L, Chen H, et al. Hemiparetic stepping to the beat: asymmetric response to metronome phase shift during treadmill gait. Neurorehabil Neural Repair. 2010;24(5):428–434.
- Shiu CH, Ng SS, Kwong PW, et al. Timed 360° turn test for assessing people with chronic stroke. Arch Phys Med Rehabil. 2016;97(4):536–544.
- Hollands KL, Pelton TA, van der Veen S, et al. A novel and simple test of gait adaptability predicts gold standard measures of functional mobility in stroke survivors. Gait Posture. 2016;43:170–175.
- Timmermans C, Roerdink M, Janssen TWJ, et al. Automatized, standardized and patient-tailored progressive walking-adaptability training: a proof-of-concept study. Phys Ther. 2019;99(7):882–892.
- Heeren A, Ooijen M, Geurts A, et al. Step by step: a proof of concept study of C-Mill gait adaptability training in the chronic phase after stroke. J Rehabil Med. 2013;45(7):616–622.
- Hollands KL, Pelton TA, Wimperis A, et al. Feasibility and preliminary efficacy of visual cue training to improve adaptability of walking after stroke: multi-centre, single-blind randomised control pilot trial. PLoS One. 2015;10(10):e0139261.
- Geerse DJ, Roerdink M, Marinus J, et al. Walking adaptability for targeted fall-risk assessments. Gait Posture. 2019;70:203–210.
- Geerse DJ, Roerdink M, Marinus J, et al. Assessing walking adaptability in Parkinson’s disease: “The Interactive Walkway”. Front Neurol. 2018;9:1096.