Abstract
Purpose
To compare stroke-specific health related quality of life in two country-regions with organisational differences in subacute rehabilitation services, and to reveal whether organisational factors or individual factors impact outcome.
Materials and methods
A prospective multicentre study with one-year follow-up of 369 first-ever stroke survivors with ischaemic or haemorrhagic stroke, recruited from stroke units in North Norway (n = 208) and Central Denmark (n = 161). The 12-domain Stroke-Specific Quality of Life scale was the primary outcome-measure.
Results
The Norwegian participants were older than the Danish (Mage= 69.8 vs. 66.7 years, respectively), had higher initial stroke severity, and longer stroke unit stays. Both cohorts reported more problems with cognitive, social, and emotional functioning compared to physical functioning. Two scale components were revealed. Between-country differences in the cognitive-social-mental component showed slightly better function in the Norwegian participants. Depression, anxiety, pre-stroke dependency, initial stroke severity, and older age were substantially associated to scale scores.
Conclusions
Successful improvements in one-year functioning in both country-regions may result from optimising long-term rehabilitation services to address cognitive, emotional, and social functioning. Stroke-Specific Quality of Life one-year post-stroke could be explained by individual factors, such as pre-stroke dependency and mental health, rather than differences in the organisation of subacute rehabilitation services.
The stroke-specific health related quality of life (SS-QOL) assessment tool captures multidimensional effects of a stroke from the perspective of the patient, which is clinically important information for the rehabilitation services.
The cognitive-social-mental component and the physical health component, indicate specific functional problems which may vary across and within countries and regions with different organisation of rehabilitation services.
For persons with mild to moderate stroke, longer-term functional improvements may be better optimised if the rehabilitation services particularly address cognitive, emotional, and social functioning.
IMPLICATIONS FOR REHABILITATION
Introduction
Stroke is a common cause of disability [Citation1], which may affect functioning in any aspect of a persons’ life [Citation2,Citation3]. Multiple functional impairments following stroke may occur separately or combined, including motor functioning, cognition, perception, visual functioning, emotional and mental health, and language problems [Citation4,Citation5]. Long-term effects are determined by the initial stroke lesion and the extent of subsequent recovery [Citation5]. Since the prevalence of stroke-related burden is expected to increase over the next decades, rehabilitation will remain a major part of post-stroke care [Citation6,Citation7]. The rehabilitation process includes aspects of professional care as well as active change, where individuals acquire the necessary knowledge and skills needed for optimum physical, psychological, and social function [Citation5]. To reduce long-term functional consequences and optimise treatment and rehabilitation outcomes, an effective and coordinated organisation with continuum of care is recommended [Citation6,Citation8,Citation9].
The long-term impact of stroke is often investigated by health-related quality of life (HRQOL) measures [Citation10–12]. The most comprehensive stroke-specific HRQOL instruments [Citation11], measure the perceived impact of stroke in several aspects of physical function, activities, and participation [Citation12,Citation13], as defined by the International Classification of Functioning, Disability and Health (ICF) [Citation14]. These self-report measures are often obtained from stroke survivors with mild to moderate strokes [Citation15–17], and cover physically related domains including mobility and self-care activities, as well as social and psychological domains, including work, language/communication, and cognition [Citation11]. The Stroke-Specific Quality of Life (SS-QOL) scale [Citation18] additionally covers domains related to fatigue, personality change, and vision. Domains rated as most affected vary across studies, with, for example, cognitive-related functions rated as both lower [Citation17] or higher [Citation15] than physical-related functions. Females [Citation19,Citation20], older individuals [Citation20–22], married patients [Citation23], patients who were self-care dependent before the stroke [Citation24,Citation25], patients with more stroke severity [Citation10,Citation26] and with psychological difficulties [Citation10,Citation21,Citation27,Citation28] have been found to have lower HRQOL. Few predictor studies have been performed with use of stroke-specific HRQOL measures [Citation25].
Whereas acute phase multidisciplinary stroke unit treatment is evidence-based [Citation29,Citation30], and described as excellent in high-income western countries [Citation31], more knowledge of service provision and rehabilitation effects in the subacute phase is needed [Citation6,Citation32]. For patients with mild to moderate consequences after stroke, evidence suggests skilled, coordinated multidisciplinary teams supporting home-based rehabilitation to increase functioning and regain independence in activities of daily living [Citation6,Citation32,Citation33]. Although continuum of care and access to multidisciplinary rehabilitation in rehabilitation units and after discharge to the community is recommended [Citation6,Citation8,Citation9], it remains unclear how to organise subacute stroke services with optimal delivery [Citation34,Citation35]. Two European studies have investigated generic HRQOL post-stroke in different countries, and found that HRQOL scores vary more than can be explained by stroke severity or sociodemographic factors alone [Citation24,Citation36]. How countries organise subacute rehabilitation services aimed at alleviating functional problems is likely to influence patients’ HRQOL; hence, studies that examine this are needed [Citation24,Citation36].
We are unaware of studies comparing stroke-specific HRQOL between country-regions characterized by a comparable welfare-system, but which differ in the organisation or delivery of the rehabilitation services following treatment in a stroke unit. We expected that stroke survivors from the Danish region, which offers a centralised service with standardised and stratified treatment, in line with recommendations, together with multidisciplinary stroke-competent community-based teams [Citation37–39], would report better HRQOL compared to participants from the North Norwegian rural region. In the latter region rehabilitation services are decentralised and locally governed. Accordingly, the aims of this comparative cohort study were to (1) describe and compare levels and profiles of the SS-QOL scale between cohorts from specified municipalities in two neighbouring countries with different organisation in subacute rehabilitation services one-year post-stroke, (2) explore whether country-region was associated with SS-QOL scores after accounting for selected covariates, and (3) to examine whether the demographic, stroke-related, or psychological factors were associated with SS-QOL scores.
Materials and methods
Setting
This was a prospective international multicentre study with participants living in the geographic area of the University Hospital of North Norway (UNN) and two municipalities in Central Denmark associated to the Aarhus University Hospital (AUH). presents an overview of the geographic and organisational differences in rehabilitation services. The Norwegian area is 23 times larger than the Danish area and includes 30 municipalities (). In Denmark, the study population was admitted to the AUH serving 1.3 million inhabitants, whereas the participants from North Norway were admitted to one of three stroke units with evident lower patient-volumes. The regions in this study were situated in high-income countries with reasonably equivalent public welfare and tax financed healthcare systems, well-organised stroke unit acute rehabilitative treatment, similar high admittance rates to stroke units (>90%), and comparable surveillance-rates post-stroke [Citation40,Citation41]. National guidelines in both countries recommend that all patients having a suspected stroke are admitted directly to stroke units. All citizens have access to specialised acute and stroke unit care with multidisciplinary treatment. The two study regions contrast distinctly in degree of treatment centralisation in stroke units as well as national recommendations of rehabilitation services organisation following stroke unit care. Rehabilitation plans are implemented in Denmark during discharge from stroke units. If further in-hospital rehabilitation is needed after stroke unit treatment at AUH, patients are transferred to a regional hospital. In the Norwegian region, admittance to in-patient rehabilitation units is judged individually, and not part of a standardized procedure. The two specialized rehabilitation departments at the hospitals “UNN Tromsø” and “UNN Harstad” have broader multidisciplinary teams and increased possibilities for medical treatment compared to the non-specialised departments. This is reflected in the admittance practice. Skilled, specialised multidisciplinary teams are implemented in the two Danish municipalities, but not in the examined area of North Norway ().
Table 1. Geographic and organisational differences in acute and subacute stroke rehabilitation in the study regions (2014–2016).
Participants
Persons with first-time stroke admitted to stroke units and included in the country’s respective Stroke Registry, were consecutively enrolled between March 2014 (Norway), or June 2014 (Denmark) through December 2015. Stroke survivors were included if they were (1) 18 years or older; (2) diagnosed with a first-time stroke according to the International Classification of Diseases, version 10 (ICD-10 I.61 or I.63); (3) admitted to the stroke unit of AUH (Denmark), or one of three stroke units at the University Hospital of North Norway, located at either Tromsø, Harstad, or Narvik; and (4) living in either Favrskov or Randers municipality in Denmark, or in the defined geographic area of North Norway. For the current study, stroke survivors had to be able to complete the questionnaires at 12-month follow-up. The exclusion criteria were patients with stroke related to brain malignancy, subarachnoid haemorrhage, or brain trauma.
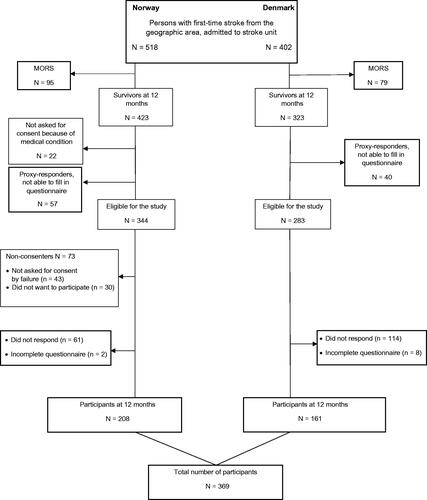
In total, 920 patients with first-time ischaemic or haemorrhage stroke (ICD10 I.63 and I.61) were potential participants for the study (Denmark N = 402 and Norway N = 518). Of those, 293 persons were excluded at 12 months follow-up (deceased n = 174; too sick to be included n = 22; consent by proxy, unable to complete the questionnaires n = 97). Of the eligible 627 stroke survivors one-year post-stroke, 73 did not consent, 175 did not respond, and 10 were excluded because of unsatisfying completion of the SS-QOL scale. A total of 369 participants were included in the study. A flowchart following the STROBE criteria is shown in .
Comparing the 73 Norwegian non-consenters with the participants showed that the non-consenters were older (Mage = 73.6, SDage = 13.5 vs. Mage = 69.8, SDage = 11.3), and significantly more were women (63% vs. 43%). Compared with participants, non-responders (n = 175) in both countries had significantly more severe acute stroke assessed with the Scandinavian Stroke Scale (SSS), (Norway: M = 47, SD = 8.6 vs. M = 44, SD = 10.4; Denmark: M = 49, SD = 9.9 vs. M = 45, SD = 14.1). In addition, the Norwegian non-responders had longer length-of-stay in stroke units than Norwegian participants (median 7 days vs. 4 days). No significant differences in age or gender were found between non-responders and participants in any of the countries.
Data collection procedures and instruments
In the preparation phase of the study, relevant and comparable variables were identified in the national stroke registries of both countries. In Norway, stroke unit nurses or health professionals informed potential participants about the study and asked for written consent either in person or by telephone. In Denmark, a health professional retrieved information directly from the National Stroke Registry on patients with stroke who were living in the respective municipalities. Those who completed the questionnaires at 12 months were included in the current analysis.
Data were collected three times: (1) at baseline we recorded pre-stroke demographic and stroke related data that was retrieved from the stroke registries and in Norway from patients’ medical journals, (2) at 3 months post-stroke, we collected information about rehabilitation services through telephone, or in Norway in connection with an outpatient visit or based on registrations from the stroke registry, and (3) at one-year post-stroke, all potential participants from both regions received a posted questionnaire package including socio-demographic questions, the SS-QOL scale, and the Hospital Anxiety and Depression Scale (HADS).
The first rehabilitation received after discharge from the stroke unit was operationalised into: inpatient rehabilitation, either in a specialised neurorehabilitation unit or less specialised rehabilitation unit; community-based rehabilitation at day centres or at home, or; no rehabilitation services after discharge from the stroke unit. After 3 months post-stroke, no further individual rehabilitation service data were collected, instead an overview of the rehabilitation services organisation in the two country-regions is presented in .
Demographic data and stroke characteristics
At baseline, data on age, gender, stroke subtype, acute treatment, and length-of-stay were obtained from both countries’ National Stroke Registries. Information on marital status (married/cohabitant or single), pre-stroke self-care dependence (living with or without assistance pre-stroke), and work status (working/studying prior to stroke) were obtained from questionnaires.
The Danish Stroke Registry used the Scandinavian Stroke Scale (SSS) to measure initial stroke severity, and the Norwegian Stroke Registry used the National Institute of Health Stroke Scale (NIHSS). Neurologic impairments were measured within 24 h and were graded in both scales, thus we chose to use the SSS, because data from the Danish National Stroke Registry were more complete (1 missing) than the National Norwegian Stroke Registry (104 missing for this study). For the Norwegian population missing NIHSS data, a classification was identified by an experienced senior physician (author, G.H) using medical records data. NIHSS scores were transformed to SSS scores using a mathematical algorithm with a reasonable to good degree of reliability [Citation42].
The HADS
The HADS consists of 14 items and can be used to reliably and validly detect the mental health states of anxiety (7 items) and depression (7 items) [Citation43], and can be used for persons with stroke [Citation44]. The response scale ranges from 0 to 3, where higher scores indicate higher severity, and subscale sum scores range from 0 to 21.
The SS-QOL scale
The outcome measure used to assess the perceived impact of stroke was the comprehensive SS-QOL scale [Citation18,Citation45]. The SS-QOL scale was previously validated in Denmark [Citation17] and recently validated for use in Norway [Citation46]. The scale was developed through interviews with stroke survivors and their closest family members. The SS-QOL scale consists of 49 items covering 12 domains: mobility, energy, upper extremity function, work and productivity, mood, self-care, social roles, family roles, vision, language, thinking, and personality. Each domain is measured by three to six items using a 5-point (1–5) Likert scale where higher scores indicate better function. An example from the language domain is “Did you have trouble finding the word you wanted to say?,” and possible replies: (1) could not do it at all, (2) a lot of trouble, (3) some trouble, (4) a little trouble, (5) no trouble at all. A previous study identified two components of the SS-QOL scale, physical and psychosocial, in a study of patients with aneurysmal subarachnoid haemorrhage [Citation47].
Index scores were generated that allowed a comparison of the relative level of each domain and total score. Reliability for the SS-QOL scale has been documented by several studies, with acceptable and good internal consistency of the domains (Cronbach’s alpha = 0.79–0.93 for Norway, and 0.81–0.94 for Denmark). Test-retest reliability of the SS-QOL scale has similarly been documented as generally good (Spearman’s rho = 0.67–0.94 for Norway, and 0.65–0.99 for Denmark) [Citation17,Citation46].
Data quality
Data quality in the completed questionnaires was good. Practical support to complete the questionnaire was accepted as long as the stroke survivor gave the replies themselves. If questionnaires were answered on behalf of an individual, the questionnaire was considered a proxy-response () and excluded from the study. In Norway, missing data was collected from participants by telephone when possible. Missing HADS items were replaced by mean subscale scores. Missing SS-QOL items were replaced by the mean scores for the corresponding domain. One or two missing items were accepted for domains with a total of five or six items, and one missing item was allowed for domains with a total of three items. We choose to exclude questionnaires that had more than five missing items in the total scale (2%), and those where we could not generate subscale scores. About 46% of the rehabilitation data were missing for the Danish participants that could not be reached by telephone.
Statistical analyses
Analyses were conducted using IBM SPSS Statistics, version 26. The descriptive data were presented as means, standard deviations (SDs), ranges or proportions. Chi-square, or Fisher’s Exact tests were used to compare categorical data, whereas independent sample t-tests, or the non-parametric Mann-Whitney U tests, were used to compare differences in continuous data. Binominal distribution (McNemar’s test) was used to detect significant dichotomous changes within each country-region. Because of high inter-correlations between the SS-QOL domain scores, we performed a principal component analysis to see if they clustered and formed more general components. The factor loadings were promax rotated (Kappa = 4).
Hierarchical linear regression analyses were conducted to identify associations in the SS-QOL scale with the between country-region of prime interest. Variables were entered in blocks, with model fit reported as adjusted R2 for each block. Four blocks of variables were specified: (1) country, (2) adjustment for age (continuous), gender (male/female), marital status (married/cohabitant vs. single), self-care independent vs. dependent prior to stroke, (3) acute stroke severity, stroke subtype, and length-of-stay in the stroke unit, and (4) HADS anxiety and HADS depression scores. Initial beta values represent each variables’ first appearance in the model, whereas final beta values represent the final model. Since the distributional properties of the SS-QOL outcome scores were highly leptokurtic and skewed, the independent T-tests as well as the regression models were bootstrapped with 5000 re-samplings to produce less biased confidence intervals. Since bootstrapping does not provide standardised beta coefficients, all variables were transformed to z-scores (M = 0, SD = 1).
Ethics
This study was conducted according to the Helsinki Declaration regarding informed consent and confidentiality. The Danish Data Protection Agency (record no. 1-16-02-363-14) and the Norwegian Committee for Medical Research Ethics (no. 2013/1461) approved the study.
Results
Descriptive data for the two samples are shown in . Most participants had mild (70%) to moderate (26%) initial stroke severity. Participants from North Norway were slightly older than participants from Central Denmark (MNorway = 69.8 years, range 38–91; MDenmark = 66.7 years, range 36–93, p < 0.05), had higher initial stroke severity (p < 0.01), and longer stroke unit length-of-stay (mean 4 vs. 2 days, respectively; p < 0.001). Anxiety and depression levels were not significantly different between the respective cohorts. More participants from Norway were widowed or single before the stroke incidence, and more participants from Denmark were working prior to their stroke.
Table 2. Demographic, stroke characteristics and treatment factors of participants with first-time stroke.
Rehabilitation pathway data after discharge from the stroke unit were available for all Norwegian participants and for 87 (54%) of the Danish participants. Non-responders at 3 months follow-up by telephone in Denmark (n = 74) did not differ significantly from the Danish participants regarding age, gender or stroke severity. As shown in , available information indicated more use of inpatient rehabilitation in North Norway, whereas Danish participants received municipality-based rehabilitation services to a higher degree either at home or in a day centre. In the total population, 39% did not have any rehabilitation after discharge from the stroke unit. Significantly more Norwegian participants had no follow-up after discharge from the stroke unit compared to Danish participants. Because a large portion of the Danish cohort had missing data for these measures, we choose to present the results only descriptively.
Comparing pre- and post-stroke data, the within country analyses demonstrated a significant decrease in self-care independence (from 93% to 80% in Denmark, and from 89% to 80% in Norway, both p’s < 0.001) and work status (from 35% to 22% in Denmark, p < 0.001; from 20% to 14% in Norway, p < 0.01).
A principal component analysis of the 12 sub-domains of the SS-QOL scale extracted two components representing more general dimensions: (1) a physical health component (PH) with strong loadings ranging between 0.89 and 0.93 (self-care, mobility, work/productivity, upper extremity function); and (2) a cognitive-social-mental component (CSM) with strong loadings ranging between 0.82 and 0.92 (thinking, personality, family roles, mood, social roles, energy). The vision and language domains were excluded because they did not correlate with either of these two components.
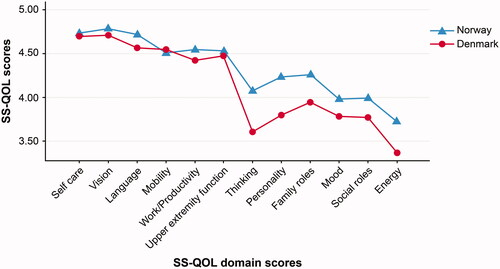
The total SS-QOL score was high in both regions (MNorway = 4.36, SD = 0.68; MDenmark = 4.19, SD = 0.76). Participants in both country-regions reported significantly more functional problems in the energy, thinking, mood, personality, social, and family roles domains ( and ) compared to the physical domains. A statistically significant difference between the country-regions emerged for five SS-QOL domains; however, these differences were of minor magnitude, with the North Norwegian region showing slightly better function (Cohen’s dEnergy = 0.26, dFamily roles = 0.28, dLanguage = 0.25, dThinking = 0.39 and dPersonality = 0.37). The SS-QOL total scale (Cohen’s d = 0.24) and the CSM component (d = 0.29) also showed a small but significant difference.
Table 3. Stroke-Specific Quality of Life scores in Norway and Denmark one-year post-stroke.
The multiple regression analysis maintained statistical significance for the country difference variable (). Adding the covariates age, pre-stroke dependency, stroke severity, anxiety, and depression explained a substantial amount of the variance in the SS-QOL total score and two component scores (adjusted R2 ranging between 0.40 and 0.59) with anxiety and, in particular, depression being the most substantial explanatory variables followed by pre-stroke self-care dependence and initial stroke severity. For the explained variance in the PH component of the SS-QOL, HADS anxiety was unimportant, and age (older) contributed slightly. In the CSM component of SS-QOL, age lost significance and stroke severity contributed with a lower magnitude. Replicating these analyses within each country showed the same significant explanatory findings in the Norwegian sample, whereas in the Danish sample age dropped out and gender came in as significant contributors (Supporting Information Table S1). There were small changes between initial and final beta values in all regression models.
Table 4. Regression analysis of the Stroke-Specific Quality of Life (SS-QOL) total scale and the two component scales. Independent variables were entered in four blocks.
Discussion
In this study of multidimensional SS-QOL across country-regions, individual factors were found to impact outcome more than region-specific characteristics like organisational differences in subacute rehabilitation services. The findings mainly cover patients with initial mild to moderate stroke severity. Both the Norwegian and Danish cohort experienced more problems within cognitive, social, energy, and emotional domains than in physical domains. A principal component analysis of the 12 SS-QOL domains extracted two components that we named the PH component and the CSM component. Compared to the participants in the North Norwegian region, participants in the Danish region reported more functional problems in the SS-QOL total scale and in the two component scales after adjustment for predefined covariates; thus, this finding did not confirm our expectation of better self-reported HRQOL in the Central Denmark region with more structured subacute multidisciplinary community-based rehabilitation services. Age, self-care dependence, stroke severity, anxiety, and depression were associated with SS-QOL scores.
Domains and profiles of the SS-QOL scale
HRQOL instruments may be assessed generically if comparisons between diseases are of prime interest, or specifically for the actual disease if distinct clinical aspects of functioning are more important. Thus, the latter represents a more comprehensive assessment of functional domains that are relevant following stroke [Citation10–12]. Compared to other studies [Citation25,Citation48], both regions scored high on the SS-QOL total scale, indicating that the populations with mild to moderate stroke severity had good functioning one-year post-stroke. The SS-QOL scale includes items across body functions, activities, and participation, as classified in the ICF [Citation12,14]. Previous studies using multidimensional instruments showed variability in scores regarding the domains related to physical and cognitive functions. In contrast to our study, a Turkish study [Citation25] found personality and thinking to be among the domains with highest scores. Also, a Swedish prospective observational study recruiting stroke survivors from stroke units [Citation15], measured HRQOL with the Stroke Impact Scale one-year post-stroke and found that the participants reported more problems with functioning in the physical domains than in the cognitive and social domains. About 87% of the sample in that study were people with initial mild to moderate strokes, and slightly more responders had severe strokes than in our study (13% vs. 4%). However, other European studies where the majority of participants had mild and moderate strokes support our findings, reporting relatively more problems in the SS-QOL cognitive, social, and emotional domains [Citation17,Citation47]. Differences and similarities in HRQOL scores may rely on the heterogeneity of the patient populations evaluated, as well as various recruitment procedures for both stroke units and for the different studies. In our study, both cohorts scored lowest in the energy domain one-year post-stroke. Fatigue is common after stroke, tends to persist, and contributes to lower QOL [Citation49,Citation50]. The SS-QOL is the only stroke-specific multidimensional instrument that includes this element to measure stroke-specific HRQOL, which may give the measure an advantage.
Components of comprehensive HRQOL measures
A previous validation study of the SS-QOL scale for patients with aneurysmal subarachnoid haemorrhage [Citation47], used principal component analysis and revealed two components of the instrument; physical and psychosocial health. However, this study is the first to report the existence of two similar components of the SS-QOL scale in stroke survivors with ischaemic and haemorrhagic stroke. As discussed by others [Citation47], using the two SS-QOL components may be useful for providing scores for PH and the CSM aspects of the HRQOL without hiding important component HRQOL findings in the total score. Additionally, with the nature of heterogenicity in stroke survivors, the SS-QOL component scores may better than SS-QOL total scores indicate specific rehabilitation needs in different populations or at an individual level.
Country-region differences
The finding that Norwegian participants had higher scores in some of the SS-QOL domains and total scores compared to the Danish participants could be a result of selection bias. First, participants from both regions had less initial stroke severity than non-participants; hence, our results are more representative of stroke survivors having a mild or moderate stroke. Second, the Danish participants were younger, more work-active prior to stroke, and more participants from Denmark were married. However, work-activity and marital status were not significantly associated with the SS-QOL scores, and younger age in this and other studies was predictive of better rather than worse HRQOL scores [Citation20–22]. Another possible explanation for different results could be the single centre inclusion in Denmark compared to multicentre inclusion in Norway. Higher treatment volumes have in some studies resulted in better stroke outcome [Citation51,Citation52], but then one would expect higher scores in SS-QOL in the Danish region, so this explanation for the observed difference is unlikely. Two other Scandinavian studies did not observe a prognostic difference between patients admitted to stroke units with different treatment volumes [Citation53,Citation54]. The organisation of the rehabilitation services may affect the overall service quality, thereby also affecting the outcome of the treated patients [Citation37,Citation55]. We thus expected improved functioning in the Danish than the Norwegian population, as the continuum of care and multidisciplinary professional support in Denmark are more systematically organised than in Norway [Citation37]. However, our findings indicate rather similar results across the countries, with doubtful clinical importance of the North Norwegian region.
The investigated geographic area in Denmark has, over the past decade, systematically developed competence in cognitive rehabilitation [Citation37,Citation56], and the Danish population in this study received more municipality-based rehabilitation services after stroke unit discharge compared to the Norwegian cohort. These conditions may matter, given stroke survivors’ insight into their own functional dilemmas regarding cognition, consequently resulting in more reported problems. As discussed by others [Citation57,Citation58], people with different expectations may report that they have a different HRQOL even when they have the same clinical condition, and current measures cannot distinguish between the individual experience of disease and expectations of health. In contrast, the apparent provision of more inpatient rehabilitation in the North Norwegian region could have a positive impact of functional cognitive abilities [Citation59]. As in previous studies comparing HRQOL across European countries [Citation24,Citation36], variations in SS-QOL scores in our study could not be entirely explained by sociodemographic factors, stroke severity, mental health, or even between country differences in rehabilitation organisation.
Factors associated with the SS-QOL scale
In accordance with most other studies, higher age, pre-stroke dependence, stroke severity, anxiety, and depression were associated with more reported functional problems [Citation10]. Findings regarding age are not fully consistent. While our study demonstrated that higher age was associated with lower SS-QOL total score and physical component scores, age was not of importance for scores in the SS-QOL CSM component. In one study [Citation60], younger stroke survivors (<65 years) reported more problems in social, emotional, vitality, and mental health domains of the Short Form Health Survey (SF-36) one-year post-stroke, as well as more problems in the PH components of SF-36 at three years post-stroke. Another study [Citation61] found no significant difference in SS-QOL scores between stroke survivors above and below 65 years following stroke. As discussed by others [Citation60], associations of HRQOL may vary over time after stroke, and may depend on whether different aspects or components of the multidimensional HRQOL are being considered.
Following a stroke, the occurrence of anxiety and depression is highly clinically significant, and frequently associated with HRQOL [Citation62,Citation63]. Anxiety has been shown to be common following stroke (23–29%), and to affect stroke survivors’ SS-QOL scores independent from depression [Citation27]. This is consistent with our findings for the SS-QOL total scale and CSM component, whereas anxiety was not a significant explanatory variable for the PH component. Post-stroke depression is a consistent determinant of HRQOL and probably the most important long-term psychosocial consequence following stroke [Citation10]. Depression after stroke has been reported with a frequency of 18–61% depending on patient selection criteria, diagnostic criteria for depression, and duration after the stroke event [Citation61].
Study strengths and limitations
This study had a prospective observational design and a fairly acceptable response rate (59%), although several limitations could limit generalisation of results. First, a non-response bias may occur if responders differ substantially from non-responders, which may be the case in this study as those with more severe stroke were less likely to respond. Results in both country-regions are limited to stroke survivors with initial mild and moderate stroke, a finding reported also in other cohort-studies of HRQOL after stroke. Given the extensive questionnaire used, results cannot be generalised to patients with severe disabilities or aphasia following stroke, and this could influence the profiles of the SS-QOL responses. Further, although the inclusion criteria were identical, the study samples differed between the country-regions. Norwegian participants were slightly older and had higher initial stroke severity than Danish participants. A possible explanation could be different stroke unit admission practices, or better recruitment of older individuals at follow-up in the North Norwegian region. Theoretically, this selection is expected to result in worse SS-QOL scores in the Norwegian than the Danish region whereas our results were in favour of the Norwegian participants. However, the overall differences were small and probably of no clinical significance. Although controlling for this type of difference is never fully possible, we expected regression analyses to account for case mix [Citation36]. Second, factorial invariance (e.g., whether the given measure is interpreted in a conceptually similar manner by respondents representing different cultural backgrounds) is a prerequisite for generalising results from patient reported outcome measures. To the best of our knowledge, the SS-QOL scale has not previously been compared across cultures and an evaluation of measurement quality related to this study has not been done, representing a limitation of our study. Although the two countries included in this study have quite similar cultures and languages, a cultural difference in interpretation of the questionnaire cannot be ruled out. Third, missing individual rehabilitation pathway data makes comparison of individual services between regions uncertain. Consequently, individual rehabilitation data was not used in more advanced statistical analyses. The missing Danish data could comprise patients with increased use of inpatient rehabilitation compared to the included participants. However, the non-responders without rehabilitation data did not differ significantly from the participants with rehabilitation data regarding age, gender or stroke severity, implying that the available results fit well with the overall descriptive data. Further, the available data supported the descriptive comparison of municipality-based rehabilitation organisation between the two country-regions. In the future, when ongoing work is completed, a more standardised classification of rehabilitation services will be available [Citation64,Citation65]. Study strengths include recruitment from stroke units in countries with high admittance rates, definition of geographic areas, few exclusion criteria, and standardised measurements in both acute care and follow-up.
In conclusion, there was a small difference in SS-QOL scores between the two country-regions, and in favour of the Norwegian participants. Given the above accounts, it is fair to conclude that country-region differences are of a negligible magnitude. This result disproved our hypothesis. The profiles of the SS-QOL scale in both country-regions may indicate requirements for long-term individualized follow-up with rehabilitation interventions that to a greater extent address cognitive, emotional, and social functioning. Our results and conclusions may generalize best to subpopulations with mild and moderate stroke, and less well to patients with a more severe stroke. Future studies including impact of individual rehabilitation pathways in natural settings are warranted.
Supplementary_Table_S1.pdf
Download PDF (101.8 KB)Disclosure statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Additional information
Funding
References
- Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120(3):439–448.
- WHO. World report on disability. Geneva: World Health Organization; 2011.
- Berzina G, Smilskalne B, Vetra A, et al. Living in Latvia after stroke: the association between functional, social and personal factors and the level of self-perceived disability-a cross-sectional study. BMJ Open. 2016;6(6):e010327.
- Abdul-Rahim AH, Quinn TJ, Alder S, et al. Derivation and validation of a novel prognostic scale (modified-stroke subtype), Oxfordshire Community Stroke Project classification, age, and prestroke modified Rankin) to predict early mortality in acute stroke. Stroke. 2016;47(1):74–79.
- Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable taskforce. Int J Stroke. 2017;12(5):444–450.
- Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377(9778):1693–1702.
- Norrving B, Barrick J, Davalos A, et al. Action plan for stroke in Europe 2018-2030. Eur Stroke J. 2018;3(4):309–336.
- Hebert D, Lindsay MP, McIntyre A, et al. Canadian stroke best practice recommendations: stroke rehabilitation practice guidelines, update 2015. Int J Stroke. 2016;11(4):459–484.
- Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98–e169.
- Carod-Artal FJ. Determining quality of life in stroke survivors. Expert Rev Pharmacoecon Outcomes Res. 2012;12(2):199–211.
- Salter KL, Moses MB, Foley NC, et al. Health-related quality of life after stroke: what are we measuring? Int J Rehabil Res. 2008;31(2):111–117.
- Teixeira-Salmela LF, Neto MG, Magalhaes LC, et al. Content comparisons of Stroke-Specific Quality of Life based upon the international classification of functioning, disability, and health. Qual Life Res. 2009;18(6):765–773.
- Silva SM, Correa JCF, Pereira GS, et al. Social participation following a stroke: an assessment in accordance with the international classification of functioning, disability and health. Disabil Rehabil. 2019;41(8):879–886.
- WHO. International classification of functioning, disability and health (ICF); 2001 [cited 2019 Aug 22]. Available from: http://www.who.int/classification/icf/en/
- Guidetti S, Ytterberg C, Ekstam L, et al. Changes in the impact of stroke between 3 and 12 months post-stroke, assessed with the Stroke Impact Scale. J Rehabil Med. 2014;46(10):963–968.
- Tornbom K, Lundalv J, Sunnerhagen KS. Long-term participation 7-8 years after stroke: experiences of people in working-age. PLoS One. 2019;14(3):e0213447.
- Muus I, Williams LS, Ringsberg KC. Validation of the Stroke Specific Quality of Life Scale (SS-QOL): test of reliability and validity of the Danish version (SS-QOL-DK). Clin Rehabil. 2007;21(7):620–627.
- Williams LS, Weinberger M, Harris LE, et al. Development of a Stroke-Specific Quality of Life scale. Stroke. 1999;30(7):1362–1369.
- Gargano JW, Reeves MJ. Paul Coverdell National Acute Stroke Registry Michigan Prototype Investigators. Sex differences in stroke recovery and Stroke-Specific Quality of Life: results from a statewide stroke registry. Stroke. 2007;38(9):2541–2548.
- Sturm JW, Donnan GA, Dewey HM, et al. Quality of life after stroke: the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke. 2004;35(10):2340–2345.
- White J, Magin P, Attia J, et al. Predictors of health-related quality of life in community-dwelling stroke survivors: a cohort study. FAMPRJ. 2016;33(4):382–387.
- Carod-Artal FJ, Egido JA. Quality of life after stroke: the importance of a good recovery. Cerebrovasc Dis. 2009;27(1):204–214.
- Kauhanen ML, Korpelainen JT, Hiltunen P, et al. Domains and determinants of quality of life after stroke caused by brain infarction. Arch Phys Med Rehabil. 2000;81(12):1541–1546.
- Sprigg N, Gray LJ, Bath PM, et al. Quality of life after ischemic stroke varies in western countries: data from the tinzaparin in Acute Ischaemic Stroke Trial (TAIST). J Stroke Cerebrovasc Dis. 2012;21(7):587–593.
- Safaz I, Kesikburun S, Adiguzel E, et al. Determinants of disease-specific health-related quality of life in Turkish stroke survivors. Int J Rehabil Res. 2016;39(2):130–133.
- Owolabi MO. What are the consistent predictors of generic and specific post-stroke health-related quality of life? Cerebrovasc Dis. 2010;29(2):105–110.
- Tang WK, Lau CG, Mok V, et al. Impact of anxiety on health-related quality of life after stroke: a cross-sectional study. Arch Phys Med Rehabil. 2013;94(12):2535–2341.
- De Wit L, Theuns P, Dejaeger E, et al. Long-term impact of stroke on patients’ health-related quality of life. Disabil Rehabil. 2017;39(14):1435–1440.
- Stroke Unit Trialists Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev. 2013;(9):CD000197.
- Indredavik B, Bakke F, Solberg R, et al. Benefit of a stroke unit: a randomized controlled trial. Stroke. 1991;22(8):1026–1031.
- Hempler I, Woitha K, Thielhorn U, et al. Post-stroke care after medical rehabilitation in Germany: a systematic literature review of the current provision of stroke patients. BMC Health Serv Res. 2018;18(1):468.
- Prvu Bettger JA, Stineman MG. Effectiveness of multidisciplinary rehabilitation services in postacute care: state-of-the-science. A review. Arch Phys Med Rehabil. 2007;88(11):1526–1534.
- Langhorne P, Baylan S, Early Supported Discharge Trialists. Early supported discharge services for people with acute stroke. Cochrane Database Syst Rev. 2017;(7):CD000443.
- Fisher RJ, Walker MF, Golton I, et al. The implementation of evidence-based rehabilitation services for stroke survivors living in the community: the results of a Delphi consensus process. Clin Rehabil. 2013;27(8):741–749.
- Putman K, De Wit L. European comparison of stroke rehabilitation. Top Stroke Rehabil. 2009;16(1):20–26.
- Ayis S, Wellwood I, Rudd AG, et al. Variations in health-related quality of life (HRQoL) and survival 1 year after stroke: five European population-based registers. BMJ Open. 2015;5(6):e007101–e007101.
- Aadal L, Pallesen H, Arntzen C, et al. Municipal cross-disciplinary rehabilitation following stroke in Denmark and Norway: a qualitative study. Rehabil Res Pract. 2018;2018:1972190.
- Danish Health Authority (Sundhetsstyrelsen). Course program for rehabilitation of adults with acquired brain injury (Forløbsprogram for rehabilitering av voksne med erhvervet hjerneskade); 2011.
- Danish Health Authority (Sundhetsstyrelsen). Brain injury rehabilitation - a health technology assessment (Hjerneskaderehabilitering - en medicinsk teknologivurdering); 2011.
- The Danish Stroke Registry (DAP) (Dansk Apoplexiregister). Annual Report 2015 (Årsrapport 2015). The Danish Clinical Registries (RKKP); 2016 [cited 2016 May 06]. Available from: https://www.sundhed.dk/content/cms/69/4669_dansk-apopleksi-register_aarsrapport_2015_finalanonym.pdf
- National Norwegian Stroke Registry (Norsk Hjerneslagregister). Annual Report 2015. (Årsrapport 2015). Nasjonalt sekretariat for Norsk Hjerneslagregister; 2016 [cited 2016 Oct 28]. Available from: https://stolav.no/Medisinskekvalitetsregistre/Norsk-hjerneslagregister/Årsrapport%20Norsk%20hjerneslagregister%202015.pdf
- Gray LJ, Ali M, Lyden PD, et al. Interconversion of the National Institutes of Health Stroke Scale and Scandinavian Stroke Scale in Acute Stroke. J Stroke Cerebrovasc. 2009;18(6):466–468.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370.
- Aben I, Verhey F, Lousberg R, et al. Validity of the beck depression inventory, hospital anxiety and depression scale, SCL-90, and Hamilton depression rating scale as screening instruments for depression in stroke patients. Psychosomatics. 2002;43(5):386–393.
- Muus I, Christensen D, Petzold M, et al. Responsiveness and sensitivity of the Stroke Specific Quality of Life Scale Danish version. Disabil Rehabil. 2011;33(25–26):2425–2433.
- Pedersen SG, Heiberg GA, Nielsen JF, et al. Validity, reliability and Norwegian adaptation of the Stroke-Specific Quality of Life (SS-QOL) scale. SAGE Open Med. 2018;6:205031211775203.
- Boosman H, Passier PE, Visser-Meily JM, et al. Validation of the Stroke Specific Quality of Life scale in patients with aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2010;81(5):485–489.
- Gillard PJ, Sucharew H, Kleindorfer D, et al. The negative impact of spasticity on the health-related quality of life of stroke survivors: a longitudinal cohort study. Health Qual Life Outcomes. 2015;13(1):159.
- Wu S, Mead G, Macleod M, et al. Model of understanding fatigue after stroke. Stroke. 2015;46(3):893–898.
- Pedersen SG, Anke A, Aadal L, et al. Experiences of quality of life the first year after stroke in Denmark and Norway. A qualitative analysis. Int J Qual Stud Health Well-Being. 2019;14(1):1659540.
- Saposnik G, Baibergenova A, O'Donnell M, et al. Hospital volume and stroke outcome: does it matter? Neurology. 2007;69(11):1142–1151.
- Svendsen ML, Ehlers LH, Ingeman A, et al. Higher stroke unit volume associated with improved quality of early stroke care and reduced length of stay. Stroke. 2012;43(11):3041–3045.
- Varmdal T, Indredavik B, Phan A, et al. Stroke in Norway 2015-16 – treatment and results. (Hjerneslag i Norge 2015-16 – Behandling og Resultater). Tidsskr Nor Legeforen. 2020. DOI:https://doi.org/10.4045/tidsskr.19.0246
- Asplund K, Sukhova M, Wester P, et al. Diagnostic procedures, treatments, and outcomes in stroke patients admitted to different types of hospitals. Stroke. 2015;46(3):806–812.
- Meyer T, Gutenbrunner C, Kiekens C, et al. ISPRM discussion paper: proposing a conceptual description of health-related rehabilitation services. J Rehabil Med. 2014;46(1):1–6.
- Arntzen C, Moe S, Aadal L, et al. Facilitating learning and change in the daily lives of stroke survivors: a comparative analysis of municipal stroke rehabilitation services in Norway and Denmark. Cogent Med. 2019;6(1):1–18.
- Carr AJ, Robinson PG. Is quality of life determined by expectations or experience? BMJ. 2001;322(7296):1240–1243.
- Harrison JK, McArthur KS, Quinn TJ. Assessment scales in stroke: clinimetric and clinical considerations. Clin Interv Aging. 2013;8:201–211.
- Cicerone KD, Langenbahn DM, Braden C, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil. 2011;92(4):519–530.
- Patel MD, McKevitt C, Lawrence E, et al. Clinical determinants of long-term quality of life after stroke. Age Ageing. 2007;36(3):316–322.
- Gunaydin R, Karatepe AG, Kaya T, et al. Determinants of quality of life (QoL) in elderly stroke patients: a short-term follow-up study. Arch Gerontol Geriat. 2011;53(1):19–23.
- Katona M, Schmidt R, Schupp W, et al. Predictors of health-related quality of life in stroke patients after neurological inpatient rehabilitation: a prospective study. Health Qual Life Outcomes. 2015;13(1):58.
- Lincoln NB, Brinkmann N, Cunningham S, et al. Anxiety and depression after stroke: a 5 year follow-up. Disabil Rehabil. 2013;35(2):140–145.
- Gutenbrunner C, Bickenbach J, Kiekens C, et al. ISPRM discussion paper: proposing dimensions for an International Classification System for Service Organization in Health-Related Rehabilitation. J Rehabil Med. 2015;47(9):809–815.
- Kiekens C, Meyer T, Gimigliano F, et al. European initiative for the application of the International Classification of Service Organization in Health-related Rehabilitation (ICSO-R). J Rehabil Med. 2020;52(1):1–318.