Abstract
Purpose
To examine any associations between postural asymmetries, postural ability, and pain for children with cerebral palsy in sitting and supine positions.
Methods
A cross-sectional study of 2,735 children with cerebral palsy, 0-18 years old, reported into the Swedish CPUP registry. Postural asymmetries, postural ability, the gross motor function classification system levels I–V, sex, age and report of pain were used to determine any relationship between these variables.
Results
Over half the children had postural asymmetries in sitting (n = 1,646; 60.2%) or supine (n = 1,467; 53.6%). These increased with age and as motor function decreased. Children were twice as likely to have pain if they had an asymmetric posture (OR 2.1–2.7), regardless of age, sex and motor function. Children unable to maintain or change position independently were at higher risk for postural asymmetries in both supine (OR 2.6–7.8) and sitting positions (OR 1.5–4.2).
Conclusions
An association was found between having an asymmetric posture and ability to change position in sitting and/or lying; and with pain. The results indicate the need to assess posture and provide interventions to address asymmetric posture and pain.
Postural asymmetries are present in children with cerebral palsy at all levels of gross motor function.
Postural asymmetries increase with age and are associated with pain.
Assessment of posture should be included in surveillance programs to enable early detection and treatment.
Implications for rehabilitation
Introduction
Cerebral palsy (CP) is the most common cause of motor impairments in childhood, with a prevalence in Europe of 2.2–2.5/1000 children [Citation1–3]. Although CP is reported as primarily a neurological motor disorder, complications can arise affecting different body systems [Citation3] and especially for those who have posture and mobility limitations compromising their ability to move, reposition and remain stable against gravity [Citation4].
Posture refers to the anatomical alignment of body segments in relation to each other and to the support surface [Citation5]. Postural ability relates to the individual’s ability to stabilize these body segments relative to each other and to the supporting surface during static and dynamic conditions; to maintain and change position in order to participate in activities [Citation5].
Approximately one-third of children with CP are non-ambulant and subsequently spend prolonged periods of time in sitting or lying [Citation1]. This can lead to postural asymmetries [Citation6], and ultimately to tissue adaptation [Citation4,Citation7], pain [Citation8,Citation9], and deformities commonly affecting the spine and lower extremities [Citation4,Citation10–12].
Pain increases with age and is more frequently located in the feet in children at Gross Motor Function Classification System (GMFCS) levels I–II and in the hips and spine in children at GMFCS levels IV–V [Citation13]. Pain can lead to reduced engagement in everyday life [Citation13–16], especially school work, sleep [Citation13,Citation17], and quality of life [Citation18], including mental health [Citation19].
Although there is a growing evidence of the prevalence and impact of postural asymmetries in adults with CP [Citation4,Citation7,Citation12,Citation20], little is known about the postural asymmetries of children, nor of their ability to change position in sitting and lying and its relationship with pain.
Against this background, the purpose of this study was to examine any associations between postural asymmetries, postural ability, and pain for children with CP in sitting and supine positions.
Materials and methods
Ethical approval and consent
The study was approved by the Medical Research Ethics Committee at Lund University (LU 383/2007) and permission to extract data was obtained from the CPUP registry. The legal caregiver of all participants consented to research based on the data held in the registry.
Study design and participants
A cross-sectional study was performed based on Swedish children followed by the National Follow-up Program for People with Cerebral Palsy (CPUP) and reported into their Registry from 1 January 2017 to 30 June 2018. All children with suspected CP are offered the opportunity to participate in this program, and over 95% participate [Citation2] and are included in the CPUP registry [Citation21].
Inclusion criteria were data reported for the primary outcome variable posture in either sitting or supine lying. Children with no data of posture were excluded. CP diagnosis was given from the age of 4 years by neuropediatricians according to the definition by Rosenbaum et al. [Citation22] with inclusion and exclusion criteria as given by the Surveillance of Cerebral Palsy in Europe [Citation23] with a brain injury before the age of 2 years and a dominating neurological symptom of either spasticity, ataxia, or dyskinesia. The 1–2% included in the registry who turn out not to have CP are then removed from it. Children are reviewed twice a year by their local occupational and physiotherapists until their sixth birthday and then annually thereafter [Citation2].
Measurements
Each child’s gross motor function was classified by their local physiotherapist using the expanded and revised version of the GMFCS [Citation24]. The GMFCS assesses the child’s ability to sit, transfer and mobilize, and is used to classify the children into one of the five levels (I–V) based upon their functional limitation and the need for mobility devices, with higher levels indicating more severe functional limitations and need for mobility devices.
The participants’ posture and ability to maintain or change position were rated by their local physiotherapist according to the Posture and Postural Ability Scale (PPAS) [Citation5,Citation25] in sitting and supine positions. The PPAS has excellent inter-rater reliability and validity for children and adults with CP [Citation5,Citation25]. Quality of posture is rated with six items from the frontal view and six items from the sagittal view, where postural alignment and symmetry gives 1 point per item while asymmetry or deviation from midline gives 0 points. These are then added to give a total score (0–6 points) for each position across each plane.
Postural ability is rated on a 7-point ordinal scale ranging from level 7 (“Able to move into and out of position”) to level 1 (“Unplaceable in an aligned position”). Rating was completed on the individual’s habitual posture on a plinth. Participants were asked to assume each of the positions (supine lying, prone lying, and sitting) as per the protocol described in Rodby-Bousquet et al. [Citation5]. When participants were unable to independently maintain a position, manual support was provided to enable them to stay in the position. Those unable to assume or move in or out of a position were placed into that position.
Postural asymmetries were grouped into the four categories “severe” (0–1 point), “moderate” (2–3 points), or “mild” asymmetries (4–5 points), or “full” symmetry/no asymmetry (6 points) meaning a symmetric posture with head midline, trunk symmetrical, pelvis neutral, legs separated and straight relative to pelvis, arms resting or feet neutral and even weight distribution, and also dichotomized into having asymmetries (0–5 points) or no asymmetry (6 points).
Likewise, postural ability was grouped into four categories, with children able to independently move into and out of supine or sitting position (PPAS level 7) grouped as “changes position”, while children with mild postural deficit (PPAS level 5–6) were grouped as “moves within position”, those with moderate postural deficit (PPAS level 3–4) were grouped as “maintains position”, while children with severe postural deficit (PPAS levels 1–2) were grouped as “cannot maintain position”. Postural ability was also dichotomized into “able to change position” (PPAS level 7) or “unable to change position” (PPAS levels 1–6).
Presence of pain was self-reported by the child or proxy (parent/primary caregiver), with the answer “yes” or “no” to the question “Do you/does the person experience pain?” [Citation26]. All current pain was included regardless of pain site and pain severity. The pain assessment has previously been validated against medical records and described in detail by Westbom et al. [Citation26]; while age was categorized into six groups (0–3, 4–6, 7–9, 10–12, 13–15, or 16–18 years old).
Statistical analyses
Categorical data were described by frequencies and percentages, n (%), while continuous data were reported as means with accompanying standard deviations (SDs). Pearson’s χ2-test and χ2-test for trend were used for tests of differences between categorical variables, while Spearman’s rank correlation rs was used for estimating correlations. Simple and multiple logistic regression models were used for estimating the magnitude of associations, with the results presented as odds ratios (ORs) with accompanying 95% confidence intervals (CIs). The OR for having pain was estimated using postural asymmetry (four categories) and inability to change position (four categories) as independent predictors, adjusted for age (categorized), sex, and GMFCS level. The OR for postural asymmetry (PPAS 0–5 points) was estimated using inability to change position (four categories), age (continuous), sex, and GMFCS level as independent predictors. Full postural symmetry (PPAS 6 points), ability to change position (PPAS level 7), lower age (0–3 years), male sex and better gross motor function (GMFCS I) were used as reference categories in the regression models. Statistical analyses were performed in IBM SPSS Statistics 26.0 and R 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria), with p-values <0.05 considered statistically significant.
Results
Participants
In total 2,735 (83%) of all 3,296 children reported into the CPUP registry were eligible for inclusion in this study. There were 1,628 boys (59.5%) and 1,107 girls with a mean (SD) age of 9.2 (4.4) years with data of posture in either sitting or supine position (). The largest group of children (n = 1,132; 41.4%), were classified at GMFCS level I, whilst the distribution varied from 10.8% to 16.5% across GMFCS levels II–V ().
Table 1. Participant demographics of the 2,735 children with cerebral palsy and distribution of postural asymmetries by the level GMFCS level, age, sex, and postural ability.
Postural asymmetries
More children (n = 1,646; 60.2%) presented with postural asymmetries in sitting than in supine (n = 1,467; 53.6%). Of the 1,646 children with postural asymmetries in sitting, 1,092 had postural asymmetries in both the frontal and sagittal view, while 1,018 of the 1,467 had asymmetries in supine lying. Severe postural asymmetries were observed in 320 children in sitting (97 frontal, 58 sagittal and 165 both) and in 329 children in supine (169 frontal, 49 sagittal, and 111 both).
Postural asymmetries were associated with GMFCS level (), and with increasing age in both supine (OR 1.07 [95% CI 1.05–1.10]) and in sitting (OR 1.05 [95% CI 1.03–1.07]), even when adjusted for ability to change position, sex, and GMFCS level (). Furthermore, boys were found to have a slightly greater tendency to have postural asymmetries in supine than girls ().
Table 2. Logistic regression analyses with odds ratios (ORs) and 95% confidence intervals (CI) for asymmetric postures (PPAS 0–5 points) in sitting and supine positions.
Postural asymmetries and pain
Pain prevalence was reported by 1,036/2,640 (39.2%) children (missing data n = 95). Pain was self-reported by 45.2% (n = 1,237) of the children and proxy reported by 52.8% (n = 1,444; missing data n = 54). Severe asymmetries in supine and sitting positions increased the risk for pain (OR 2.1–2.7) (). There was also higher ORs for pain in children unable to change position (1.5–2.3) and with higher age OR 1.08 (95% CI 1.06–1.10).
Figure 1. Simple logistic regression analyses with odds ratios (ORs) and 95% confidence intervals for pain in children with mild to severe postural asymmetries and inability to change position (postural ability). (A) Asymmetries in supine frontal view; (B) asymmetries in supine sagittal view; (C) asymmetries in sitting frontal view; (D) asymmetries in sitting sagittal view.
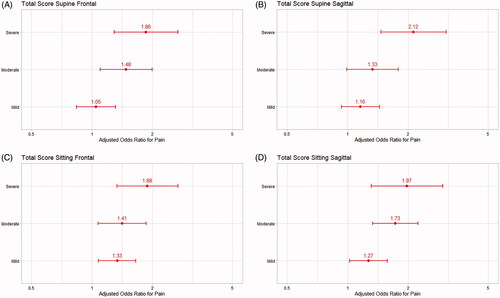
Postural asymmetries and ability to change position
In total, 824 (30.1%) children were unable to move independently in and out of a supine position and 995 (36.4%) in sitting. A clear majority of the children that were unable to change position also had an asymmetric posture in supine (760/824) and in sitting (919/995) (). Inability to change position doubled the probability of having postural asymmetries; in supine, OR (95% CI): frontal 2.0 (1.3–3.1), sagittal 1.9 (1.1–3.2); in sitting, OR (95% CI): frontal 2.5 (1.6–3.9); sagittal 2.8 (1.8–4.2), even when adjusted for age, sex and GMFCS level ().
Table 3. Postural asymmetries relative to postural ability in supine and sitting.
Postural asymmetries and GMFCS level
Postural asymmetries were seen in children at all GMFCS levels, with a strong negative correlation between GMFCS level and the total score of posture (rs −0.65 to −0.68) as well as their ability to change position in supine (rs −0.79) and in sitting (rs −0.85), indicating more asymmetries at increasing levels of functional limitations and need for the use of mobility devices. Children at GMFCS levels I–II more frequently had symmetric postures or mild asymmetries, whilst those at GMFCS level III showed mild to moderate, GMFCS level IV moderate, and GMFCS V severe postural asymmetries in both supine and sitting (). The most common asymmetry in supine lying was located at the upper and lower extremities for children at GMFCS levels I–IV and to the head and trunk for children with GMFCS level V (), while postural asymmetries in sitting particularly involved the trunk, pelvis, and weight distribution for children at GMFCS levels I–IV and the whole body from the head to the feet for children with GMFCS level V ().
Table 4. Distribution of postural asymmetries according to the Posture and Postural Ability Scale (PPAS) in Supine and Sitting for children at each level of the Gross Motor Function Classification System (GMFCS).
Discussion
This study reports on the prevalence of postural asymmetries, postural ability and pain in a large population of Swedish children with CP. To our knowledge, this is the first study exploring postural asymmetries and postural ability in children with CP. In this cross-sectional study of 2,735 children with CP at all GMFCS levels, postural asymmetries were observed in just over half of the cohort (60.2% sitting; 53.6% supine), and were evident in sitting and supine, in both sagittal and frontal planes. There were differences in the presence of postural asymmetries found in relation to gross motor function at all levels, and in relation to sex, boys had an increased risk of asymmetry (24%) solely in the supine position.
Consistent with previous findings in adults [Citation4] a clear association was found between gross motor function and the presence of postural asymmetries. Specifically, as children’s gross motor function decreased, the presence of postural asymmetries increased. For those with GMFCS level V these postural asymmetries tended to be more severe in both sitting and supine than for the other groups. The results support Ágústsson et al. [Citation7] who also reported finding more postural asymmetries in adults with lower levels of motor function.
Several studies have reported postural asymmetries and deformities in adults with CP [Citation4,Citation11,Citation12,Citation20]. We found that the risk for postural asymmetries increased with age (OR 1.05 and 1.07 per year) in both supine and sitting. Nevertheless, we identified postural asymmetries also in the youngest children. This is consistent with findings by Fulford and Brown [Citation27] and Porter et al. [Citation6] and may indicate that postural asymmetries may originate early in childhood. The results support the need for early postural interventions to monitor range of motion (ROM) and the development of postural asymmetries from an early age, and continuously throughout life [Citation4,Citation27]. The presence of postural asymmetries remains a concern, as previous studies have reported how they most likely result in soft tissue adaption, contractures, further postural deformities [Citation4,Citation7,Citation12,Citation20] and pain [Citation28]. These changes may in turn lead to reduced functional participation [Citation29] and decreased quality of life [Citation30,Citation31].
Postural asymmetries in sitting where more frequent than in supine lying and most commonly affected the trunk and pelvis, whereas in supine they more frequently involved the upper and lower extremities. Possibly, having more asymmetries in sitting may be a result of gravity having greater impact when trying to maintain a stable sitting position over a smaller base of support compared to maintaining symmetry in lying. Although this may not be the whole reason as Rodby-Bousquet et al. [Citation4] reported more postural asymmetries in sitting compared with standing which has an even smaller base of support. Children with GMFCS level IV and V have more severe asymmetries affecting the whole body. Indeed, they may require postural support for several body segments to facilitate a more neutral postural alignment such as a spinal orthosis, and adequate supports within the wheelchair, seating system and in bed.
The postural asymmetries identified in children with GMFCS levels I and II most commonly affect only one or two body segments such as the feet or arms. This group of children can independently change position and subsequently are less likely to develop severe fixed deformities. However, clinicians could consider orthotics for the upper extremities or ankle–foot orthosis to facilitate biomechanical alignment and activities in everyday life. For all children, it is vital that any intervention does not negatively impact upon their ability to participate in everyday life.
The inability to change position increased the risk of having postural asymmetries in both sitting and lying, and with pain. Indeed, children unable to change position when in supine or when sitting were twice as likely to have severe postural asymmetries than those able to change position independently. This is concerning as this lack of ability to change position coupled with the amount of time spent in that position is reported to possibly result in an escalation of these postural asymmetries [Citation4], and the risk of potentially life-threatening complications, reducing ability to participate in everyday activities and worsening quality of life [Citation32].
Since just over 50% of children with moderate and severe postural asymmetries (56% frontal; 51.5% sagittal) required support to achieve an aligned sitting posture, adaptive seating systems may be a vital provision for these children to provide them with stability and enable them to participate in activities of daily life. This is echoed in earlier work by Sahinoglu et al. [Citation33] who found that both adjustable seating and custom molded seating was required for the children with more postural asymmetries, and Neilson et al. [Citation34] who reported on improved quality of life and sitting posture through the provision of customized seating for adults with profound postural disabilities. Furthermore, the use of spinal orthoses may improve head control, stability, and arm–hand function of children with CP [Citation35] and ultimately promote participation in activity and reduce pain [Citation36].
The prevalence of pain was reported by 39% of the children and/or their caregivers in this study, similar to Alriksson-Smidt and Hägglund [Citation9], who found that approximately one third of Swedish children with CP aged 1–14 years had pain, and that the occurrence of pain increased with age. In contrast, however, Parkinson et al. [Citation29] reported that the prevalence of pain was 74% in their sample of 13–17 year olds. Further, we found a strong association between pain and postural asymmetries, with children with severe postural asymmetries being twice as likely to have pain as any other children. It is vital that pain is addressed possibly through the prevention or management of postural asymmetries, as it is well recognized that pain has a negative impact on both quality of life [Citation18,Citation30,Citation37] and participation in activities of daily living [Citation14,Citation17,Citation29]. Further studies are required to determine optimum postural interventions to pre-emptively manage pain for these children.
Limitations
The cross-sectional design of this study means that all measurements reflect only a single time point, albeit the most recent for each participant. Although this design does not look at how postural asymmetries develop or evolve over time, it does suggest that postural asymmetries can be identified already in children as young as 0–3 years of age, and a trend toward there being more children having postural asymmetries in the older age-groups, as well as the presence of pain. Although a strong agreement in pain prevalence rating between the young person and their proxy has been reported [Citation8], assessment of pain is a challenge especially in children at a low age and in children with less efficient communication. Therefore, we decided to only include pain prevalence and defined any current pain reported by the child or proxy as having pain. This study did not explore whether the pain was acute or chronic in nature, the origin, severity or location of the reported pain.
Generalizability
This large sample study reports on prospectively collected data of a large population of Swedish children with CP as registered in the Swedish CPUP registry. In Sweden, there is a proactive approach to healthcare delivery and generally utilization of 24-h postural management aiming to monitor and prevent postural asymmetry and contractures before they occur, and specifically on preventing hip dislocations in children [Citation10]. Hence, these results may be slightly different from those in countries which tend to be more reactive in the management of posture and postural asymmetry in children with CP. Nonetheless, this study serves to highlight the importance of regular monitoring of children to permit early identification of postural asymmetries and subsequent treatment to ameliorate or prevent deterioration.
Conclusions
In conclusion, in this population-based study 60.2% of children with CP had postural asymmetries in sitting, and 53% in supine, while 39.2% were reported to have current pain. Postural asymmetries increased as age increased, and gross motor function decreased. Children with severe postural asymmetries in either sitting or supine position were twice as likely to have pain and children unable to change position in supine were twice as likely to have postural asymmetries. Future research should explore any relationship between distribution of postural asymmetries and pain, as well as the effectiveness of postural management interventions in managing postural asymmetries of children with cerebral palsy.
Availability of data and material
The dataset analyzed during the current study is part of the CPUP registry.
Table 1. Continued.
Disclosure statement
The authors report no conflicts of interests. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- (SCPE) SoCPiE. Prevalence and characteristics of children with cerebral palsy in Europe. Dev Med Child Neurol. 2002;44:633–640.
- Westbom L, Hägglund G, Nordmark E. Cerebral palsy in a total population of 4–11 year olds in southern Sweden. Prevalence and distribution according to different CP classification systems. BMC Pediatr. 2007;7:41.
- Himmelmann K, Hagberg G, Uvebrant P. The changing panorama of cerebral palsy in Sweden. X. Prevalence and origin in the birth-year period 1999–2002. Acta Paediatr. 2010;99(9):1337–1343.
- Rodby-Bousquet E, Czuba T, Hagglund G, et al. Postural asymmetries in young adults with cerebral palsy. Dev Med Child Neurol. 2013;55(11):1009–1015.
- Rodby-Bousquet E, Agustsson A, Jonsdottir G, et al. Interrater reliability and construct validity of the Posture and Postural Ability Scale in adults with cerebral palsy in supine, prone, sitting and standing positions. Clin Rehabil. 2014;28(1):82–90.
- Porter D, Michael S, Kirkwood C. Is there a relationship between preferred posture and positioning in early life and the direction of subsequent asymmetrical postural deformity in non-ambulant people with cerebral palsy? Child: Care Health Dev. 2008;34(5):635–641.
- Ágústsson A, Sveinsson Þ, Rodby-Bousquet E. The effect of asymmetrical limited hip flexion on seating posture, scoliosis and windswept hip distortion. Res Dev Disabil. 2017;71:18–23.
- Fairhurst C, Shortland A, Chandler S, et al. Factors associated with pain in adolescents with bilateral cerebral palsy. Dev Med Child Neurol. 2019;61(8):929–936.
- Alriksson-Schmidt A, Hagglund G. Pain in children and adolescents with cerebral palsy: a population-based registry study. Acta Paediatr. 2016;105(6):665–670.
- Hägglund G, Alriksson-Schmidt A, Lauge-Pedersen H, et al. Prevention of dislocation of the hip in children with cerebral palsy: 20-year results of a population-based prevention programme. Bone Joint J. 2014;96–B(11):1546–1552.
- Porter D, Michael S, Kirkwood C. Patterns of postural deformity in non-ambulant people with cerebral palsy: what is the relationship between the direction of scoliosis, direction of pelvic obliquity, direction of windswept hip deformity and side of hip dislocation? Clin Rehabil. 2007;21(12):1087–1096.
- Holmes C, Brock K, Morgan P. Postural asymmetry in non-ambulant adults with cerebral palsy: a scoping review. Disabil Rehabil. 2019;41(9):1079–1088.
- Eriksson E, Hägglund G, Alriksson-Schmidt AI. Pain in children and adolescents with cerebral palsy – a cross-sectional register study of 3545 individuals. BMC Neurol. 2020;20(1):15.
- Penner M, Xie WY, Binepal N, et al. Characteristics of pain in children and youth with cerebral palsy. Pediatrics. 2013;132(2):e407–e413.
- Hodgkinson I, Jindrich ML, Duhaut P, et al. Hip pain in 234 non-ambulatory adolescents and young adults with cerebral palsy: a cross-sectional multicentre study. Dev Med Child Neurol. 2001;43(12):806–808.
- Dang VM, Colver A, Dickinson HO, et al. Predictors of participation of adolescents with cerebral palsy: a European multi-centre longitudinal study. Res Dev Disabil. 2015;36:551–564.
- Tedroff K, Gyllensvärd M, Löwing K. Prevalence, identification, and interference of pain in young children with cerebral palsy: a population-based study. Disabil Rehabil. 2019. DOI:https://doi.org/10.1080/09638288.2019.1665719
- Findlay B, Switzer L, Narayanan U, et al. Investigating the impact of pain, age, Gross Motor Function Classification System, and sex on health-related quality of life in children with cerebral palsy. Dev Med Child Neurol. 2016;58(3):292–297.
- Ramstad K, Jahnsen R, Skjeldal OH, et al. Mental health, health related quality of life and recurrent musculoskeletal pain in children with cerebral palsy 8–18 years old. Disabil Rehabil. 2012;34(19):1589–1595.
- Ágústsson A, Sveinsson T, Pope P, et al. Preferred posture in lying and its association with scoliosis and windswept hips in adults with cerebral palsy. Disabil Rehabil. 2019;41(26):3198–3202.
- Alriksson-Schmidt AI, Arner M, Westbom L, et al. A combined surveillance program and quality register improves management of childhood disability. Disabil Rehabil. 2017;39(8):830–836.
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;49:8–14.
- (SCPE) SoCPiE. Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol. 2000;42:816–824.
- Palisano RJ, Rosenbaum P, Bartlett D, et al. Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol. 2008;50(10):744–750.
- Rodby-Bousquet E, Persson-Bunke M, Czuba T. Psychometric evaluation of the Posture and Postural Ability Scale for children with cerebral palsy. Clin Rehabil. 2016;30(7):697–704.
- Westbom L, Rimstedt A, Nordmark E. Assessments of pain in children and adolescents with cerebral palsy: a retrospective population-based registry study. Dev Med Child Neurol. 2017;59(8):858–863.
- Novak I. Stand up and be counted. Dev Med Child Neurol. 2013;55(11):974.
- Marcström A, Hägglund G, Alriksson-Schmidt AI. Hip pain in children with cerebral palsy: a population-based registry study of risk factors. BMC Musculoskelet Disord. 2019;20(1):62.
- Parkinson KN, Dickinson HO, Arnaud C, SPARCLE Group, et al. Pain in young people aged 13 to 17 years with cerebral palsy: cross-sectional, multicentre European study. Arch Dis Child. 2013;98(6):434–440.
- Dickinson HO, Parkinson KN, Ravens-Sieberer U, et al. Self-reported quality of life of 8–12-year-old children with cerebral palsy: a cross-sectional European study. Lancet. 2007;369(9580):2171–2178.
- Jarl J, Alriksson-Schmidt A, Rodby-Bousquet E. Health-related quality of life in adults with cerebral palsy living in Sweden and relation to demographic and disability-specific factors. Disabil Health J. 2019;12:460–466.
- Crawford S, Stinson M. Management of 24-h-body positioning. In: Söderback I, editor. International handbook of occupational therapy interventions. Cham: Springer International Publishing; 2015. p. 189–203.
- Sahinoğlu D, Coskun G, Bek N. Effects of different seating equipment on postural control and upper extremity function in children with cerebral palsy. Prosthet Orthot Int. 2017;41(1):85–94.
- Neilson A, Bardsley G, Rowley D, et al. Measuring the effects of seating on people with profound and multiple disabilities – a preliminary study. J Rehabil Res Dev. 2001;38(2):201–213.
- Pettersson K, Rodby-Bousquet E. Prevalence and goal attainment with spinal orthoses for children with cerebral palsy. J Pediatr Rehabil Med. 2019;12(2):197–203.
- Bolas J, Boyle P. Parental views regarding seating and participation for young children with cerebral palsy. J Occup Ther Schools Early Interv. 2017;10(3):254–265.
- McKinnon CT, Meehan EM, Harvey AR, et al. Prevalence and characteristics of pain in children and young adults with cerebral palsy: a systematic review. Dev Med Child Neurol. 2019;61(3):305–314.
