Abstract
Purpose
Previously we demonstrated the feasibility of a six-week-long combination of high-intensity interval endurance and strength training (HIT/HIRT) for women with nonmetastatic breast cancer leading to improvements in psychological well-being and performance. Now we report results of a 24-month follow-up.
Methods
Previous intervention (IG, n = 10; 58.7 ± 8.4yrs) and control group (CG, n = 9; 58.8 ± 6.6yrs) were asked for follow-up examinations 12 (T12) and 24 months (T24) after cessation of the supervised training (POST). Medical history, mental well-being, performance and immunological variables were analyzed with respect to intervention start (PRE).
Results
IG maximum oxygen consumption (⩒O2peak) 12%-improved POST (p = 0.05) and declined to baseline values T24, while CG ⩒O2peak increased 12% T24 (p = 0.01). IG strength (1RM) increased 31% POST (p < 0.001) and remained above baseline level T24 (p = 0.003), whereas CG 1RM slightly improved T24 (+19%, p = 0.034). IG Anxiety and Depression decreased POST and did not change until T24. IG C-reactive protein decreased POST and increased to pre-exercise levels T24. CG immunological/inflammatory/life quality markers did not change.
Conclusions
Six weeks of HIT/HIRT by breast cancer patients can induce similar beneficial effects like two years of convalescence, but outcomes were unstable and showed a fast backslide in aerobic capacity, activity level and in pro-inflammatory state within 12 months.
High-intensity interval endurance and strength training (HIT/HIRT) for female breast cancer patients was shown to improve psychological well-being and performance, but long-term effects/adherence are unknown.
Significant backslides in aerobic capacity, activity level as well as in the pro-inflammatory response after one and two years are observed and should be monitored.
Continuous supervision and/or support of breast cancer patients before, during, and after medical care due to poor training adherence when voluntarily executed is recommended.
IMPLICATIONS FOR REHABILITATION
Introduction
Breast cancer therapy exerts high mental [Citation1,Citation2] and physiological [Citation3,Citation4] stress on patients before, during and after treatment. To alleviate certain symptoms like increases in depression, fatigue and declines in daily physical activities, exercise in general and specialized training interventions may be non-invasive options besides drug treatment [Citation5–8]. Several guidelines that recommend exercise for cancer therapy were established in the last years. They recommend endurance and strength training at moderate intensity to support the convalescence after therapy [Citation9,Citation10]. Up to now, alternatives to moderate aerobic intensity exercise in cancer therapy like high intensity exercises for strength, endurance or in combination are only rarely examined [Citation11,Citation12]. In addition, there are even fewer studies that followed breast cancer patients over years [Citation13], although it was shown that lifestyle modifications could prevent about one-third of all cases of breast cancer [Citation14]. Adherence to different training regimes also need further examination [Citation15–17].
We previously demonstrated in a randomized controlled trial the feasibility of a six-week supervised combination of high-intensity interval endurance and strength training (HIT/HIRT) in a group of female breast cancer patients (Intervention study) who trained either during or after therapy, experienced beneficial physiological and psychological adaptations with HIT/HIRT [Citation18]. Briefly, the intervention group (IG, n = 15) exhibited increased aerobic capacity and strength while anxiety and depression concomitantly decreased. On the other hand, the control group (CG, n = 11) performed only moderate-intensity continuous training (MICT) like, for example, (water-) gymnastics, cycling, or swimming and did not show any changes of the aforementioned variables. After the six-week initial intervention program, the importance of continuing with exercise activities to maintain the health benefits gained was explained to all study participants. Participants received information how to perform HIT/HIRT on their own, e.g., by running and cycling activities or strength training using body weight. However, the adherence to regular training following the intervention is normally not monitored as well as the sustainability of the training induced adaptions. Furthermore, several studies have shown that the adherence to perform previous activity level significantly decreases after cancer treatment [Citation19–21].
To this end, we were (1) interested about continuation of study participants to our beneficial HIT/HIRT, but without supervised instructions. (2) We aimed to evaluate sustainability of fitness and health markers in a follow-up period of 24 months (Follow up).
Materials and methods
After Intervention study (PRE = baseline, POST = after six weeks HIT/HIRT) all female breast cancer patients were asked to participate in Follow-up with examinations after 12 (T12) and 24 months (T24). Inclusion criteria in the first instance were female breast cancer patients aged 18-65 years and a cancer diagnose within the last two years. Certain locations within the breast, tumor subtypes of breast cancer or treatments (ongoing or finished) of the disease were no exclusion criteria. Randomization was performed by stratified quota sampling. The first participants were nonrandomly selected into IG until a sufficient number was reached. Then all following patients got allocated to CG. However, patients aged <18 years and >65 years, with metastases, symptomatic cardiac disease or any other indication that might contradict maximal exercise testing or training were not included in our study. Ten out of 15 individuals from the IG and nine out of eleven from the CG agreed to participate in Follow-up. Medical history was reported each year during follow-up as well as health status, medical treatments and general physical activities. All study examinations were performed identical to Intervention study including the same variables and the data analyzed regarding to the new number of participants: aerobic capacity, strength, mental well-being, immunological and inflammatory variables. This study was registered in the German Register for Clinical Studies (DRKS) with the ID: DRKS00011410. Ethical approval was granted by the Ethical Committee of Ulm University, Germany (application number: 141/14). All participants gave written informed consent prior participation. visualizes time points of data collection (PRE-T24), participants’ allocation, study progress and variables.
Figure 1. Flowchart of Intervention study and Follow-up including participant number, performed training method and data collection time points. Only data collected from participants that participated at all data collecting time points during Intervention study and Follow-up were included into the analysis: Pre-intervention (PRE), post-intervention (POST), one year post-intervention (T12) and two years post-intervention (T24). HIT/HIRT: high-intensity interval endurance and strength training; MICT: moderate-intensity continuous training; IG: intervention group; CG: control group; ⩒O2peak: peak oxygen consumption; 1RM = one-repetition maximum test; HADS-D: Hospital Anxiety and Depression Scale.
Mental well-being
Anxiety and Depression as markers for mental well-being were assessed using the Hospital Anxiety and Depression Scale (HADS-D, German version; [Citation22]). Questionnaires for determination of HADS-D score were analyzed with reference to established protocols of Glaesmer et al. and Herrmann and Buss [Citation22,Citation23].
Aerobic capacity and strength
Endurance performance was determined by Cardiopulmonary exercise testing (CPX) with peak oxygen consumption (⩒O2peak) as main predictor for aerobic capacity [Citation24]. Briefly, a step protocol (start at 25 W, increase of 25 W each 3 min) on a bicycle ergometer (LodeVR Corival, Groningen, the Netherlands) was chosen according to international standards [Citation25]; the average oxygen uptake of the last 10 s before test abortion was defined as the ⩒O2peak and no submaximal cardiopulmonary exercise testing variables were assessed.
To calculate total body strength changes, one-repetition maximum testing (1RM) at six training devices machines for upper and lower body were performed [Citation24]. The 1RM was defined as the training weight that could be voluntarily executed not more than one time. The six training devices (TechnogymVR, Cesena, Italy) consisted of upper and lower body training machines: leg press, rowing machine, one-legged leg bender and stretcher, latissimus pulldown and chest press. Arithmetic mean of these six exercises (1RMmean) were calculated by normalizing weight data, where the result of PRE was accounted for 100%.
Inflammation and immunology
Laboratory markers for inflammatory and immune status were high sensitivity C-reactive Protein (hsCRP), leucocytes (LEU) and neopterin (NEO). Neopterin (IBL international, RE59321, Switzerland) and hsCRP (IBL international; EU59151, Switzerland) were analyzed using commercial ELISA kits according to the manufacturers’ instructions (hsCRP CVmean 5%; NEO CVmean 5%). LEU concentration was evaluated with an automatic blood profile analyzer (Sysmex XN, Sysmex, Japan).
Assessment of activity
The individual activity level was recorded with the adjusted activity score (AAS) from the retrospective questionnaire “human activity profile” by Fix and Daughton, which covers an adaptable time frame of several weeks to one year [Citation26]. For PRE to POST, last six weeks were assessed, whereas for POST to T12 and T12 to T24 the last year was queried. The AAS measures the usual daily activities. It is calculated by counting how many activities with lower values than the maximum activity score (MAS) the respondent has ‘‘stopped doing’’ and subtracting this from the MAS with AAS maximum is 94 [Citation26,Citation27].
To analyze if medical treatment or individual activity level during Follow-up had any effect on performance or molecular parameters, data were analyzed with respect to participants which received medical treatment or no treatment and participants with an adjusted activity score AAS ≥74 (high activity level) or <74 (low activity level) [Citation26,Citation27].
Statistics
Statistical analysis was performed using the SPSS software package (SPSS Statistics 21.0, IBM, Ehningen, Germany) and GraphPad PRISM (Version 6.04, La Jolla, USA). Data were tested for Gaussian distribution by Shapiro–Wilk normality test. Intra-group differences with one-way ANOVA with repeated measures followed by post hoc tests to determine statistical significance over time in relation to baseline data collection time point PRE. All data in our data sets were normally distributed, so that ANOVA was never violated by non-normal distributed data. To analyze the influence of individual activity level and occurrence of medical treatments on the main outcome variables, data of all 19 participants were pooled and individual activity level and occurrence of medical treatment were included as covariates into the model. Descriptive statistics of all data are presented as arithmetic mean ± standard deviation. Statistical differences were considered to be significant for p values <0.05.
Result
Medical history
Anthropometric data, medical treatments and physical activity of IG and CG during Intervention study and Follow-up are summarized in . Body composition was comparable to POST, but in total, IG received more medical treatment from POST to T24 than CG. High daily physical activity declined simultaneously by 38% in IG and CG at T24.
Table 1. Anthropometric data, medical treatments and physical activity of the female intervention group and control group during the original intervention study and during the 24-month follow-up period.
Aerobic capacity and strength
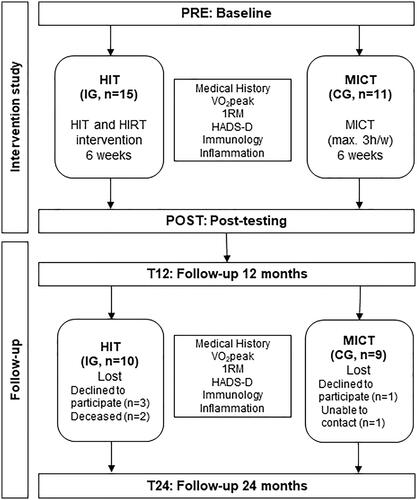
⩒O2peak increased from 20.9 ± 4.6 ml/min/kg at PRE to 23.5 ± 6.8 ml/min/kg at POST (+12%, p = 0.05) in IG (). During follow-up, ⩒O2peak did not change versus PRE to 20.3 ± 6.5 ml/min/kg at T12 (-3%, p = 0.626) without changing at T24 (+1%, p = 0.797). In CG, ⩒O2peak increased significantly by 12% over time from PRE to T24 ((), 23.0 ± 3.7 to 25.9 ± 4.2 ml/min/kg; p = 0.011).
Figure 2. Aerobic capacity and strength of intervention and control group during Intervention study and Follow-up. (A) Observed increase of ⩒O2peak at POST in IG compared to PRE could not be observed at T12 and T24. ⩒O2peak of CG significantly increased from to PRE to T24. (B) Normalized Mean 1RM (starting point PRE = 100%) of IG increased at POST, decreased significantly at T12 but remained increased regarding PRE during Follow-up. Significant differences to PRE could be measured at POST and T24 for CG with increased values.
1RMmean representing total body strength of all six training machines after normalization to baseline values at PRE, increased by 31 ± 10% (pmean<0.001) in IG at POST, was 17 ± 11% (pmean=0.001) higher at T12 and 22 ± 18% (pmean=0.003) higher at T24. In CG, where strength did not significantly change at POST (3 ± 13%, pmean=0.459), 1RMmean increased likewise during Follow-up by 18 ± 19% (pmean=0.022) at T12 and 19 ± 22% at T24 (pmean=0.034), respectively (1RMmean are visualized in , detailed and raw strength data are listed in Supplementary Table S1).
Mental well-being: Anxiety and depression
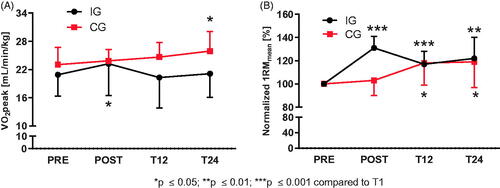
HADS-D revealed lower anxiety values at all-time points for IG compared to PRE (pPOST=0.033, pT12=0.008, pT24=0.017) and decreased depression values at POST (p = 0.019) and T24 (p = 0.037). In CG, rating of depression and anxiety values did not change over time. HADS-D scores for both variables and groups were classified as non-cases (score < 8) at all data collection time points ().
Figure 3. Anxiety and Depression scores for intervention and control group assessed by the Hospital Anxiety and Depression Scale (HADS-D). (A) Anxiety (Arbitrary Units, AU) experienced by IG decreased after PRE and remained significantly reduced at T12 and T24. Anxiety in CG did not differ over all-time points. (B) Depression (AU) determination revealed decreased scores for IG at POST and T24 compared to PRE. CG values remained unchanged.
Immunology and inflammation
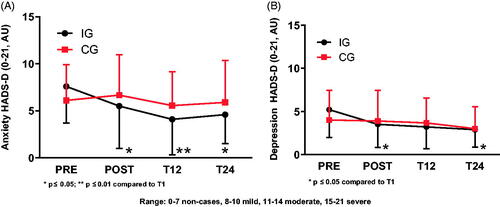
Neopterin () and leucocyte () concentrations remained unchanged in both IG and CG at all-time points. HsCRP () decreased in IG at POST (p = 0.046), but without significant differences at T12 and T24 compared to PRE. HsCRP values of CG did not differ at any time points.
Figure 4. Neopterin, high-sensitive C-reactive protein and leucocyte concentration as markers for the immunological and inflammatory status. (A) Neopterin did not differ at all-time points for IG and CG. (B) HsCRP concentration of IG decreased at POST but did no longer differ at T12 and T24 compared to PRE. CG hsCRP concentration did not show any differences at all-time points. (C) Leucocyte concentrations for IG and CG showed no difference at all-time points.
Whole group analyses
Analyses of all 19 participants regarding respective individual activity level and occurrence of medical treatment as covariates showed that high activity as well as occurrence of medical treatment was associated with performance status and inflammation. A high AAS was positively related with ⩒O2peak (POST p = 0.039; T12 p = 0,609; T24 p = 0.044) and with strength (POST p = 0.001; T12 p < 0.001; T24 p < 0.001) compared to PRE, while AAS and hsCRP (POST p = 0.033; T12 p = 0.047; T24 p = 0.0737) were negatively associated. Regarding medical treatment there was a negative interdependence observable between ⩒O2peak and medical treatment (POST p = 0.039; T12 p = 0,609; T24 p = 0.041) and between medical treatment and strength (POST p = 0.001; T12 p < 0.001; T24 p < 0.001) compared to PRE, which indicates that the more medical treatment received, the lower was the strength and aerobic capacity gain in the patients. Immune variables NEO and LEU did not exhibit any interdependence with respect to AAS or medical treatment (data not shown) to starting time point PRE.
Discussion
The follow-up examinations 12 and 24 months after cessation of our preceding Intervention study, consisting of either a feasible six-week long combined HIT/HIRT (intervention group) or MICT (control group) in breast cancer patients, which induced beneficial adaptations in aerobic capacity and strength as well as in the mental well-being of the IG, showed subsequently decreased activity and aerobic performance in a home-based setting after the intervention.
The main finding was a significant backslide in aerobic capacity, activity level as well as in the pro-inflammatory response. Instead, the separately examined control group increased slowly in aerobic performance and strength and maintained a stable mental well-being during Follow-up. Hence, although physiological benefits were undoubtedly induced during the course of Intervention study and explained to the study participants, such desirable outcomes are remarkably instable and already 12 months after the improvement of analytical markers almost all variables except strength reached pre-exercise levels again. Therefore, it was difficult for the patients to integrate HIT/HIRT into daily life on their own. There is only a limited number of studies that followed breast cancer patients over 1–2 years reporting inflammation, performance and well-being and even less, which monitored such data after HIT/HIRT [Citation13].
Our IG started with non-significantly lower baseline performance capacity as CG (⩒O2peak 20.9 ± 4.6 and 23.0 ± 3.7 ml/min/kg, respectively), but the intended amelioration of the general strength status in IG by 31% was considerably higher with respect to 1RM load. Performance improvements suffered a backslide as soon as the supervised and guided intervention program ceased, and study participants were advised to continue with the exercise program on their own. The percentage amelioration of the strength during the entire period of 24 months was lower for participants in CG than for those in IG but still positive over time as shown by their 1RM load. Instead, inflammation and immune parameters of the participants were only slightly affected.
Neopterin, which belongs to the chemical group known as pteridines and is synthesized by human macrophages upon stimulation with the cytokine interferon-gamma, is indicative of a pro-inflammatory immune status, and thus serves as a marker of cellular immune system activation [Citation28]. In both IG and CG values remained unchanged at every time point, indicating no increased pro-inflammatory effect of HIT/HIRT on patients and furthermore resulted in comparable immune status at all four different study time points. Another parameter which is affected by cancer treatment is leucocyte count and differentiation: treatment may induce pathological conditions like leucopenia [Citation29]. Leucocyte complexity indirectly provides insight into breast cancer survival [Citation30] and can be modulated by exercise [Citation31]. Our unchanged total leucocyte count during Intervention study and Follow-up was also observed in a study by Kim et al, where the effect of a 12-week walking exercise on the immune cells was examined [Citation32] which is in accordance with our neopterin data. The experimental values in IG in leucocyte count was also accompanied by a significantly decreased and presumably beneficial hsCRP response. This interdependence is known [Citation33], and the cessation of HIT/HIRT reversed these adaptations as seen in Follow-up.
This reversion was also observed for IG with respect to aerobic capacity. The values for participants in this group were almost at pre-intervention levels. When the impacts of medical cancer treatments gradually faded and the patients could once more perform regular daily activities, the increase in strength was comparable to CG over time (). This development could also be observed after analyzing of covariates AAS and medical treatment which both showed an association to aerobic capacity and strength at POST and T24, respectively. This may be explained by training between PRE and POST and the general convalescence during Follow-up. Thus, a 6-week of HIT/HIRT probably induced same beneficial adaptions like two years of convalescence, but adherence to this beneficial and feasible training program and individual additional support seem mandatory for continuous success [Citation34].
HIT/HIRT executed like in the Intervention study can theoretically very easily be integrated in daily life because of its time-saving training duration [Citation35], but it does need access to training machines including bicycle ergometers and strength machines. CG, on the other hand, performed moderate intensity exercise without any additional devices, and may thus have been able to integrate similar training into their daily life. In addition, the general improved fitness of CG may have led to the continuation of training postintervention. Group analysis of participants with medical treatment during Follow-up showed that treatment per se had a slight influence on aerobic and strength performance over time as medical treatment seem to negatively affect aerobic capacity and strength gain. In addition, although a high activity level was associated with higher ⩒O2peak and 1RM, there was no significant change during Follow-up observable. It can thus be speculated that continuation of HIT/HIRT on their own behalf despite the initial success may be hampered by lack of supervision and concomitant decreased motivation and lack of training possibilities [Citation36,Citation37].
Besides an improved post-interventional training regime for cancer patients, a high pre-operative fitness is desirable to alleviate recursive mechanisms after postoperative exercise programs. Despite the positive outcome for IG at the end of supervised training, the backslide of fitness levels after its cessation was significant.
Finally the inverse proportional association between cardiorespiratory fitness and cancer mortality became evident in several studies [Citation38,Citation39]. Analysis of recent data led to the estimation, that successful lifestyle changes could prevent 25% to 30% of all cases of breast cancer [Citation14]. A recent study by Kirsten et al showed that there is an inverse relationship between mortality during cancer therapy and ⩒O2peak [Citation40]. These data underline the importance of regular exercise training to diminish the risk for cancer. Therefore, prehabilitation may be a viable option before each cancer treatment. It describes the concept of optimizing the postoperative outcome through preoperative intervention [Citation41]. A higher cardiorespiratory fitness, including both aerobic capacity and strength, can alleviate adverse physiological symptoms induced by diverse cancer therapies, and therefore improve patients quality of life [Citation42–44].
To help patients to maintain and/or improve their physiological and psychological status by collecting and integrating individual training performance, medical treatment and trainer supervision, data have to be integrated continuously into daily life [Citation45]. A chance may lie in the current technological developments, which enables home-based guidance, supervision and support beside the “classical model” of trainer and performer. Many apps for cancer patients have been developed which include data analysis, motivation improvement and regular feedback [Citation46], supporting patients and their trainers alike. Communal sport activities (i.e., team sports, training groups) [Citation47,Citation48] can support adherence to training programs in cancer patients and help lower drop-out rates due to recursive symptoms or treatment. In a meta-analysis, Qiu et al. showed that the use of a step counter is associated with reduced sedentary time in adults and may thus support aerobic and health capacity [Citation49]. Health and performance status before and after breast cancer treatment per se are important factors contributing to training progression and continuation as well as feedback about, health and performance status. At best, training groups should start as soon as possible at the beginning of the therapy to increase motivation and training adherence during all stages of medical treatment: prehabilitation, medical treatment, rehabilitation and after the end of clinical supervision.
Limitations
As this study was conducted as a follow-up of a previous pilot study, it is limited to four collection data time points and a relatively small sample size. Nevertheless, it underlines the importance of an exercise program for aerobic capacities and strength in a group of breast cancer patients in a longer aftercare.
A further possible limitation observed in our Follow-up was that after the cessation of the training the occurrence of medical treatment in IG was more profound than in CG, in total, which may lead to limited training time and thus performance. Medical treatment often has a detrimental effect on mental factors, for example, anxiety and depression [Citation49,Citation50] and the physical fitness [Citation51–53]. These outcomes were not observed during the Follow-up in either IG or CG. A possible explanation may be that anxiety and depression scores of our study population right at the beginning of the intervention could be classified as non-cases [Citation54], so that the patients did not suffer any high mental stress pre- or post-exercise. But the feasibility of the HIT/HIRT for breast cancer was already shown in the Intervention study for adherence and performance parameters and is furthermore supported by the immunological status of the patients.
Due to randomization effects, IG showed non-significantly (p = 0,200) lower basal pre-interventional aerobic capacity than CG, but the HIT/HIRT intervention resulted in a steep increase of ⩒O2peak values and adjustment of both groups and overreached even the CG by 11% at POST despite lower basal values with regard to strength. Only one questionnaire was used to measure activity levels, but the human activity profile used gives a good estimate of physical activity for people with various diagnosis and age groups [Citation55–57]. Finally, possible confounding parameters like nutrition or daily exercise monitoring were not accessed as no continuous nutrition/training diary was kept during the two-year long follow-up.
Conclusions
This two-year follow-up of our Intervention study consisting of a six-week-long combination of HIT/HIRT with breast cancer patients showed, that despite the feasibility and benefits for health and performance parameters, these outcomes had no long-term effect on adherence, aerobic capacity and molecular markers. Six weeks of HIT/HIRT seems to induce similar beneficial effects like two years of general convalescence, but this process may be influenced by former and ongoing activity level as well as complications like therapy. The lack of supervision when patients are released to voluntarily executed training may lead to poor training adherence and therefore to the reversion to pre-exercise values. Small-meshed supervision including technical tools like apps, pedometers or integrating a club sport can be opportunities to support cancer patients in behavior change towards activity and health checks. In addition to therapy the solution is thus not only to “adjust one screw”, but to integrate a supervised training program/team, beginning with diagnosis and ending in patient’s daily life after medical care.
Acknowledgments
The authors gratefully acknowledge to all patients that participated and the team at the Division of Sports and Rehabilitation Medicine at Ulm University Hospital. We thank Jana Leger1 for support during the data collection process and Birgit Fink1 for proofreading. This research received no external funding.
Disclosure statement
The authors declare no financial or non-financial competing interests.
Data availability statement
Raw data were generated at Ulm University Hospital, Division of Sports and Rehabilitation Medicine. Derived data supporting the findings of this study are available from the corresponding author (S.V.W.S) on request.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Asher A. Cognitive dysfunction among cancer survivors. Am J Phys Med Rehabil. 2011;90.
- Burish TG, Tope DM. Psychological techniques for controlling the adverse side effects of cancer chemotherapy: Findings from a decade of research. J Pain Symptom Manage. 1992;7(5):287–301.
- Hidding JT, Beurskens CHG, van der Wees PJ, et al. Treatment related impairments in arm and shoulder in patients with breast cancer: a systematic review. PLoS One. 2014;9(5):e96748.
- Kayl AE, Meyers CA. Side-effects of chemotherapy and quality of life in ovarian and breast cancer patients. Curr Opin Obstet Gynecol. 2006;18(1):24–28.
- Mehnert A, Veers S, Howaldt D, et al. Effects of a physical exercise rehabilitation group program on anxiety, depression, body image, and health-related quality of life among breast cancer patients. Onkologie. 2011;34(5):248–253.
- Tomlinson D, Diorio C, Beyene J, et al. Effect of exercise on cancer-related fatigue: a meta-analysis. Am J Phys Med Rehabil. 2014;93(8):675–686.
- Agudelo LZ, Femenia T, Orhan F, et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159(1):33–45.
- Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3(7):961–968.
- Schmitz KH, Campbell AM, Stuiver MM, et al. Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69(6):468–484.
- Iyengar NM, Jones LW. Development of exercise as interception therapy for cancer: a review. JAMA Oncol. 2019;5(11):1620–1627. [cited 2020 Jan 9]
- Baumann FT, Bloch W, Weissen A, et al. Physical activity in breast cancer patients during medical treatment and in the aftercare – a review. Breast Care (Basel). 2013;8(5):330–334. Available from: 10.1159/000356172.
- Lahart IM, Metsios GS, Nevill AM, et al. Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database Syst Rev. 2018;(1):CD011292.
- Roine E, Sintonen H, Kellokumpu-Lehtinen P-L, et al. Health-related quality of life of breast cancer survivors attending an exercise intervention study: a five-year follow-up. In Vivo. 2020;34(2):667–674.
- Harvie M, Howell A, Evans DG. Can diet and lifestyle prevent breast cancer: what is the evidence? Am Soc Clin Oncol Educ Book. 2015:e66-73.
- Heinrich KM, Patel PM, O’Neal JL, et al. High-intensity compared to moderate-intensity training for exercise initiation, enjoyment, adherence, and intentions: an intervention study. BMC Public Health. 2014;14(1):789.
- Lund LW, Ammitzbøll G, Hansen DG, et al. Adherence to a long-term progressive resistance training program, combining supervised and home-based exercise for breast cancer patients during adjuvant treatment. Acta Oncol. 2019;58(5):650–657.
- Mazzoni A-S, Brooke HL, Berntsen S, et al. Exercise adherence and effect of self-regulatory behavior change techniques in patients undergoing curative cancer treatment: secondary analysis from the phys-can randomized controlled trial. Integr Cancer Ther. 2020;19:153473542094683.
- Schulz SVW, Laszlo R, Otto S, et al. Feasibility and effects of a combined adjuvant high-intensity interval/strength training in breast cancer patients: a single-center pilot study. Disabil Rehabil. 2018;40(13):1501–1508.
- Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med. 1997;3(3):215–226.
- Courneya KS. Exercise in cancer survivors: an overview of research. Med Sci Sports Exerc. 2003;35(11):1846–1852.
- Song L, Guan T, Guo P, et al. Cardiovascular disease, risk factors, and health behaviors among cancer survivors and spouses: A MEPS Study. Cancer Med. 2020;9(18):6864–6874.
- Herrmann C, Buss U. Vorstellung und validierung einer deutschen Version der "Hospital Anxiety and Depression Scale" (HAD-Skala). Diagnostica. 1994;40:143–154.
- Glaesmer H, Hoyer J, Klotsche J, et al. Die Deutsche Version des Life-Orientation-Tests (LOT-R) zum dispositionellen Optimismus und Pessimismus. Zeitschrift Für Gesundheitspsychologie. 2008;16(1):26–31.
- Scharhag-Rosenberger F, Becker T, Streckmann F, et al. Studien zu körperlichem Training bei onkologischen Patienten: Empfehlungen zu den Erhebungsmethoden. Dtsch Z Sportmed. 2014;2014(11):304–313.
- Balady GJ, Arena R, Sietsema K, Interdisciplinary Council on Quality of Care and Outcomes Research, et al. Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225.
- Fix AJ, Daughton D. Human Activity Profile: Professional Manual. Odessa (Ukraine): Psychological Assessment Resources; 1988. Print.
- Herzberg PY, Heussner P, Mumm FHA, et al. Validation of the human activity profile questionnaire in patients after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16(12):1707–1717.
- Murr C, Widner B, Wirleitner B, et al. Neopterin as a marker for immune system activation. Curr Drug Metab. 2002;3(2):175–187.
- Larsson AM, Roxa A, Leandersson K, et al. Impact of systemic therapy on circulating leukocyte populations in patients with metastatic breast cancer. Sci Rep. 2019;9(1):19–49943.
- DeNardo DG, Brennan DJ, Rexhepaj E, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1(1):54–67.
- Zimmer P, Baumann FT, Bloch W, et al. Impact of a half marathon on cellular immune system, pro-inflammatory cytokine levels, and recovery behavior of breast cancer patients in the aftercare compared to healthy controls. Eur J Haematol. 2016;96(2):152–159.
- Kim JJ, Shin YA, Suk MH. Effect of a 12-week walking exercise program on body composition and immune cell count in patients with breast cancer who are undergoing chemotherapy. J Exerc Nutrition Biochem. 2015;19(3):255–262.
- Huang ZS, Lo SC, Tsay W, et al. Revision in reference ranges of peripheral total leukocyte count and differential leukocyte percentages based on a normal serum C-reactive protein level. J Formos Med Assoc. 2007;106(8):608–616.
- Kirkham AA, Bonsignore A, Bland KA, et al. Exercise Prescription and Adherence for Breast Cancer: One Size Does Not FITT All. Med Sci Sports Exerc. 2018;50(2):177–186.
- Weston M, Weston KL, Prentis JM, et al. High-intensity interval training (HIT) for effective and time-efficient pre-surgical exercise interventions. Perioper Med (Lond)). 2016;5:2–26.
- Meneses-Echavez JF, Gonzalez-Jimenez E, Ramirez-Velez R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: a systematic review and meta-analysis. BMC Cancer. 2015;15:15–1069.
- Bolam KA, Mijwel S, Rundqvist H, et al. Two-year follow-up of the OptiTrain randomised controlled exercise trial. Breast Cancer Res Treat. 2019;175(3):637–648.
- Lago C, Sung HJ, Ma W, et al. p53, Aerobic Metabolism, and Cancer. Antioxid Redox Signal. 2011;15(6):1739–1748.
- Lakoski S, Willis B, Barlow C, et al. Midlife Cardiorespiratory Fitness, Incident Cancer, and Survival After Cancer in Men: The Cooper Center Longitudinal Study. JAMA Oncol. 2015;1(2):231.
- Kirsten J, Wais V, Schulz SV, et al. Sarcopenia screening allows identifying high-risk patients for allogenic stem cell transplantation. Cancers (Basel. 2021;13(8):1771.
- Durrand J, Singh SJ, Danjoux G. Prehabilitation. Clin Med (Lond)). 2019;19(6):458–464.
- Gillis C, Li C, Lee L, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121(5):937–947.
- Johannsson H. Prehabilitation is positive for patients. BMJ. 2019;367:l5830.
- Beaudry RI, Kirkham AA, Thompson RB, et al. Exercise intolerance in anthracycline-treated breast cancer survivors: the role of skeletal muscle bioenergetics, oxygenation, and composition. oncologist. 2020;25(5):e852-e860.
- Lunde P, Nilsson BB, Bergland A, et al. The effectiveness of smartphone apps for lifestyle improvement in noncommunicable diseases: systematic review and meta-analyses. J Med Internet Res. 2018;20(5):e162.
- Prochaska JJ, Coughlin SS, Lyons EJ. Social media and mobile technology for cancer prevention and treatment. Am Soc Clin Oncol Educ Book. 2017;37:128–137.
- Newton RU, Galvão DA. Exercise in prevention and management of cancer. Curr Treat Options Oncol. 2008;9(2-3):135–146.
- Sweegers MG, Boyle T, Vallance JK, et al. Which cancer survivors are at risk for a physically inactive and sedentary lifestyle?: Results from pooled accelerometer data of 1447 cancer survivors. Int J Behav Nutr Phys Act. 2019;16(1):19–820.
- Qiu S, Cai X, Ju C, et al. Step Counter Use and Sedentary Time in Adults: A Meta-Analysis. Medicine (Baltimore). 2015;94(35):e1412.
- Oberste M, Schaffrath N, Schmidt K, et al. Protocol for the "Chemobrain in Motion - study" (CIM - study): a randomized placebo-controlled trial of the impact of a high-intensity interval endurance training on cancer related cognitive impairments in women with breast cancer receiving first-line chemotherapy. BMC Cancer. 2018;18(1):18–4992.
- Schmitz KH, DiSipio T, Gordon LG, et al. Adverse breast cancer treatment effects: the economic case for making rehabilitative programs standard of care. Support Care Cancer. 2015;23(6):1807–1817.
- Dieli-Conwright CM, Orozco BZ. Exercise after breast cancer treatment: current perspectives. Breast Cancer. 2015;7:353–362.
- Klassen O, Schmidt ME, Ulrich CM, et al. Muscle strength in breast cancer patients receiving different treatment regimes. J Cachexia Sarcopenia Muscle. 2017;8(2):305–316.
- Zigmond AS, Snaith RP. The Hospital Anxiety And Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370.
- Bastone AC, Moreira BS, Vieira RA, et al. Validation of the human activity profile questionnaire as a measure of physical activity levels in older community-dwelling women. J Aging Phys Act. 2014;22(3):348–356.
- Davidson M, Morton N. d. A systematic review of the Human Activity Profile. Clin Rehabil. 2007;21(2):151–162.
- Teixeira-Salmela LF, Devaraj R, Olney SJ. Validation of the human activity profile in stroke: a comparison of observed, proxy and self-reported scores. Disabil Rehabil. 2007;29(19):1518–1524.
