Abstract
Purpose
To examine the nature and severity of impaired self-awareness (ISA) and denial of disability (DD) in a community-dwelling traumatic brain injury (TBI) population. Additionally, to investigate reliability, internal consistency, and feasibility of the Clinician’s Rating Scale for evaluating Impaired Self-Awareness and Denial of Disability after brain injury (CRS-ISA-DD).
Materials and methods
ISA and DD were studied using the CRS-ISA-DD in a cross-sectional study with 78 TBI patients (3.1 years post-injury).
Results
42% of individuals approached consented to participate in this study. Most participants showed one or more symptoms of ISA and DD, but severity scores were in the lower range (ISA: 13.2 ± 16.2; DD: 9.4 ± 10.7). The CRS-ISA-DD takes <10 min to complete, has excellent inter-rater reliability (ISA: ICC(2,1) = 0.928; DD: ICC(2,1) = 0.835), and acceptable-good internal consistency (ISA: α = 0.819; DD: α = 0.645). ISA severity correlated with neuropsychological test scores (rs = −0.30 to −0.47) and injury severity. DD severity correlated with anxiety (rs = −0.22) but not with avoidance coping or defense mechanisms.
Conclusions
Low levels of ISA and DD occurred in this sample of TBI patients. The CRS-ISA-DD is a reliable and feasible instrument. We recommend using it as a diagnostic tool to differentiate between ISA and DD once self-awareness problems have been identified.
Unawareness of deficits can persist into the chronic stage when rehabilitation treatment has ended.
The two main factors of unawareness, impaired self-awareness and denial of disability, are related to different neurological and psychological mechanisms.
The Clinician’s Rating Scale for evaluating Impaired Self-Awareness and Denial of Disability after brain injury (CRS-ISA-DD) can be used to distinguish the two main factors of unawareness.
IMPLICATIONS FOR REHABILITATION
Introduction
Traumatic brain injury (TBI) can have neuropsychological consequences for which many people receive rehabilitation treatment. Unfortunately, a proportion of TBI patients suffer from problems of impaired self-awareness. Unawareness of deficits refers to the inability to appraise one’s strengths and weaknesses as well as understand the implications these have for daily life activities [Citation1,Citation2]. These consequences can persist into the chronic stage, long after patients have been discharged home [Citation3]. Patients who are unaware of their deficits are prone to less independence in complex everyday tasks, worse psychological and/or emotional adjustment, and less favorable employment outcomes compared to patients with good awareness of their deficits [Citation4,Citation5]. Moreover, TBI patients’ self-awareness problems can affect their significant others. For example, self-awareness deficits in the chronic stage correlate with relatives’ subjective burden [Citation6]. Therefore, it is important for both the patients as well as their significant others that self-awareness problems are addressed. However, to determine what type of care is most suitable, it is important to know what type of self-awareness deficits they have.
The nature of unawareness of deficits is not completely understood but it is recognized that biological, psychological, and socio-environmental factors are involved [Citation7]. Biological aspects include neurocognitive factors originating from brain dysfunction. Neurocognitive impaired self-awareness of deficits (ISA) is thought to be a direct result of a neurologically based deficiency [Citation8]. This type of unawareness of deficits has been associated with impaired executive functions [Citation9] and injury severity [Citation10–12]. The psychological factor associated with unawareness of deficits, denial of disability (DD), is thought to be the result of psychological avoidance or defensive coping mechanisms [Citation13]. Denial as a coping mechanism protects patients from emotional distress, particularly anxiety [Citation8,Citation14]. At the socio-environmental level, factors include interactions with friends, family, and colleagues, such as opportunities to experience and recognize changes in functioning [Citation7].
In the past, efforts have been made to distinguish neurocognitive and psychological factors that influence impairments in awareness of deficits. This is especially relevant for clinical practice, but also important in a community context. At first glance, patients with ISA and DD can present alike, reporting no or few problems, but the different types of unawareness might require different treatment approaches. For example, several case studies suggest that patients with ISA require a different therapeutic approach compared to patients with DD. Katz, Fleming, and colleagues [Citation15] describe three case studies with various and complex presentations of unawareness. They report that a patient with unawareness due to neurocognitive disturbance profits from challenging tasks in which they can discover abilities and weaknesses with the help of therapist feedback, while these challenging tasks could risk breaking down defense mechanisms in patients with high levels of DD [Citation15]. The patients with high levels of DD probably benefit most from a more psychotherapeutic approach that involves developing a therapeutic alliance, providing opportunities to seek support, and taking into account the patient’s readiness and cognitive impairment [Citation16].
Most instruments used to assess awareness, like the Patient Competency Rating Scale (PCRS), can indicate a general impairment in awareness of deficits but do not differentiate between ISA and DD. To our knowledge, the Clinician’s Rating Scale for evaluating Impaired Self-Awareness and Denial of Disability after brain injury (CRS-ISA-DD) developed by Prigatano and Klonoff [Citation8] is the only instrument specifically designed to differentiate between ISA and DD. It consists of two subscales, each enlisting ten behaviors indicative of either ISA or DD. Prigatano and Klonoff [Citation8] investigated the inter-rater reliability and construct validity of the CRS-ISA-DD in a clinical TBI population. In general, inter-rater reliability was high (r = 0.77). The initial test of construct validity was encouraging since results showed that patients clinically judged as primarily ISA patients scored significantly higher on the ISA scale than DD patients and vice versa [Citation8]. So far, the scale has only been used in a few studies with clinical populations [Citation8,Citation15,Citation17]. Since problems related to impaired self-awareness can persist after discharge home, it is important to investigate the usefulness of this instrument in TBI patients who are reintegrating into the community.
The aim of this study was twofold: [Citation1] to investigate the nature and severity of ISA and DD symptoms in a community-dwelling TBI sample and [Citation2] to investigate psychometric properties of the CRS-ISA-DD. It was hypothesized that the ISA and DD scales would both show a positive association with a frequently used measure of general awareness of deficits, namely, the Patient Competency Rating Scale. In addition, we hypothesized that high ISA scores would be associated with poorer neuropsychological functioning but not, or to a lesser extent, to psychological factors such as anxiety. In contrast, we expected that the DD scale would be associated with measures of avoidance coping, use of defense mechanisms, and anxiety while not, or to a lesser extent, with poorer neuropsychological functioning.
Methods
Participants
Participants were former patients of three rehabilitation centers in the Netherlands and their significant others. The patients had all received in- and/or outpatient rehabilitation but were no longer in active treatment at the moment of recruitment. Recruitment took place from July to November 2013 at Adelante, Libra, and Zuyderland Medical Center, and from March 2017 to June 2018 at Libra and Adelante. This second cohort was added in an attempt to gather data with more variability for the construct validity analysis. Inclusion criteria were a minimum age of 18; moderate to severe traumatic brain injury as measured by Post Traumatic Amnesia (PTA) >1 h or a Glasgow Coma Scale (GCS) score 3–12 or Loss of Consciousness (LOC) >1 h [Citation18]; between 6 months and 6 years post-injury; discharged home after rehabilitation. Exclusion criteria were a premorbid psychiatric disorder that required treatment; language and communication problems; absence of a significant other willing to participate; the presence of a statement of refusal to participate in scientific research in the medical file.
A significant other was defined as a person who is close to the patient and knows the patient well, such as a partner or family member. Significant others were eligible for participation if they were 18 years or older and were excluded if they had language and communication problems based on clinical judgment.
Measures
Demographic and injury-related information
Socio-demographic data and injury characteristics were collected from medical files. These included age, sex, level of education, date of injury, cause of injury, and initial severity of the injury. The level of education was dichotomized into high education, including senior secondary education, university preparatory education, higher professional education and university, and low education, which was primary education or less. Injury severity was reported using the GCS [Citation19], duration of PTA, or duration of LOC [Citation18], depending on which clinical data were available.
Impaired self-awareness and denial of disability
The Clinician’s Rating Scale for Evaluating Impaired Self-Awareness and Denial of Disability (CRS-ISA-DD) consists of two subscales: Impaired Self-Awareness (ISA) and Denial of Disability (DD). Each subscale consists of ten items on typical behaviors of ISA or DD. For this study, all original items were translated and revised to clarify their meaning (Supplementary Appendix 2). The assessor rates the presence and severity of the behaviors based on contact with the patient and significant other. In this study, this was based on the interview with the patient and significant other, as well as the patient’s verbal and nonverbal reactions during neuropsychological testing and feedback. If a behavior is rated as present, the severity of the behavior is scored. Severity scores can range from 1 (very mild) to 10 (severe). If a characteristic is not present, the severity score is 0. The total severity score of each subscale was used in the analyses and can range from 0 to 100 with a higher score representing more severe ISA or DD [Citation8].
Feasibility
To assess the feasibility of the CRS-ISA-DD, patients and significant others were asked how uncomfortable and how confronting the assessment procedure was. These items were rated on a scale ranging from 1 (very uncomfortable or confronting) to 5 (not at all uncomfortable or confronting). In addition, the researchers recorded the time needed to complete the CRS-ISA-DD and they rated the ease to complete the scale. Scores ranged from 1 (very easy) to 5 (very hard).
Awareness of deficits
The Patient Competency Rating Scale (PCRS) is a 30-item self-report instrument [Citation20]. The items measure the patient’s degree of difficulty on a variety of tasks and functions on a scale from 1 (can’t do) to 5 (can do with ease); the total score range is 30–150. Both the patient and significant other completed the PCRS. The measure of awareness of deficits is the discrepancy score (patient’s total score – significant other’s total score) and can range from −120 to 120. The greater the discrepancy, the more impaired the patient’s self-awareness of deficits. A negative discrepancy score indicates an underestimation of competencies by the patient, while a positive discrepancy score indicates an overestimation of competencies.
Neuropsychological assessment
The neuropsychological tasks used were chosen to reflect diverse cognitive functions and give an indication of impairments in these cognitive functions (and underlying brain networks). The Letter Digit Substitution Test (LDST) [Citation21] was used to assess information processing speed. Patients were instructed to match digits with letters according to a key, as many as possible within 90 seconds. There was a written and a verbal version. The number of digits correctly reported after 60 seconds was used as an outcome measure. The Visual Verbal Learning Test (VVLT) [Citation22] was administered as a measure of memory performance. Patients were shown a list of 15 words repeated over five trials and were asked to repeat them after each trial (immediate recall) and after 20 minutes (delayed recall). The total number of correct responses over five trials and the number of correct responses on the delayed recall were used as outcome measures. The Halstead Finger Tapping Test (HFTT) [Citation23] measures finger tapping speed and is thought to provide information about brain dysfunction [Citation23–25]. The index finger is placed on a lever and all other fingers rest on the board. Patients were given seven 10-second trails for both the dominant and non-dominant hand. After three trials patients switched hands to avoid fatigue [Citation26]. The outcome measure was the average of seven trials per hand. Phonemic fluency was used as a measure of executive functioning [Citation27,Citation28]. There were three rounds in which patients had 60 seconds to name as many words as possible starting with either the letter D, A, or T. The number of correct items generated was used as an outcome measure. The Zoo map test, a measure of planning and executive functioning, is part of the behavioural assessment of the dysexecutive syndrome [Citation29]. It involves plotting a route through a map following a set of rules. Scores range from 0 to 16 with higher scores indicating better performance. The Block Design test, part of the Wechsler Adult Intelligence Scale-IV (WAIS-IV) [Citation30], was administered but scores were not calculated. Instead, participants’ reactions to difficult situations and confrontations were noted and used to score the CRS-ISA-DD.
Defense mechanisms
The Defense Mechanism Manual (DMM) was employed to assess the use of defense mechanisms in Thematic Apperception Test (TAT) stories (Cards 2, 3BM, 4, 12F) [Citation31]. The stories were audio-recorded, transcribed, and translated into English in order to be independently scored by two trained coders (JP and RK) on the presence of three defense mechanisms: denial, projection, and identification. Scores were summed to obtain a DMM total score with higher scores indicating more use of overall defense mechanisms. Relative scores per defense mechanism were calculated by dividing the scores of each defense score by the sum of all three defense scores. The method has good inter-rater reliability scores (r = 0.82 for Denial, r = 0.71 for Projection, r = 0.82 for Identification) [Citation31].
Avoidance coping
The COPE Inventory [Citation32] is a 60-item self-report questionnaire measuring coping responses with scores from 1 (I usually don’t do this at all) to 4 (I usually do this a lot). Items are organized into 15 separate scales. In this study, three subscales were used: denial, mental disengagement, and behavioral disengagement. Together these subscales form the second-order factor avoidance coping which has a score range of 12–48 and higher scores represent more use of avoidance coping [Citation17].
Anxiety
The anxiety subscale of the Hospital Anxiety and Depression Scale (HADS-A) was used to determine patients’ levels of anxiety [Citation33]. The HADS-A contains seven items scored on a 4-point scale ranging from 0 to 3. Higher scores reflect a higher amount of reported anxiety symptoms.
Study procedures
The patients’ former treating physician or psychologist screened the patient files for eligibility. They informed patients and significant others and asked for interest in participation. If they were interested, a researcher contacted the patient, gave more information if necessary, and, if both patient and significant other agreed, planned a visit. After obtaining written informed consent, a researcher carried out the assessment procedure at the patient’s home.
The assessment procedure was held in Dutch. First, a standardized neuropsychological interview with the patient and significant other was held that lasted approximately 30 minutes (Supplementary Appendix 1). They were asked to share their views on changes due to the TBI with a focus on the patient’s cognitive impairment, emotional changes, and problems in daily functioning. This was videotaped to allow two researchers to independently analyze it. Next, patients completed the neuropsychological tests and questionnaires. Significant others completed a questionnaire about the patient’s functioning. Oral feedback about performance on the neuropsychological tests was given immediately and patients’ verbal and nonverbal reactions during neuropsychological testing and feedback were noted. Finally, patients and significant others completed a short feasibility questionnaire. Both researchers noted the CRS-ISA-DD administration time and completed a questionnaire on its feasibility.
Ethical approval for this study was obtained from the ethics committee of the Maastricht University Medical Center (MUMC) with reference number NL42752.068.12. The study was conducted according to the principles of the Declaration of Helsinki (World Medical Association, October 2008) and in accordance with the Medical Research Involving Human Subjects Act (WMO).
Statistical analyses
All statistical analyses were conducted using SPSS 25 for Windows. Results were considered significant if p ≤ 0.05.
Demographic and injury-related information
Descriptive statistics were used to describe the demographic and injury-related parameters. The following neuropsychological test scores were transformed to standardized z- and t-scores derived from norm data: LDST [Citation21], VVLT [Citation34], HFFT [Citation35], and phonemic fluency [Citation36]. In line with the scoring instructions, data of the COPE were discarded if more than one item on a subscale was missing. PCRS and HADS-A data were discarded if more than 25% of the items were missing. If for any scale the number of missing items was within the allowed range, the total score was calculated by extrapolating the total score of the items available ((Total score/Number of completed items) × Total number of items on the scale).
Nature and severity ISA and DD
Descriptive statistics were used to describe the presence of one or more symptoms of ISA and DD, as well as the severity of these symptoms.
Inter-rater reliability
Two raters, both neuropsychologists, completed the CRS-ISA-DD independently of each other in order to determine inter-rater reliability using intra-class correlations (ICC). The appropriate type of ICC, that is, ICC(2,1), was determined based on the description by Shrout and Fleiss [Citation37]. The estimate for absolute agreement between raters was reported. An ICC >0.74 is considered excellent, 0.74–0.60 is considered good, 0.59–0.40 is considered fair and <0.40 is considered poor [Citation38]. This was completed on the first cohort only. The rater for the second cohort was also a rater in the first cohort.
Internal consistency
Internal reliability of the ISA and DD scales was assessed using Cronbach’s α. A Cronbach’s α ≥ 0.8 was considered good, 0.70–0.80 moderate, 0.60–0.70 acceptable and <0.60 was considered as poor internal consistency [Citation39].
Floor and ceiling effects
Floor or ceiling effects were considered present if more than 15% of the patients had the highest or lowest possible score on either the ISA or DD scale [Citation40].
Feasibility
To explore the feasibility, the mean time of the CRS-ISA-DD assessment and frequency counts of the different answers on the feasibility questionnaire were calculated.
Correlations with other measures
Because of violations of the normality assumption, Spearman’s correlation coefficients were used to investigate associations between the CRS-ISA-DD subscales and injury severity, PCRS discrepancy score, neuropsychological test scores, COPE Avoidance, DMM, and HADS-A. Correlations were considered good if ≥0.60, moderate between 0.30 and 0.60, and poor for correlations ≤0.30 [Citation39].
Results
Participants
Seventy-eight patients and their significant others consented to participate, corresponding to a 42% response rate. Although not explicitly asked, some patients and significant others who refused to participate gave reasons for refusal. They found it too time-consuming, too confronting, did not see a need to participate, or thought the injury was too long ago and wanted to close that chapter of their life. In some cases, there were no significant others available or willing to participate. Some significant others anticipated a quarrel with the patient since they disagreed about the experienced difficulties and, therefore, chose not to participate.
Patient characteristics are presented in . The majority of the significant others were partners (n = 43; 55%) or parents (n = 23; 30%). Of the remaining significant others, five were the patient’s children, three were siblings, two were neighbors and one was an ex-partner. With respect to missing values, the following amount of data was discarded because the number of missing items was out of range: two PCRS patient questionnaires, two HADS-A questionnaires, and three COPE avoidance scales. Feasibility data was missing and discarded for three patients and seven significant others. There were no missing values in the CRS-ISA-DD data.
Table 1. Sample characteristics (n = 78).
Nature and severity ISA and DD
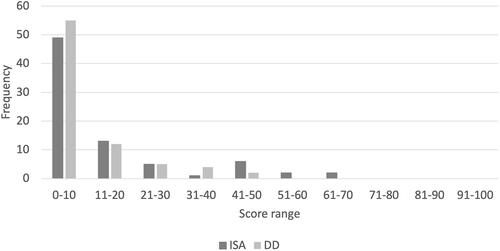
Regarding the scores on the CRS-ISA-DD, at least one item was scored as a present for most participants on the ISA scale (n = 57) as well as the DD scale (n = 58). Eleven participants showed the presence of more than three items on ISA, and seven participants showed the presence of more than three items on DD. If present, severity was scored. As can be seen in , the severity scores on the ISA and DD scales were skewed to the left. The majority of the patients had a severity score in the lower range on both scales, indicating that most patients had little awareness deficits and only a few patients showed severe ISA and/or DD. The correlation between the ISA and DD scales was not significant (rs(76) = −0.07, p = 0.54).
Inter-rater reliability
For the first cohort of participants included in the study, a second rater independently rated the CRS-ISA-DD data (n = 57). Inter-rater reliability was excellent on both scales. The ICC(2,1) absolute agreement coefficient was 0.928 (p < 0.00, 95% CI = 0.881–0.957) for the ISA scale and 0.835 (p < 0.00, 95% CI = 0.735–0.899) for the DD scale.
Internal consistency
The internal consistency of the ISA scale was good (Cronbach’s α = 0.819). The DD scale had an acceptable internal consistency (Cronbach’s α = 0.645).
Floor and ceiling effects
Indications of floor effects were found on both scales. On the ISA scale, 19 patients (24.4%) scored the lowest possible score. On the DD scale, 20 patients (25.6%) scored the lowest possible score. None of the participants obtained the highest possible score on the ISA or DD scale, indicating no ceiling effects.
Feasibility
The majority of the participants who filled out the feasibility questionnaires did not experience the assessment procedure as uncomfortable (patients: 84%, n = 63; significant others: 93%, n = 66; score >3) or confronting (patients: 73%, n = 55; significant others 79%, n = 56; score >3).
For the subset of 57 participants assessed by two raters, assessment time was noted. The neuropsychological interview and assessment took approximately 2.5 hours in total. To complete the CRS-ISA-DD, the raters on average needed 8.3 minutes (SD = 3.2, range 5–17.5). Once familiar with the scale, the rating duration decreased. Seventy-seven percent of the ratings were made within 5–10 minutes. The two raters indicated that for the majority of the ratings (65%) the CRS-ISA-DD was easy to very easy (score < 3) to score.
Correlations with other measures
The associations between the ISA and DD scale and the injury severity measures, PCRS discrepancy scores, neuropsychological assessment, COPE Avoidance, DMM, and HADS-A scores can be found in .
Table 2. Relationships between the ISA and DD scales and other measures.
Injury severity measures
It was uncommon for all three measures of injury severity to be noted in the medical files. In eight cases, the GCS, PTA, as well as LoC scores, were available. In 32 cases, only two of these were available, in 35 cases, only one was available, and in three cases, none was available. Classifying patients into groups based on different measurements can lead to inconsistencies. Therefore, each injury severity measure was used as a continuous variable and separately correlated with ISA and DD. The ISA scale showed moderate significant associations with GCS score (rs(51) = −0.32, p = 0.02), longer duration of PTA (rs(37) = 0.37, p = 0.02) as well as LOC (rs(29) = 0.42, p = 0.02). These results indicate that the more severely injured someone was, the higher their ISA scores. The DD scale, on the other hand, was not significantly associated with GCS (rs(51) = −0.04, p = 0.77), duration of PTA (rs(37) = 0.08, p = 0.63) or duration of LOC (rs(29) = −0.13, p = 0.47).
Awareness of deficits
No associations were found between the PCRS discrepancy scores and either ISA (rs(74) = −0.03, p = 0.78) or DD scales (rs(74) = −0.01, p = 0.92). In general, PCRS discrepancy scores were low (M = 3.0, SD = 12.5) indicating that, on average, patients had good awareness of their deficits.
Neuropsychological assessment
With respect to the neuropsychological measures, significant negative associations were found between the ISA scale and the written version of the LDST (rs(74) = −0.30, p = 0.01), the VVLT total score (rs(76) = −0.47, p < 0.001), VVLT delayed recall score (rs(76) = −0.45, p < 0.001) and the Zoo map test (rs(75) = −0.39, p < 0.001). These moderate associations indicate that worse cognitive performance is associated with more severe ISA. No significant associations were found between the ISA scale and the verbal version of the LDST (rs(74) = −0.22, p = 0.06), phonemic fluency (rs(76) = −0.21, p = 0.06) or the HFFT (left rs(75) = −0.17, p = 0.14; right rs(76) = −0.08, p = 0.51). None of the neuropsychological measures correlated significantly with the DD scale: written version of the LDST (rs(74) = 0.01, p = 0.94), verbal version of the LDST (rs(74) = 0.07, p = 0.58), the VVLT total score (rs(76) = 0.05, p = 0.68), VVLT delayed recall score (rs(76) = 0.07, p = 0.55), HFFT (left rs(75) = 0.07, p = 0.52; right rs(76) = 0.03, p = 0.77), phonemic fluency (rs(76) = 0.04, p = 0.70), Zoo map test (rs(75) = 0.17, p = 0.15).
Avoidance coping
No significant associations were found between the COPE Inventory Avoidance factor and either ISA (rs(73) = 0.11, p = 0.37) or DD scales (rs(73) = −0.14, p = 0.22).
Defense mechanisms
Total defense mechanism scores were not significantly correlated with either ISA (rs(74) = −0.15, p = 0.19) or DD scale (rs(74) = 0.05, p = 0.67). On the DMM subscales, ISA showed low but significant associations with the use of denial (rs(74) = 0.24, p = 0.04) and projection (rs(74) = −0.24, p = 0.03), and no significant associations with identification (rs(74) = −0.04, p = 0.71). Thus, more severe ISA was associated with more use of denial and less use of projection as a defense mechanism. None of the DMM subscales correlated significantly with the DD scale (denial (rs(74) = −0.18, p = 0.11); projection (rs(74) = 0.04, p = 0.74); identification (rs(74) = 0.20, p = 0.09)).
Anxiety
HADS-A scores were not significantly associated with ISA scores (rs(74) = −0.05, p = 0.67). However, the correlation between anxiety scores and DD scores was significant (rs(74) = −0.22, p = 0.05) and indicates that patients with more severe DD report fewer anxiety symptoms.
Discussion
The aim of this study was to investigate the nature and severity of ISA and DD symptoms in a community-dwelling TBI sample and to assess the psychometric properties of the CRS-ISA-DD. The current sample showed symptoms of impaired self-awareness. The majority of the participants showed the presence of at least one ISA or DD behavior, indicating ISA and DD occur in community-dwelling TBI patients. Sometimes the ISA or DD was fairly severe. Nonetheless, there were not many participants with extreme severity scores, especially regarding DD.
The variability in scores on the ISA and DD scales was low. Our sample consisted of, mostly, patients with good awareness and only a few who showed higher levels of ISA and/or DD. The floor effect for the ISA and DD scales also indicates a reduced variability in the gathered data. The lack of patients with more severe awareness deficits in our sample may have influenced the statistical power. Denial could serve as a mechanism to protect oneself against the immediate impact of a life-changing event, such as TBI, and could act as a buffer during which more adaptive coping strategies can be developed [Citation41]. Even though some patients with DD were present in the current sample, it is possible that the more extreme cases were missed or that DD is more frequently present in the acute post-injury phase. In the case of the latter, the CRS-ISA-DD might be less suitable for use as a screening tool in chronic community-dwelling patients and more suitable earlier in the rehabilitation trajectory. As such, this adds essential new information on the future use of this instrument.
In terms of administration time and level of confrontation, the CRS-ISA-DD is a feasible instrument. Assessment of the psychometric properties indicates excellent absolute agreement on both the ISA and DD scales, as reflected by the high ICC scores. This is in line with other studies that report high inter-rater reliability of the CRS-ISA-DD [Citation8,Citation17]. The internal consistency was good for the ISA scale and acceptable for the DD scale. The collection of information necessary to complete the CRS-ISA-DD, such as the neuropsychological assessment and interview with the patient and significant other, was time-consuming in this particular research setting. However, once all information is gathered and one is familiar with the scale, the CRS-ISA-DD can be completed within 10 minutes. Raters judged the scale as easy to use.
The patients showed overall good awareness of deficits, reflected in the low average discrepancy scores on the PCRS and the low average scores on the ISA and DD scales of the CRS-ISA-DD. Unexpectedly, PCRS discrepancy scores were not significantly correlated to ISA or DD. A possible explanation is that they measure more distinct aspects of awareness than we hypothesized. Recognizing a problem when it occurs and anticipating future problems require higher levels of awareness than merely understanding changes in one’s level of functioning [Citation42]. The PCRS is a self-report measure addressing the understanding of difficulties patients are dealing with, while the CRS-ISA-DD is a clinician-rated instrument based on the patient’s behavior. Besides differentiating between ISA and DD, it also includes observations of patients during tasks and can assess their reactions and awareness while making mistakes.
Participants who were more severely injured showed more severe levels of ISA while injury severity had no associations with DD. Furthermore, in line with other studies [Citation9,Citation43], patients with more severe ISA performed worse on tests for processing written information, memory, and planning. Injury severity has been associated with less efficient brain network functioning [Citation44]. In turn, injury to networks such as the frontoparietal control network has been associated with impaired self-awareness [Citation45]. The neuropsychological tasks used in the study indicate impairments in different cognitive functions and underlying brain networks. The correlations of ISA, but not DD, with poor performance on these tasks, suggest ISA is associated with neurocognitive factors while DD is not.
There was a significant negative correlation between DD and anxiety symptoms. This is in line with previous research [Citation9] and suggests that denial of disability is a psychological mechanism to protect against emotional distress [Citation8,Citation14]. No evidence for a positive association between DD and avoidance coping was found. This is possibly due to a lack of anxiety to drive the avoidance behavior. However, this finding is in contradiction to previous studies [Citation17]. Mean avoidance coping scores were much lower in the current sample and time since injury was longer. As mentioned before, DD and avoidance behavior might be mostly present in the more acute phases following TBI. An assumption is that those who participated are generally functioning at a reasonable level in society and do not need to engage in other defense mechanisms. There is no information on societal participation (e.g., return to work/education, independent living, and supervision) in the current study. It would be interesting to take this into account in future studies.
The use of defense mechanisms was not in line with our hypotheses. Patients with more severe ISA made more use of the denial defense and less use of the projection defense relative to the other defenses, while DD was not associated with any of the defenses. It is important to note that the process of translating the TAT stories before scoring might have influenced the results. Moreover, denial is scored when patients fail to respond to certain aspects of the pictures [Citation46]. Individuals with high ISA scores simply might not attend to all relevant stimuli or do not perceive them as threatening due to their cognitive impairments. Furthermore, defense mechanisms can be placed on a developmental continuum with denial being the most immature defense, followed by projection and then identification [Citation46]. Since cognitive performance was more compromised in patients with more severe ISA, their ability to use more mature defenses may have been reduced as well. This is in line with the findings of Cramer [Citation46], who found that ‘cognitive weakness’ was one of several behavioral correlates in the use of denial in young adults.
There was an unexpected sampling bias; only 42% of patients approached agreed to participate. ISA and, in particular, DD may be uncommon problems in chronic community-dwelling TBI populations. However, it has been shown that self-awareness problems persist into the chronic phase [Citation47]. The true prevalence of ISA and DD in community-dwelling TBI patients could be higher than detected. Individuals with higher levels of ISA and DD might have participated but might still have been unwilling to discuss their problems during the assessment. Alternatively, they might have refused participation altogether since they found it too confronting or anticipated a quarrel. This is a paradox: the behavior we want to quantify is the reason that we cannot measure it. A more accurate representation of ISA and DD prevalence could be detected by changing the sampling procedure. For example, potential participants could be recruited through significant others instead of through former treating physicians or psychologists. Furthermore, contacting the participants at a shorter time since injury might be beneficial to increase participation. This way, relevance for the participants themselves increases as well as the chance of enrolling patients with higher levels of DD. However, this would lead to a different patient population with more acute TBI. In order to investigate ISA and DD in a chronic community-dwelling TBI population, making the assessment procedure less confronting between patients and significant others could enhance response rates. Although it was necessary for this study to observe patients’ reactions to significant others’ opinions, it might help if the interviews are held separately.
A limitation of this study is that despite the considerable sample size, the final sample did not include a large variability of ISA and DD scores. Nonetheless, the results provide useful new information on measuring self-awareness in a community-dwelling TBI population. The ISA and DD scores obtained in the current study were lower than the scores in the original study of construct validation of the CRS-ISA-DD [Citation8]. It is important to realize that the CRS-ISA-DD was developed in a clinical setting. In such a setting, a clinician would decide to use the instrument after suspecting a self-awareness problem. In a community setting, such as in the current study, the patient and significant other are asked to observe their own behavior and determine whether there is a self-awareness problem. However, especially in the presence of self-awareness problems, this might be difficult, if not impossible, for them to assess. Thus, despite the feasibility of the CRS-ISA-DD, the results indicate the instrument is not very useful as a general screening instrument when administered by someone not familiar with the patient. The instrument might be more useful when awareness problems are evident and when administered by someone who is familiar with the patient. This would often be in a clinical setting. Determining which type of treatment is necessary should be done as soon as possible. Therefore, the instrument would be of great use early in the rehabilitation trajectory. However, if awareness problems persist until later in the rehabilitation trajectory, this instrument can still be effective in determining which type of awareness problems are most prominent and how to best deal with them. By conducting an initial screening and recruiting only those patients with impaired self-awareness problems, the usefulness of CRS-ISA-DD as a diagnostic tool to separate ISA from DD can be further explored.
In conclusion, despite a low variability of scores on the CRS-ISA-DD, ISA and DD seem to occur in a population of community-dwelling TBI patients. The prevalence in the current study might be an underrepresentation. Overall, the CRS-ISA-DD is a feasible instrument with excellent inter-rater reliability and acceptable good internal consistency. The data suggests that the ISA and DD scales measure different phenomena. The ISA subscale correlates with injury severity and cognitive problems and seems to be able to distinguish unawareness of deficits as a result of a neurologically based deficiency. In the current sample, it remains unclear whether the instrument can also dissociate a DD group that correlates with psychological denial factors. The CRS-ISA-DD has potential as a diagnostic tool but the current study suggests it is less applicable as a screening tool for self-awareness problems in a community-dwelling TBI population. However, once a self-awareness problem has been identified, the instrument could be very useful to differentiate between ISA and DD, in both the clinic and at home. For scientific purposes, it is desirable to have more variability in the ISA and DD scores in order to draw more reliable conclusions. Therefore, in future research, it would be interesting to use the CRS-ISA-DD as a diagnostic tool in a community-dwelling TBI patient population in which self-awareness problems have already been established.
Supplemental_file.docx
Download MS Word (23 KB)Acknowledgments
Authors would like to thank participants and employees at Adelante (Hoensbroek), Libra Zorggroep (Blixembosch and Leijpark, Eindhoven), Zuyderland Medical Center (Sittard) associated with the study. We would specifically like to thank Ingrid Brands for her contributions to the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Fleming JM, Strong J, Ashton R. Self-awareness of deficits in adults with traumatic brain injury: how best to measure? Brain Inj. 1996;10(1):1–15.
- Prigatano GP, Schacter DL. Introduction. In Prigatano GP, Schacter DL, editors. Awareness of deficit after brain injury: clinical and theoretical issues. New York (NY): Oxford University Press, Inc.; 1991.
- Millis SR, Rosenthal M, Novack TA, et al. Long-term neuropsychological outcome after traumatic brain injury. J Head Trauma Rehabil. 2001;16(4):343–355.
- Leung DP, Liu KP. Review of self-awareness and its clinical application in stroke rehabilitation. Int J Rehabil Res. 2011;34(3):187–195.
- Ownsworth T, Clare L. The association between awareness deficits and rehabilitation outcome following acquired brain injury. Clin Psychol Rev. 2006;26(6):783–795.
- Chesnel C, Jourdan C, Bayen E, et al. Self-awareness four years after severe traumatic brain injury: discordance between the patient’s and relative’s complaints. Results from the PariS-TBI study. Clin Rehabil. 2018;32(5):692–704.
- Ownsworth T, Clare L, Morris R. An integrated biopsychosocial approach to understanding awareness deficits in Alzheimer’s disease and brain injury. Neuropsychol Rehabil. 2006;16(4):415–438.
- Prigatano GP, Klonoff PS. A clinician’s rating scale for evaluating impaired. Self-awareness and denial of disability after brain injury. The Clinical Neuropsychologist. 1998;12(1):56–67.
- Ownsworth T, Fleming J, Strong J, et al. Awareness typologies, long-term emotional adjustment and psychosocial outcomes following acquired brain injury. Neuropsychol Rehabil. 2007;17(2):129–150.
- Hurst FG, Ownsworth T, Beadle E, et al. Domain-specific deficits in self-awareness and relationship to psychosocial outcomes after severe traumatic brain injury. Disabil Rehabil. 2020;42(5):651–659.
- Prigatano GP, Altman IM. Impaired awareness of behavioral limitations after traumatic brain injury. Arch Phys Med Rehabil. 1990;71(13):1058–1064.
- Sherer M, Hart T, Whyte J, et al. Neuroanatomic basis of impaired self-awareness after traumatic brain injury: findings from early computed tomography. J Head Trauma Rehabil. 2005;20(4):287–300.
- Belchev Z, Levy N, Berman I, et al. Psychological traits predict impaired awareness of deficits independently of neuropsychological factors in chronic traumatic brain injury. Br J Clin Psychol. 2017;56(3):213–234.
- Prigatano GP, Morrone-Strupinsky J. Management and rehabilitation of persons with anosognosia and impaired self-awareness. In: Prigatano GP, editor. The study of anosognosia. New York: Oxford University Press; 2010. p. 495–516.
- Katz N, Fleming J, Keren N, et al. Unawareness and/or denial of disability: implications for occupational therapy intervention. Can J Occup Ther. 2002;69(5):281–292.
- Ownsworth T. The impact of defensive denial upon adjustment following traumatic brain injury. Neuropsychoanalysis. 2005;7(1):83–94.
- Kortte KB, Wegener ST, Chwalisz K. Anosognosia and denial: their relationship to coping and depression in acquired brain injury. Rehabil Psychol. 2003;48(3):131–136.
- Hannay H. Neuropathology for neuropsychologists. In: Lezak MD, Howieson DB, Loring DW, editors. Neuropsychological assessment. 4th ed. New York (NY): Oxford University Press; 2004. p. 157–285.
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84.
- Prigatano GP. Neuropsychological rehabilitation after brain injury. Baltimore (MD): Johns Hopkins University Press; 1986.
- van der Elst W, van Boxtel MP, van Breukelen GJ, et al. The letter digit substitution test: normative data for 1,858 healthy participants aged 24-81 from the Maastricht Aging Study (MAAS): influence of age, education, and sex. J Clin Exp Neuropsychol. 2006;28(6):998–1009.
- Brand N, Jolles J. Learning and retrieval rate of words presented auditorily and visually. J Gen Psychol. 1985;112(2):201–210.
- Reitan RM. Manual for administration of neuropsychological test batteries for adults and children. Indianapolis (IN): Indiana University Medical Center; 1955.
- Halstead WC. Brain and intelligence; a quantitative study of the frontal lobes. Chicago (IL): University of Chicago Press; 1947.
- Vega A, Jr, Parsons OA. Cross-validation of the Halstead-Reitan tests for brain damage. J Consult Psychol. 1967;31(6):619–625.
- Prigatano GP, Borgaro SR. Qualitative features of finger movement during the Halstead Finger Oscillation Test following traumatic brain injury. J Int Neuropsychol Soc. 2003;9(1):128–133.
- Baldo JV, Schwartz S, Wilkins D, et al. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc. 2006;12(6):896–900.
- Henry JD, Crawford JR. A meta-analytic review of verbal fluency performance in patients with traumatic brain injury. Neuropsychology. 2004;18(4):621–628.
- Wilson BA, Alderman N, Burgess PW, et al. Behavioural assessment of the dysexecutive syndrome. Bury St Edmunds (UK): Thames Valley Test Company; 1997.
- Wechsler D. Wechsler Adult Intelligence Scale-Fourth edition: administration and scoring manual. San Antonio (TX): Psychological Corporation; 2008.
- Cramer P. Personality change in later adulthood is predicted by defense mechanism use in early adulthood. J Res Personality. 2003;37(1):76–104.
- Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56(2):267–283.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370.
- van der Elst W, van Boxtel MP, van Breukelen GJ, et al. Rey’s Verbal Learning Test: normative data for 1855 healthy participants aged 24-81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsychol Soc. 2005;11(3):290–302.
- Heaton R, Miller S, Taylor MJ, et al. Revised comprehensive norms for an expanded Halstead-Reitan battery: demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz (FL): Psychological Assessment Resources; 2004.
- Schmand B, Groenink SC, van den Dungen M. [Letter fluency: psychometric properties and Dutch normative data]. Tijdschr Gerontol Geriatr. 2008;39(2):64–76. Dutch.
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428.
- Cicchetti DV. The precision of reliability and validity estimates re-visited: distinguishing between clinical and statistical significance of sample size requirements. J Clin Exp Neuropsychol. 2001;23(5):695–700.
- Visser-Meily JM, Post MW, Riphagen II, et al. Measures used to assess burden among caregivers of stroke patients: a review. Clin Rehabil. 2004;18(6):601–623.
- Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34–42.
- Gainotti G. Emotional and psychosocial problems after brain injury. Neuropsychol Rehabil. 1993;3(3):259–277.
- Crosson B, Barco PP, Velozo CA, et al. Awareness and compensation in post-acute head injury rehabilitation. J Head Trauma Rehabil. 1989;4(3):46–54.
- Bogod NM, Mateer CA, Macdonald SW. Self-awareness after traumatic brain injury: a comparison of measures and their relationship to executive functions. J Int Neuropsychol Soc. 2003;9(3):450–458.
- Raizman R, Tavor I, Biegon A, et al. Traumatic brain injury severity in a network perspective: a diffusion MRI based connectome study. Sci Rep. 2020;10(1):1–12.
- Ham TE, Bonnelle V, Hellyer P, et al. The neural basis of impaired self-awareness after traumatic brain injury. Brain. 2014;137(2):586–597.
- Cramer P. Defense mechanisms, behavior, and affect in young adulthood. J Pers. 2002;70(1):103–126.
- Pettemeridou E, Kennedy MRT, Constantinidou F. Executive functions, self-awareness and quality of life in chronic moderate-to-severe TBI. NeuroRehabilitation. 2020;46(1):109–118.