Abstract
Purpose
To obtain information on characteristics, management, current objective nutritional status and perception of nutritional status of children with cerebral palsy (CP) from healthcare professionals (HCPs) and caregivers.
Materials and methods
A detailed survey of several items on eight main topics (general characteristics, motor function, comorbidities, therapies, anthropometry, feeding mode and problems and perceived nutritional status) was developed and tested for the study. Correlation between nutritional status and Gross Motor Function Classification System (GMFCS) levels was assessed using continuous variables (Z-scores for weight-for-age, height-for-age, weight-for-height, and body mass index-for-age), and categorical variables (being malnourished, stunted, or wasted). HCP and caregiver perceptions of the child’s nutritional status as well as agreement between perceived and objective nutritional status and agreement between perceived nutritional status and concerns about the nutritional status were analyzed.
Results
Data were available for 497 participants from eight European countries. Poorer nutritional status was associated with higher (more severe) GMFCS levels. There was minimal agreement between perceived and objective nutritional status, both for HCPs and caregivers. Agreement between HCP and caregiver perceptions of the child’s nutritional status was weak (weighted kappa 0.56). However, the concerns about the nutritional status of the child were in line with the perceived nutritional status.
Conclusions
The risk of poor nutritional status is associated with more severe disability in children and adolescents with CP. There is a mismatch between HCP and caregiver perceptions of participants’ nutritional status as well as between subjective and objective nutritional status. Our data warrant the use of a simple and objective screening tool in daily practice to determine nutritional status in children and adolescents with CP. Clinical trial registration: ClinicalTrials.gov Identifier: NCT03499288 (https://clinicaltrials.gov/ct2/show/NCT03499288).
Use of the ESPGHAN recommendations and simple screening tools in daily practice is needed to improve nutritional care for individuals with CP.
Attention should be paid to the differences in the perception of nutritional status of individuals with CP between professionals and caregivers to improve appropriate referral for nutritional support.
Objective measures rather than the professional’s perception need to be used to define the nutritional status of individuals with CP.
IMPLICATIONS FOR REHABILITATION
Introduction
Numerous medical problems affecting children and adolescents with cerebral palsy (CP) are related to the primary neurologic disability and secondary complications that may be associated with modifiable factors, such as nutrition [Citation1]. Impaired nutritional status can have long-term implications for growth and development [Citation2–7]. The relationship between CP and nutritional status is complex and affected by multiple factors beyond dietary intake [Citation2,Citation3]. These factors include gross motor function classification system (GMFCS) level [Citation8–11], oral-motor dysfunction and feeding skills [Citation3], gastrointestinal disorders (such as dysphagia, gastro-esophageal reflux and constipation [Citation2]), physical activity levels as well as altered energy requirements [Citation5,Citation10,Citation12–15]. Last but not least the availability of healthcare resources and nutritional support can influence the nutritional status of patients with CP [Citation3,Citation4,Citation16].
The frequent feeding difficulties and complex nature of CP led to an increasing attention for a systematic approach to the management of feeding and nutrition in the CP population in the past decade. The Eating and Drinking Ability Classification System (EDACS) was developed to classify eating and drinking ability of CP patients. EDACS is a 5-level classification system ranging from level I (eats and drinks safely and efficiently) to level V (unable to eat or drink safely – tube feeding may be considered to provide nutrition) used in several countries [Citation17]. Using the EDACS aids communication between people with CP, their parents, and different healthcare professionals (HCPs) working in different settings [Citation17]. To facilitate a systematic approach to the care of feeding and nutritional difficulties in CP patients, the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) has developed uniform guidelines to manage nutritional problems in children with neurological impairment [Citation18]. The ESPGHAN guidelines, among other things, include red-flag warning signs to identify undernutrition based on physical signs, weight-for-age Z-score (< −2), triceps skinfold thickness (<10th centile for age and sex), mid-upper arm fat or muscle area (<10th centile), and faltering weight and/or failure to thrive.
Several observational studies have highlighted the burden of nutrition-related problems among children and adolescents with CP [Citation8,Citation9,Citation19–27]. More severe disability (higher GMFCS level) is associated with a risk for undernutrition and negative changes in body composition, such as lower fat-free mass index levels and greater body fat percentage, even among children and adolescents with access to nutritional support [Citation10,Citation19,Citation20,Citation24,Citation25]. Well-nourished children with CP are at risk for overweight/obesity associated with low levels of daily activity [Citation9], better functional oral motor performance [Citation23], and, in individuals with quadriplegia and low resting energy expenditure, the use of tube feeding [Citation9].
Although these studies emphasized specific issues for individual countries, they have not yet provided a complete picture showing how nutritional parameters are related to other factors, such as gastrointestinal problems, feeding difficulties, and other comorbidities. There remains a need for broader international research into the nutritional challenges facing the CP population. Therefore, the aims of the multi-country PURPLE N (Profiling Children and Youth with Cerebral Palsy in Relation to Feeding and Nutrition) study were
obtaining information on the current nutritional status, management, and characteristics of children and adolescents with CP;
comparing the child’s objective nutritional status with the nutritional status of the child as perceived by the HCP and caregiver;
comparing the child’s perceived nutritional status by HCP and caregiver, and
evaluating concerns about the child’s nutritional status expressed by HCPs and caregivers.
Materials and methods
Participants
Eligible participants were enrolled from hospital-based clinical practice (out-patient care). Participants had a confirmed diagnosis of CP, were younger than 18 years, and had visited their HCP within the past 12 months. The diagnosis of CP was obtained and confirmed by the participant’s HCP from the patient’s hospital dossier. Individuals with neurodegenerative diseases and acute infections (meningitis, encephalitis, or poliomyelitis) were excluded. Ethical approval was obtained from institutional review boards and written informed consent was provided by parents/legal representatives according to local laws. The study was conducted in line with the Declaration of Helsinki.
Study design
This multicenter, observational, cross-sectional study was conducted in the Czech Republic, Greece, Hungary, Italy, the Netherlands, Poland, Slovakia, and Turkey between April 2017 and June 2018. A final selection of participating countries was made based on their geographical location (Greater Europe) and the willingness of hospitals and HCPs to participate in the study. Because of the descriptive nature of this study, no formal sample size calculation for the main outcome parameters was performed. The chosen sample size (n > 500) was based on recommendations from expert HCPs and literature review.
A detailed survey of several items on eight main topics (general characteristics, motor function, comorbidities, therapies, anthropometry, feeding mode, nutritional status as well as caregivers’ participation) was developed by the study team including child neurologists, rehabilitation specialists, dieticians and speech therapists. Next, it was pilot tested for both HCPs and caregivers, after which questions and expressions were corrected where needed. After this step, surveys were translated into the language of each participating country and translated surveys were pilot tested locally. The final (English) survey is included in the Supplementary material.
Separate surveys were completed online as electronic case report forms by HCPs and caregivers to obtain information on general characteristics, anthropometric measurements, comorbidities, type and frequency of physical, occupational, and speech and language therapy, feeding mode, stress and problems, and perceived nutritional status. HCP survey data were recorded during a study visit or obtained from the last regular doctors visit (i.e., retrospectively from patient dossier; visit maximum 1 year ago). HCPs were asked to use their real-world methods in the assessment of children with CP. Caregivers completed their survey at home or on site (without the HCP being present in the room in order to ensure an unbiased environment). Surveys had to be submitted within 30 days after signed informed consent was provided, with a maximum of 30 days allowed between HCP and caregiver surveys.
The study was sponsored by Danone Nutricia Research, Utrecht, the Netherlands.
Data analysis
Participants were categorized according to their GMFCS level as provided by the HCP [Citation28]. Anthropometric measurements were used as proxies for children’s nutritional status and comprised continuous variables (Z-scores) and categorical variables based on WHO Child Growth Standards (Supplementary Table 1). In this context, the term malnourished is used synonymously with undernourished both for objective assessment of nutritional status and subjective assessment by HCPs or caregivers. The measurements analyzed were weight-for-age (WFA; participants aged 0–18 years), height-for-age (HFA; participants aged 0–18 years), body mass index-for age (BMIFA; continuous variable for participants aged 0–18 years and categorical variable for participants aged 5–18 years), and weight-for-height (WFH; for participants aged 0–5 years). BMIFA was calculated only if weight and height were measured on the same date. HCPs and caregivers were asked separately to indicate if they had a concern about feeding and/or nutritional status of participants. Data from HCPs were used for all analyses unless stated otherwise. When data were not available from HCPs, data from caregivers were used if possible; when height data were missing (n = 25), height was calculated based on tibial length (TL) using the Stevenson formula: height = (3.26 × TL) + 30.8. When this was not possible (n = 5), data provided by the caregiver were used. For the children with missing weight data (n = 8), data provided by the caregiver were used. Analyses were done according to a predefined statistical plan.
Statistical analysis
All statistics were reported descriptively. Continuous data were shown as the number of participants, mean, standard deviation, median, minimum and maximum. The least-squares means, standard deviations, and 95% confidence interval (CI) were shown for variables analyzed using AN(C)OVA procedures. Logistic regression analyses were done using nutritional status measures as categorical parameters. All statistical inferential procedures were performed at a significance level of 5% (unless otherwise indicated). The association between GMFCS level and nutritional status was analyzed with a crude model (without confounding variables) and an adjusted model including variables considered a priori as potential confounders (age, sex, gestational duration, and presence of comorbidities). To investigate potential intermediate effects, models were performed including the variables intensity of physical therapy, presence of oro-motor dysfunction, presence of feeding problem or stress of long feeding times. To test for potential effect modifying factors, the variables age group, country of residence, and use of tube feeding were added to the model as interaction terms; a p-value <0.10 for an interaction term was considered a signal for an effect modifier. Agreement between nutritional status perceived by HCPs and caregivers was investigated using Cohen’s kappa inter-rater reliability test [Citation29], where the levels of agreement were: none (0–0.20), minimal (0.21–0.39), weak (0.40–0.59), moderate (0.60–0.79), strong (0.80–0.90), and almost perfect (>0.90). Statistical analyses were performed by Danone Nutricia Research using SAS® (SAS version 9.4 or higher) for Windows, SAS Institute Inc., Cary, NC.
Results
Study participants
Data are available for 497 of 498 participants, including 430 participants with completed HCP and caregiver surveys and 67 with completed HCP surveys only. HCPs were mostly pediatric neurologists (78%) followed by pediatricians (11%). HCPs from Turkey, Poland, and Italy contributed the largest number of participants (75%).
summarizes baseline demographics, clinical characteristics, and feeding profiles of participants. The most common age group was 6–12 years (38%) followed by 2–4 years (21%), over 12 years (17%), 4–6 years (15%), and under 2 years (9%); 58% of participants were male. Half the study population had GMFCS level IV (18%) or V (32%), 84% had spastic motor type, and 55% had quadriplegia, thus representing a more severe form of CP. The median gestational age was higher in children with GMFCS V than in those with other levels.
Table 1. Demographic, clinical and feeding profile of study participants according to HCPs.
Comorbidities were reported by HCPs in most participants and comprised cognitive problems (66%), active epilepsy (54%), visual abnormalities (51%), gastrointestinal disturbances (43%), drooling (41%), behavioral problems (33%), oropharyngeal dysphagia (27%), respiratory and sleep problems (both 22%), pain (13%), and hearing abnormalities (9%). The most commonly reported gastrointestinal problem was constipation (74%) followed by gastrointestinal reflux (40%), vomiting (23%), and retching (8%). Gastrointestinal problems, oropharyngeal dysphagia, and drooling were generally more frequent in those with GMCFS V ().
Figure 1. Gastrointestinal problems, oropharyngeal dysphagia (OPD) and drooling according to GMFCS level.
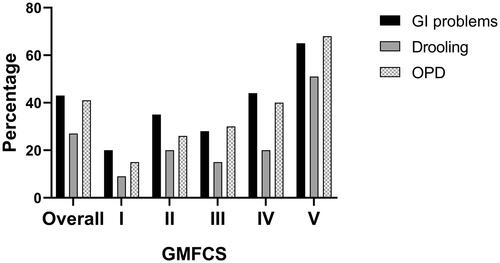
Most participants were fed orally, either through self-feeding or assisted-feeding, and reported feeding problems increased with GMFCS level ().
Nutritional status
Nutritional status data are presented using continuous variables (Z-scores for WFA, HFA, WFH, and BMIFA) and categorical variables according to WHO definitions for being malnourished, stunted, or wasted (Supplementary Table 1).
Supplementary Figure 1A shows that Z-scores generally declined with GMFCS level for most nutritional parameters (WFA, HFA, and BMIFA). Further details on the mean differences in Z-scores between the GMFCS levels can be found in the Supplementary material.
Perceived versus objective nutritional status
There was weak agreement between HCPs and caregivers in their perceptions of nutritional status based on subjective assessment of participants as severely or mildly/moderately malnourished, normal, or mildly/moderately or severely overweight/obese (weighted kappa 0.56). Caregivers seemed more inclined than HCPs to perceive their child as having a normal nutritional status (64% vs. 51%). HCP and caregiver perceptions of nutritional status were different from objective parameters of nutritional status as well. There was only minimal agreement between perceived and objective nutritional status based on WFH in children <5 years and BMIFA for children >5 years when analyzed for HCPs (weighted kappa = 0.38 and 0.35, respectively) or caregivers (weighted kappa = 0.37 and 0.33, respectively).
Concerns about nutritional status
Most caregivers (71%) and HCPs (66%) were not concerned about the nutritional status of the participants (). Overall, HCP concerns about poor nutritional status agreed with HCP perceptions of nutritional status; however, 7% of participants about whom HCPs had expressed concern were in fact perceived as having a “normal” nutritional status by the HCPs. Overall, HCPs expressed concerns about nutritional status in 167 of 497 participants (34%); among these participants, HCPs thought their concern was addressed personally or by a colleague in 77% of cases. Concerns were expressed by caregivers about nutritional status in 124 of 428 participants (29%); among the caregivers who expressed concerns, 84% discussed their concerns with the participant’s HCP and 72% felt their concern was addressed.
Table 2. Concerns expressed according to perceptions of the nutritional status of participants. Data are reported independently for HCPs and caregivers.
Discussion
PURPLE N is the first multi-country study to provide a comprehensive analysis of nutritional status and management of children and adolescents with CP, including insights into perceived and objective nutritional status and a comparison of the different perspectives of HCPs and caregivers. In agreement with previous studies, the PURPLE N study showed an association between a higher GMFCS level and risk of malnutrition in children and adolescents with CP. However, HCPs and caregivers did not consistently agree in their subjective assessment of nutritional status and their perceptions were different from the objective nutritional status of participants. This important new finding has implications for the management of individuals with CP in terms of shared nutritional goals, education, and improving communication channels.
PURPLE N confirmed previous studies showing an association between higher GMFCS level and poor nutritional status [Citation8–11,Citation19,Citation25,Citation26]. Nutritional status can be determined in different ways.
In line with the ESPGHAN guidelines and recommendations, in the PURPLE N study, data were requested for WFA, triceps skinfold thickness and mid-upper arm circumference. The results of our study showed that WFA was measured frequently in the hospital-based study setting whereas triceps skinfold thickness and mid-upper arm circumference were rarely assessed. This raises questions about the practicality of ESPGHAN red flags in routine practice, particularly for HCPs who are not nutritional experts.
The vast majority of HCPs who filled out the surveys in this study were considered to be the so-called “gatekeeping HCPs” in the respective countries, meaning that these HCPs see the children on a regular basis and are responsible for flagging health issues in different domains and referring to other specialist HCPs. As these gatekeeping HCPs are often not experts within the field of nutrition, they might not be aware of the existence of the ESPGHAN red flags (which in part might also be related to the fact that the official publication of the guidelines occurred during data collection for this study) and/or not always sufficiently trained to measure the parameters that go beyond weight and height. Lack of time can also be a limiting factor when it comes to measuring nutritional status parameters as HCPs have to examine multiple facets of health of the child in limited time. However, as these HCPs have an important role in signaling of, amongst others, nutritional problems, it is important to create awareness amongst these HCPs about the existence of the ESPGHAN red flags and/or another screening tool. In this way, children with CP can be properly screened for nutritional problems and referred to a nutritional specialist (e.g., dietician, gastroenterologist, speech-language therapist; depending on the type of problem and availability of specialists in a country) who can do further assessment and provide a suitable intervention. Good nutritional status is an essential basis for good functioning and rehabilitation and is thus an important area for gatekeeping HCPs to screen for potential problems.
When HCPs do not have the possibility or the time to screen for nutritional problems, they, understandably, often rely on their expert opinion and perception. Until now, it remained unclear how well this perception of nutritional status matches the objective nutritional status. To the best of our knowledge, this is the first study that compared objective parameters of nutritional status with the perception of nutritional status. In the analysis of perceived versus objective nutritional status, BMIFA was used for participants aged 5–18 years because the objectively defined categories (severely malnourished, moderately malnourished, normal, overweight, and obese) are analogous to those used for perceived nutritional status. Similarly, WFH was used for children below 5 years of age, although the categories include the term “wasted” rather than “malnourished.” Despite the known difficulties with the use of BMI in children with CP, it was nevertheless chosen to use BMIFA/WFH for the comparison between perceived and objective nutritional status. Both WFA and HFA only focus on the malnourished/stunted part of the spectrum and did thus not match with the categories for perceived nutritional status. Interestingly, we found minimal agreement between perceived nutritional status and objective parameters based on anthropometric measurements. This mismatch was apparent both for HCP and caregiver perceptions of nutritional status, which may reflect a disparity in the subjective category cut-offs that HCP and caregivers have in mind compared with WHO-defined parameters used for nutritional status categories (see Supplementary Table 1). This study also looked into the difference in perception of nutritional status between HCPs and caregivers. The analysis showed only weak agreement in the perception of nutritional status between HCPs and caregivers. The reasons for poor agreement are not clear but may reflect the subjective nature of assessments, the stigma of labeling children as malnourished, and intrinsic differences in reference points for malnutrition or overweight/obesity used by HCPs and caregivers. This is an important insight as both HCPs and caregivers play an important role in the care of children with CP and thus in signaling potential problems. Looking at the concerns both parents and HCPs had with respect to the nutritional status of the children, their concerns were most of the time in line with their perceived nutritional status of the child and in most cases both HCPs and parents indicated that their concerns were addressed in the care of the child. However, knowing that there was a minimal agreement between the objective and perceived nutritional status, this shows again the importance of the use of good objective (screening) measures for nutritional status in this population. These measures can then be used as a basis for communication between HCPs and parents and as a starting point for a systematic approach to the nutritional care of these children.
This study has some limitations. Due to its multi-country design, assessment methods might be different among countries and these hospital-based samples are evidently not representative of the complete CP population in the various countries. The multi-country and multicenter design, however, might bring a broader overview of CP-related issues in Europe. The study was also designed to provide a broad profile of individuals with CP. Consequently, participants had a large age range and therefore, the overall data comprise information from individuals with different nutritional requirements. Indeed, age was shown to be a significant effect modifier in the association between GMFCS and WFA, but not for other nutritional parameters. Finally, the data were not always collected during a newly scheduled visit to the HCP but could have been collected during an earlier visit (within one year before the study started). Z-scores were therefore based on participant’s age at the date of measurement. Despite these recognized limitations, the dataset was sufficiently robust to allow for analysis according to the predefined statistical plan.
The findings of the PURPLE N study have important implications for managing individuals with CP. The study highlighted that children and adolescents with CP remain at risk for malnutrition and stunted growth. The risk is greatest for those with more severe CP and as children get older, particularly for those aged 12–18 years. This is well recognized in literature, but still in many clinics there is little attention for nutrition and growth in this population due to varying reasons. HCPs and caregivers should be alert to possible warning signs of nutritional problems. However, further research is warranted to explore why HCPs do not routinely measure all the parameters recommended in the ESPGHAN guidelines and to examine simpler measures that could be used as red-flag warning signs in daily practice. There is a need to introduce a validated and simple screening tool to help HCPs and parents to identify children with potential nutritional problems. Such a tool was developed recently in Australia [Citation30] and is being validated in other countries.
The lack of agreement between perceived and objective nutritional status also has potential implications for clinical practice. Firstly, a malnourished child perceived to have a normal nutritional status is unlikely to be referred by the HCP for further assessment. Particularly, in situations where objective measurements are rarely or not performed, HCPs often rely on their perception of whether a child is malnourished or not (anecdotal evidence). This study shows that this perception is often not in line with the objective nutritional status. Secondly, a difference in opinion between caregivers and HCPs could also potentially be a barrier to appropriate referral. Perception of nutritional status is important, but as this study shows, objective measures are better tools for managing individuals with CP.
In conclusion, careful and coordinated attention to nutritional status by HCPs and caregivers can help to identify individuals with CP at risk for malnutrition or overweight/obesity and will encourage prompt referral for tailored nutritional advice.
Supplementary_materialcomplete_R1.pdf
Download PDF (2.2 MB)Acknowledgements
Parents and children who participated in the study.
Current and former Danone Nutricia Research employees: Marjolein Alvares, Ada Geerts, Jochem Hogenhuis, Reina den Hollander, Cédric Koolschijn, Barbara Mourmans, Ary Savitri, Sophie Swinkels, Paul Vos, Kim IJspeert, Zoë van Zyl.
Disclosure statement
ARPS is an employee of Danone Nutricia Research Utrecht, The Netherlands. The sponsor was involved in the set-up of the study, data analysis, data interpretation (together with the authors of this manuscript), and supported writing of the manuscript.
Additional information
Funding
References
- Graham HK, Rosenbaum P, Paneth N, et al. Cerebral palsy. Nat Rev Dis Primers. 2016;2:15082.
- Penagini F, Mameli C, Fabiano V, et al. Dietary intakes and nutritional issues in neurologically impaired children. Nutrients. 2015;7(11):9400–9415.
- Rempel G. The importance of good nutrition in children with cerebral palsy. Phys Med Rehabil Clin N Am. 2015;26(1):39–56.
- Scarpato E, Staiano A, Molteni M, et al. Nutritional assessment and intervention in children with cerebral palsy: a practical approach. Int J Food Sci Nutr. 2017;68(6):763–770.
- Oftedal S, Davies PS, Boyd RN, et al. Longitudinal growth, diet, and physical activity in young children with cerebral palsy. Pediatrics. 2016;138(4):e20161321.
- Melunovic M, Hadzagic-Catibusic F, Bilalovic V, et al. Anthropometric parameters of nutritional status in children with cerebral palsy. Mater Sociomed. 2017;29(1):68–72.
- Stallings VA, Charney EB, Davies JC, et al. Nutritional status and growth of children with diplegic or hemiplegic cerebral palsy. Dev Med Child Neurol. 1993;35(11):997–1006.
- Herrera-Anaya E, Angarita-Fonseca A, Herrera-Galindo VM, et al. Association between gross motor function and nutritional status in children with cerebral palsy: a cross-sectional study from Colombia. Dev Med Child Neurol. 2016;58(9):936–941.
- Hurvitz EA, Green LB, Hornyak JE, et al. Body mass index measures in children with cerebral palsy related to gross motor function classification: a clinic-based study. Am J Phys Med Rehabil. 2008;87(5):395–403.
- Walker JL, Bell KL, Stevenson RD, et al. Differences in body composition according to functional ability in preschool-aged children with cerebral palsy. Clin Nutr. 2015;34(1):140–145.
- Benfer KA, Weir KA, Bell KL, et al. Oropharyngeal dysphagia in children with cerebral palsy: comparisons between a high- and low-resource country. Disabil Rehabil. 2017;39(23):2404–2412.
- Carlon SL, Taylor NF, Dodd KJ, et al. Differences in habitual physical activity levels of young people with cerebral palsy and their typically developing peers: a systematic review. Disabil Rehabil. 2013;35(8):647–655.
- Garcia Iniguez JA, Vasquez Garibay EM, Garcia Contreras AA, et al. Energy expenditure is associated with age, anthropometric indicators and body composition in children with spastic cerebral palsy. Nutricion Hospitalaria. 2018;35(4):909–913.
- Keawutan P, Bell KL, Oftedal S, et al. Longitudinal physical activity and sedentary behaviour in preschool-aged children with cerebral palsy across all functional levels. Dev Med Child Neurol. 2017;59(8):852–857.
- Williams SA, McFadden LM, Blackmore AM, et al. Do adolescents with cerebral palsy meet recommendations for healthy weight and physical activity behaviours? Disabil Rehabil. 2020;42(9):1227–1232.
- Samson-Fang L, Fung E, Stallings VA, et al. Relationship of nutritional status to health and societal participation in children with cerebral palsy. J Pediatr. 2002;141(5):637–643.
- Sellers D, Mandy A, Pennington L, et al. Development and reliability of a system to classify the eating and drinking ability of people with cerebral palsy. Dev Med Child Neurol. 2014;56(3):245–251.
- Romano C, van Wynckel M, Hulst J, et al. European society for paediatric gastroenterology, hepatology and nutrition guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with neurological impairment. J Pediatr Gastroenterol Nutr. 2017;65(2):242–264.
- Aydin K. A multicenter cross-sectional study to evaluate the clinical characteristics and nutritional status of children with cerebral palsy. Clin Nutr Espen. 2018;26:27–34.
- Finbraten AK, Martins C, Andersen GL, et al. Assessment of body composition in children with cerebral palsy: a cross-sectional study in Norway. Dev Med Child Neurol. 2015;57(9):858–864.
- Henderson RC, Grossberg RI, Matuszewski J, et al. Growth and nutritional status in residential center versus home-living children and adolescents with quadriplegic cerebral palsy. J Pediatr. 2007;151(2):161–166.
- Kakooza-Mwesige A, Tumwine JK, Eliasson AC, et al. Malnutrition is common in Ugandan children with cerebral palsy, particularly those over the age of five and those who had neonatal complications. Acta Paediatr. 2015;104(12):1259–1268.
- Pinto VV, Alves LAC, Mendes FM, et al. The nutritional state of children and adolescents with cerebral palsy is associated with oral motor dysfunction and social conditions: a cross sectional study. BMC Neurol. 2016;16:55.
- Sangermano M, D'Aniello R, Massa G, et al. Nutritional problems in children with neuromotor disabilities: an Italian case series. Ital J Pediatr. 2014;40:61.
- Tuzun EH, Guven DK, Eker L, et al. Nutritional status of children with cerebral palsy in Turkey. Disabil Rehabil. 2013;35(5):413–417.
- Wang F, Cai Q, Shi W, et al. A cross-sectional survey of growth and nutritional status in children with cerebral palsy in west China. Pediatr Neurol. 2016;58:90–97.
- Hariprasad PG, Elizabeth KE, Valamparampil MJ, et al. Multiple nutritional deficiencies in cerebral palsy compounding physical and functional impairments. Indian J Palliat Care. 2017;23(4):387–392.
- Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223.
- McHugh ML. Interrater reliability: the kappa statistic. Biochem Med. 2012;22(3):276–282.
- Bell KL, Benfer KA, Ware RS, et al. Development and validation of a screening tool for feeding/swallowing difficulties and undernutrition in children with cerebral palsy. Dev Med Child Neurol. 2019;61(10):1175–1181.
