Abstract
Purpose
To determine which factors are associated with physical inactivity in hospitalized adults of all ages.
Methods
A cross-sectional sample of 114 adults admitted to a gastrointestinal surgery, internal medicine or cardiology hospital ward (median age 60, length of stay 13 days) were observed during one random day from 8 am to 8 pm using wireless accelerometers and behavioral mapping protocols. Factors (e.g., comorbidities, self-efficacy, independence in mobility, functional restraints) were collected from medical records, surveys, and observations.
Results
Patients were physically active for median(IQR) 26 (13–52.3) min and were observed to lie in bed for 67.3%, sit for 25.2%, stand for 2.5%, and walk for 5.0% of the time. Multivariable regression analysis revealed that physical inactivity was 159.87% (CI = 89.84; 255.73) higher in patients dependent in basic mobility, and 58.88% (CI = 10.08; 129.33) higher in patients with a urinary catheter (adjusted R2 = 0.52). The fit of our multivariable regression analysis did not improve after adding hospital ward to the analysis (p > 0.05).
Conclusions
Independence in mobility and urine catheter presence are two important factors associated with physical inactivity in hospitalized adults of all ages, and these associations do not differ between hospital wards. Routine assessments of both factors may therefore help to identify physically inactive patients throughout the hospital.
Healthcare professionals should be aware that physical inactivity during hospital stay may result into functional decline.
Regardless of which hospital ward patients are admitted to, once patients require assistance in basic mobility or have a urinary catheter they are at risk of physical inactivity during hospital stay.
Implementing routine assessments on the independence of basic mobility and urine catheter presence may therefore assist healthcare professionals in identifying physically inactive patients before they experience functional decline.
IMPLICATIONS FOR REHABILITATION
Introduction
Physical inactivity during hospital stay is a large problem, and in elderly patients this has been associated with hospitalization-associated functional decline [Citation1–4]. In turn, hospitalization-associated functional decline leads to prolonged length of hospital stay and increased mortality [Citation5]. Given that hospitalization-associated functional decline occurs frequently and is not limited to adults aged 60 and older [Citation6–9], more emphasis on preventing functional decline is paramount.
Interventions that increase in-hospital physical activity have proven to be effective in preventing hospitalization-associated functional decline [Citation10–12]. These interventions have also proven to be effective in reducing the length of stay [Citation13,Citation14], improving the level of independence in daily activities [Citation15,Citation16], and improving the likelihood of returning home [Citation5,Citation15]. Still, many hospitalized patients continue to spend the most time in bed and barely spend time physically active [Citation13,Citation17–20]. If we can identify these physically inactive patients, we might be able to better translate effective interventions increasing in-hospital physical activity into local intervention strategies.
Previous studies have identified a history of falls [Citation13], use of medical equipment [Citation19], use of walking aids [Citation19], low level of pre-admission mobility [Citation13], low level of pre-admission cognitive function [Citation18], and low level of physical function during admission [Citation16,Citation18] to be associated with physical inactivity in hospitalized patients; however, these studies solely focused on older hospitalized patients. Two recent studies quantified the physical activity levels of hospitalized adults of all ages admitted to a variety of hospital wards [Citation17,Citation21], and only one of those studies also examined the factors associated with physical inactivity in adults [Citation21]. This study identified in a sample of n = 39 that pain levels, functional independence and functional restraints are related to time lying in bed during the day [Citation21]. If, however, the factors associated with physical inactivity are assessed in a larger sample of hospitalized adults of all ages, we may be able to examine more factors related to physical inactivity. This might provide healthcare professionals with more guidance on how to optimally identify physically inactive adults of all ages in clinical care.
To our knowledge, no larger studies have investigated the factors associated with physical inactivity in hospitalized adults of all ages while taking into account the case mix of gastrointestinal surgery, internal medicine and cardiology hospital wards. To this end, this study conducted a thorough assessment of the physical activity levels at five hospital wards at a university hospital in Amsterdam and aimed to answer the following primary research question: Which factors are associated with physical inactivity in hospitalized adults of all ages?
Materials and methods
Study design
This cross-sectional, observational study was conducted in five hospital wards – two gastrointestinal surgery, internal medicine haematology, internal medicine infectious diseases, and cardiology – at Amsterdam University Medical Centres (Amsterdam UMC) - location Academic Medical Centre, a 1002-bed tertiary university hospital in Amsterdam. Each hospital ward had nursing-to-patient ratios of 1:3 or 1:4, depending on the patients’ acuity. Allied health staffing consisted of 0.5–1 physiotherapists to each hospital ward. The Medical Ethical Review Committee of the Amsterdam UMC assessed and approved this study (reference number W17_479 # 18.003), and this study has been conducted according to the principles of the Declaration of Helsinki. Participants gave verbal and written informed consent to participate in the study. The study was reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Participants
Patients in this study were admitted at the gastrointestinal surgery ward for acute or elective gastrointestinal surgery (including re-admissions due to postoperative complications), at the haematology ward for investigations and treatment of blood or bone marrow disorders, at the infectious diseases ward for a variety of medical conditions (e.g., pneumonia, complicated infections), and at the cardiology ward for the diagnostics and treatment of heart disorders. Patients were included during an audit at the two gastrointestinal surgery wards between 15 January 2018 and 11 February 2018, at the haematology and infectious diseases wards between 13 August 2018 and 9 September 2018, and at the cardiology ward between 29 April 2019 and 26 May 2019. Inclusion criteria were: aged 18 and older, able to make an active independent bed-chair transfer before hospitalization, Dutch or English speaking and reading proficiency, and admission for more than 24 h. Patients with obligatory bed rest, expected to be discharged before noon on the day of observation, delirious on either the day of inclusion or observation and those receiving end-of-life care were excluded. Patients were observed from 8 am to 8 pm on either a weekday or a weekend day. One or two days before each day of observation, a random sample of hospitalized patients was approached to participate. The selection of potential participants was performed using a computer-generated list based on the room number. In the case of refusal, the investigator approached the patient in the next hospital room on the computer-generated list.
Outcome measures
Physical activity
Wireless accelerometers (Physical Activity Monitor (PAM) AM400, PAM BV, Oosterbeek, The Netherlands, 2018) were used to measure the total amount of physical activity in minutes objectively (> 1.4 Metabolic Equivalent Tasks (METs) [Citation22]) between 8 am and 8 pm. Also, the PAM compares each second of physical activity with the following three pre-defined intensity zones: light physical activity intensity (1.4–3.0 MET), moderate physical activity intensity (3.0–7.0 MET), and vigorous physical activity intensity (>7.0 MET), and measures the derivative of calculated energy expenditure for 24 h physical activity (PAM-score). The PAM is a 2 cm wide coin, water-proof, and was attached to the ankle. The PAM contains a sensor with sensitive elements in all three directions (x, y, and z), measures accelerations 10 times per second and integrates it to one second. The number of time accelerations were measured above > 1.4 MET were accumulated to the total amount of physical activity in minutes. Each of these accelerations was also converted to the PAM-score, representing the ratio of the energy spent according to METs compared resting metabolism (PAM-score = (METs − 1) × 100 averaged over the day). The validity and reliability of the PAM in healthy adults is moderate-to-good in assessing the estimate of energy expenditure [Citation23,Citation24].
Behavioural mapping protocols were used in which structured observations revealed the percentage of time patients spent at each type of activity (i.e., lying, sitting, standing, walking) and location in the hospital (i.e., hospital room, hallway, not observed) [Citation17]. In detail, participants in each room were observed for a 1-min period every 10 min. This way, every participant was observed 72 times. The observations were performed by trained physical therapy graduate students using a predetermined set of mutually exclusive levels of activity (lying in bed, sitting on the edge of the bed or chair, making a transfer from bed to chair or standing, walking, or using the ergometer) and locations (patient room, toilet/bathroom, hallway, lounge, other). For an equal amount of time during the minute of observation, the activity with the highest intensity was recorded. The observations were directly recorded in the online Castor Electronic Data Capture database (Ciwit BV, Amsterdam, The Netherlands, 2018).
Factors
We collected demographic information from medical records. In addition, we used the medical records to assess the type of admission (i.e., acute or elective), to identify whether the participant had surgery during current hospital admission, to assess the number of comorbidities using the Charlson Comorbidity Index (CCI) [Citation25], and to calculate the number of days between the day of admission and observation (i.e., as a derivative of length of hospital stay). We also extracted the Katz-ADL score, which describes the level of independence in ADL 2-weeks preadmission and ranges from 0 (completely ADL dependent) to 6 (completely ADL independent) [Citation26]. On the day of observation, we used the Activity Measure for Post-Acute Care (AM-PAC) “6-clicks” Basic Mobility short form to assess the level of independence in basic mobility and the AM-PAC “6-clicks” Daily Activity short form to assess the level of independence in ADL [Citation27,Citation28]. Both contain six items, which are scored on a scale of 1 (unable to do or total assistance required) to 4 (no assistance required). The first five questions of the AM-PAC “6-clicks” Basic Mobility were used to distinguish between patients not requiring any help (score = 20/20) with basic mobility activities and patients requiring assistance (score < 20). Muscle strength was assessed by measuring handgrip strength using the JAMAR handheld-dynamometer [Citation29,Citation30]. Using a survey, we assessed the patients’ perceived self-efficacy related to mobility using seven questions (i.e., getting out of bed, getting out of a chair, showering, walking stairs, walking in the neighbourhood, doing the groceries and going to a social activity) which was be scored using a five-point Likert scale (0 to 4) and ranged from 0 (minimal) to 28 (maximal). We used the Short Falls Efficacy Scale-International (Short FES-I) to derive these seven mobility-related self-efficacy questions [Citation31]. Lastly, the number of functional restraints (i.e., drains, urine catheter, IV-lines, hospital isolation precautions) was assessed by direct observation.
Data analysis
All analyses were conducted using IBM-SPSS Statistics version 25 (IBM Corp, Armonk, New York). Descriptive data are given as means with standard deviations (SD) or medians with interquartile ranges (IQRs). Normality was evaluated by visually inspecting histograms and Q-Q plots. Multiple imputations was used to impute missing factors. Frequency distributions and summary statistics were used to summarize the accelerometer data of all participants and for each hospital ward individually. Data of patients who wore the PAM during the entire observation period (8 am–8 pm) was used. Frequency distributions and summary statistics were also used to calculate the number of times a patient was observed at each possible location and type of activity. We used these to calculate percentages of time spent patients spent between 8 am and 8 pm per observed item.
To explore which factors were associated with physical inactivity, a univariable linear regression analysis was used to evaluate the relationship between the total number of minutes of physical activity and patient factors (e.g., age, type of admission), hospital ward, physical performance measures, and functional restraints. Based on the univariable linear regression analyses results, we performed a multivariable regression model to test if age, surgery, IV-lines, urine catheter, independence in basic mobility, and mobility-related self-efficacy were associated with physical inactivity. Independence in ADL was omitted from the multivariable regression model due to collinearity with independence in basic mobility. In addition, the influence of the hospital ward was evaluated using a mixed linear model; however, no improved fit (p > 0.05) was observed. All parameter estimates were expressed with a 95% confidence interval (CI). Because of the residuals’ non-normally distribution, we performed a natural logarithmic transformation of the dependent variable before performing the regression models. To be able to interpret the amount of change for each variable within the multivariable regression model, we transformed the regression coefficients (β), to change percentages using: change percent = 100(e^(β) − 1).
Results
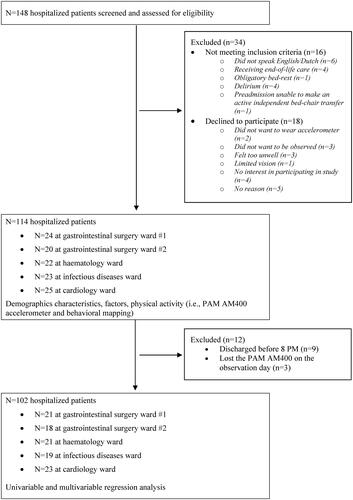
One hundred and forty-eight patients were considered for inclusion. Of those, 16 patients did not meet the inclusion criteria, and 18 patients declined to participate. This resulted in 114 patients divided over the 5 wards (). Nine patients were discharged before 8 pm, and three patients lost the accelerometer during the day of observation. Eighty-three patients were observed on a weekday and 31 patients on a weekend day. The median (IQR) age of the included sample was 60 (46.8–70.3), and 72 (63.2%) were male. The median (IQR) length of stay was 13 days (8–25) days. The observation day was performed at median (IQR) 8 (3–14.5) days after admission to the hospital. Of the 114 patients, 96 (84.2%) patients were completely independent in basic ADL (Katz-ADL score = 0/6) before hospitalization. One hundred-and-three (90.4%) had at least one tether (i.e., IV-line, drain). Seventy-four (64.9%) patients were observed to be independent in basic mobility (AM-PAC “6-clicks” Basic Mobility short form questions 1–5 = 20). All demographics and factors are presented in . Hospital ward specific presentation of the demographics and factors can be found in Supplemental Online Material S1.
Table 1. Demographic characteristics and factors.
Level of physical activity
Patients were physically active for a total number of median (IQR) 26 (13–52) min during the 12-h observation period. When divided over the three intensity zones, patients were physically active with light intensity for median (IQR) 21 (11–36) min, moderate for 4 (2–13) min, and vigorous for 0 (0–0) min. The median (IQR) PAM-score was 2.34 (1.30–5.40). The total number of minutes physical activity in patients observed on a weekday was median (IQR) 24 (12–50), compared to 27 (14–62) on a weekend day. Hospital ward specific presentation of the accelerometer data can be found in Supplemental Online Material S2.
There were 7095 observations of a type of activity and location (median 67 per patient, IQR 62–70). Patients were observed to lie in bed for mean (SD) 67.3% (23.5), sitting 25.2% (19.9), standing/transfer 2.5% (2.6), and walking/ergometer 5.0% (5.6) of the time. Additionally, patients were observed to spend 92.7% (11.3) of their time at the patient room, 1.6% (2.0) at the toilet/bathroom, 2.7% (4.2) at the hallway, 2.6% (7.3) at the patient lounge and 0.4% (1.6) at unspecified locations (e.g., medical examination rooms). Hospital ward specific presentation of the behavioural mapping data can be found in Supplemental Online Material S3.
Factors associated with physical inactivity
In the univariable regression analyses, higher age, being admitted to surgery ward #2, having surgery during admission, more IV-lines, a urine catheter, dependence in basic mobility and ADL on the day of observation and less mobility-related self-efficacy were all significantly (p < 0.05) associated with more physical inactivity. Multivariable regression analysis revealed that being dependent on basic mobility on the day of observation and having a urinary catheter were the only two predictors that were significantly associated with physical inactivity (). The overall fit of the multivariable regression model was adjusted R2 = 0.52 (p < 0.001). We found that physical inactivity is 159.87% (CI 89.84–255.73) higher in patients who are dependent on basic mobility. We also found that physical inactivity is 58.88% (CI 10.08–129.33) higher in patients with a urinary catheter.
Table 2. Linear regression with factors associated with physical inactivity (−ln[physical activity in minutes]) using the imputed dataset.
Discussion
This cross-sectional observational study illustrated that adults of all ages admitted to gastrointestinal surgery, internal medicine or cardiology hospital wards were physically active for only 26 min (13–53) and spent most of their time during the day lying in bed (67.3%). Using a multivariable regression model, we determined that (1) dependence in basic mobility and (2) urine catheter presence were significantly associated with physical inactivity. These two factors were the only remaining factors in our multivariable regression analysis, indicating that they may be of more importance in identifying physically inactive patients than age, self-efficacy, IV-lines, and surgery. Additionally, we observed that the fit of our multivariable regression model did not significantly change after adding hospital ward to the analysis, indicating that the associations found within our study did not differ between hospital wards.
The amounts of objectively assessed physical activity in our study were considerably lower than reported in comparable studies [Citation15,Citation16,Citation18]. Possible explanations may be the difference in patient population or the accelerometers used to measure physical activity. For example, previous studies defined physical activity as the time that patients stand or walk via the patient’s postural position, while the PAM AM400 accelerometer solely measures physical activity via three-dimensional accelerations and, therefore, will not include the time patients stand till in the total amount of physical activity [Citation15,Citation16,Citation18]. Only one study measured the time patients walk separately from the time that patients standstill and observed elderly patients to be walking for only median 4–10 min a day [Citation20]. Considering that slow walking (±2 mph/3.2 kph) would be classified as physical activity when using the PAM, we may assume that patients on our hospital wards are relatively more physically active than patients aged 65 years and older who have been admitted to an acute geriatric ward.
We also found that patients were lying in bed for considerably higher amounts of time when we compared the results of our behavioural mapping data with comparable hospital wards from other studies [Citation17,Citation32]. For example, Mudge et al. described that patients were in bed for 53.3–65.1% of the time, whereas we observed patients lie in bed on comparable wards for 67.5–79.6% of the time [Citation17]. These differences might result from the considerably longer observation period (8 am–8 pm versus 10 am–6 pm). However, they may also reflect a difference in case mix, culture, or ward environment. Despite the differences in percentages, our study emphasizes that the same pattern occurs in adults of all ages admitted to gastrointestinal surgery, internal medicine, and cardiology wards: patients remain largely in bed during hospitalization, but the exact amounts vary between hospital wards.
The importance of the association between independence in basic mobility and in-hospital physical activity has been highlighted by many authors [Citation18,Citation19,Citation21]. In addition to the previous studies, we observed in our sample adults of all ages requiring assistance in basic mobility is by far the strongest factor associated with physical inactivity. This finding suggests that routine assessments of independence in basic mobility are the starting point to identify physically inactive patients of all ages before a functional decline occurs. Previous research in the John Hopkins Hospital has shown that mobility assessments in routine clinical practice can best be performed using the valid and reliable AM-PAC “6-clicks” inpatient Basic Mobility short form [Citation27,Citation28,Citation33].
In addition, our findings also revealed that patients who have urine catheters are significantly more physically inactive than patients who do not have a urine catheter. These findings may suggest that by registering urinary catheters in addition to the routine assessments of mobility, the accuracy of identifying physically inactive patients can be improved. This finding is in line with the study of Koenders et al. [Citation21], who showed that both urine catheter use and drain use were significantly associated with time spent lying in bed. Although both studies indicate that functional restraints can be used to identify physically inactive patients, we were unable to conclude that these functional restraints are the impeding causes. Interventions should therefore not only consider removing functional restraints but also should look more broadly at what is needed to counter physical inactivity. This is substantiated by a recent synthesis of qualitative evidence showing that physical inactivity during hospital stay is primarily caused by a multifaceted and complex phenomenon, whereby multiple issues should be tackled at the same time to be able to counter physical inactivity in hospitalized patients effectively [Citation34].
Finally, the finding that both associations do not differ between hospital wards is new and suggests that routine assessments in clinical care have added value across an entire hospital. Assuming that many hospitals already register the urine catheter presence in their electronic medical record, implementing the AM-PAC “6-clicks” inpatient Basic Mobility short form in the electronic medical record may offer healthcare professionals and policymakers with new opportunities to systematically identify physically inactive patients throughout the hospital.
Strengths and limitations of the study
The strengths of this research is the comprehensive assessment of physical activity through both behavioural mapping and accelerometers, the random selection of participants on each hospital ward and the extensive inclusion of factors which may be associated with in-hospital physical activity. We also recognize some limitations of this study. First, data were collected on one random day during the patients’ admission. The physical activity data may therefore not reflect overall physical activity during the entire hospital stay. However, we included the number of days that patients were admitted until physical activity measurement as a factor and determined that other factors were more strongly associated with physical inactivity. Second, concerns remain present regarding the most appropriate criterion measure to define light, moderate, and vigorous physical activity. Considering that the PAM AM400 has been validated in healthy adults, our description of the absolute physical activity intensities might be underestimated in hospitalized adults [Citation23,Citation24]. Third, independence in ADL was omitted from the multivariable regression model due to collinearity with independence in basic mobility. This choice was based on the results of the univariable regression analysis and the applicability of the measure in clinical practice. Still, readers should note that the variance explained with independence in basic mobility might also be largely explained by assessing independence in ADL.
Conclusions
The results of this study show that physical inactivity in hospitalized adults of all ages is significantly associated with dependence on basic mobility and urine catheter presence. And, furthermore, that both associations do not differ between hospital wards. These findings imply that regardless of which hospital ward patients are admitted to, once patients require assistance in basic mobility or have a urinary catheter they are at risk of physical inactivity. Also, these results imply that through routine assessments of basic mobility and urine catheter presence, healthcare professionals may be able to identify physically inactive patients before these patients experience a functional decline. A possible next step would be to translate the effective interventions from the literature into local intervention strategies to improve physical activity in the identified physically inactive patients. Future research is particularly needed to investigate the relationship between (1) social (e.g., family, visitors, healthcare professionals) and environmental context and (2) physical inactivity. Understanding how the social and environmental context influences the patient’s physical activity behaviour may offer healthcare professionals new interventions to sustainably prevent physical inactivity during hospital stay. Furthermore, future research should focus on identifying normative values for physical activity during hospital stay, so that hospital wards can more easily include physical activity as a goal in clinical practice.
DR_SupplementalOnlineMaterial3_Geelen_Factors.pdf
Download PDF (89.5 KB)DR_SupplementalOnlineMaterial2_Geelen_Factors.pdf
Download PDF (104.7 KB)DR_SupplementalOnlineMaterial1_Geelen_Factors.pdf
Download PDF (132.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, S. J. G. Geelen, upon reasonable request.
Additional information
Funding
References
- Zisberg A, Shadmi E, Sinoff G, et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59(2):266–273.
- Zisberg A, Shadmi E, Gur-Yaish N, et al. Hospital-associated functional decline: the role of hospitalization processes beyond individual risk factors. J Am Geriatr Soc. 2015;63(1):55–62.
- Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: "she was probably able to ambulate, but I'm not sure". JAMA. 2011;306(16):1782–1793.
- Sourdet S, Lafont C, Rolland Y, et al. Preventable iatrogenic disability in elderly patients during hospitalization. J Am Med Dir Assoc. 2015;16(8):674–681.
- Portegijs E, Buurman BM, Essink-Bot ML, et al. Failure to regain function at 3 months after acute hospital admission predicts institutionalization within 12 months in older patients. J Am Med Dir Assoc. 2012;13(6):569.e1–7.
- Chodos AH, Kushel MB, Greysen SR, et al. Hospitalization-associated disability in adults admitted to a safety-net hospital. J Gen Intern Med. 2015;30(12):1765–1772.
- Ehlenbach WJ, Larson EB, Curtis JR, et al. Physical function and disability after acute care and critical illness hospitalizations in a prospective cohort of older adults. J Am Geriatr Soc. 2015;63(10):2061–2069.
- Van Ancum JM, Scheerman K, Jonkman NH, et al. Change in muscle strength and muscle mass in older hospitalized patients: a systematic review and meta-analysis. Exp Gerontol. 2017;92:34–41.
- van Seben R, Reichardt LA, Aarden JJ, et al. The course of geriatric syndromes in acutely hospitalized older adults: the hospital-ADL study. J Am Med Dir Assoc. 2019;20(2):152–158.e2.
- Volpato S, Onder G, Cavalieri M, et al. Characteristics of nondisabled older patients developing new disability associated with medical illnesses and hospitalization. J Gen Intern Med. 2007;22(5):668–674.
- Saez de Asteasu ML, Martinez-Velilla N, Zambom-Ferraresi F, et al. Physical exercise improves function in acutely hospitalized older patients: secondary analysis of a randomized clinical trial. J Am Med Dir Assoc. 2019;20(7):866–873.
- Resnick B, Boltz M. Optimizing function and physical activity in hospitalized older adults to prevent functional decline and falls. Clin Geriatr Med. 2019;35(2):237–251.
- Fisher SR, Goodwin JS, Protas EJ, et al. Ambulatory activity of older adults hospitalized with acute medical illness. J Am Geriatr Soc. 2011;59(1):91–95.
- Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: a quality-improvement project. J Hosp Med. 2016;11(5):341–347.
- Brown CJ, Redden DT, Flood KL, et al. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660–1665.
- Evensen S, Sletvold O, Lydersen S, et al. Physical activity among hospitalized older adults – an observational study. BMC Geriatr. 2017;17(1):110.
- Mudge AM, Mcrae P, Mchugh K, et al. Poor mobility in hospitalized adults of all ages. J Hosp Med. 2016;11(4):289–291.
- Pedersen MM, Bodilsen AC, Petersen J, et al. Twenty-four-hour mobility during acute hospitalization in older medical patients. J Gerontol A Biol Sci Med Sci. 2013;68(3):331–337.
- Tasheva P, Kraege V, Vollenweider P, et al. Accelerometry assessed physical activity of older adults hospitalized with acute medical illness – an observational study. BMC Geriatr. 2020;20(1):382.
- Villumsen M, Jorgensen MG, Andreasen J, et al. Very low levels of physical activity in older patients during hospitalization at an acute geriatric ward: a prospective cohort study. J Aging Phys Act. 2015;23(4):542–549.
- Koenders N, Weenk M, van de Belt TH, et al. Exploring barriers to physical activity of patients at the internal medicine and surgical wards: a retrospective analysis of continuously collected data. Disabil Rehabil. 2021;43(13):1883-1889.
- Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American college of sports medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434.
- Van der Weegen S. Self-management support using mobile technology. A monitoring and feedback tool embedded in a counselling protocol to increase physical activity of patients with COPD or type 2 diabetes in primary care: the it’s LiFe! study [PhD thesis]. Maastricht: University of Maastricht; 2015.
- Vooijs M, Alpay LL, Snoeck-Stroband JB, et al. Validity and usability of low-cost accelerometers for internet-based self-monitoring of physical activity in patients with chronic obstructive pulmonary disease. Interact J Med Res. 2014;3(4):e14.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial FUNCTION. JAMA. 1963;185:914–919.
- Geelen SJG, Valkenet K, Veenhof C. Construct validity and inter-rater reliability of the Dutch activity measure for post-acute care "6-clicks" basic mobility form to assess the mobility of hospitalized patients. Disabil Rehabil. 2019;41(21):2563–2569.
- Jette DU, Stilphen M, Ranganathan VK, et al. Validity of the AM-PAC "6-Clicks" inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94(3):379–391.
- Bohannon RW. Muscle strength: clinical and prognostic value of hand-grip dynamometry. Curr Opin Clin Nutr Metab Care. 2015;18(5):465–470.
- Bohannon RW. Considerations and practical options for measuring muscle strength: a narrative review. Biomed Res Int. 2019;2019:8194537.
- Kempen GI, Yardley L, van Haastregt JC, et al. The short FES-I: a shortened version of the falls efficacy scale-international to assess fear of falling. Age Ageing. 2008;37(1):45–50.
- Kuys SS, Dolecka UE, Guard A. Activity level of hospital medical inpatients: an observational study. Arch Gerontol Geriatr. 2012;55(2):417–421.
- Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133–142.
- Koenders N, Marcellis L, Nijhuis-van der Sanden MWG, et al. Multifaceted interventions are required to improve physical activity behaviour in hospital care: a Meta-ethnographic synthesis of qualitative research. J Physiother. 2021;67(2):115–123.