Abstract
Purpose
Measuring the physical activity of adults with progressive muscle diseases is important to inform clinical practice, for activity recommendations and for outcomes meaningful to participants in clinical trials. Despite its wide use, the measurement properties of the International Physical Activity Questionnaire (IPAQ) have not been established in a muscle disease population.
Materials and methods
The sample of 103 adults with progressive muscle diseases included independently mobile participants and wheelchair users. Their home-based activity measured by the IPAQ was compared to simultaneous weeks of accelerometer activity data collected remotely in a longitudinal, measure evaluation study. Validity, reliability, and responsiveness were evaluated for the IPAQ alone, and for the IPAQ used in conjunction with a smart activity monitor.
Results
The IPAQ did not demonstrate satisfactory criterion validity, reliability or responsiveness and it systematically overestimated moderate and vigorous physical activity time by 161 minutes per week. Measurement properties of the IPAQ were improved when it was used in combination with a smart activity monitor.
Conclusions
The IPAQ did not have satisfactory measurement properties compared to accelerometry in adults with progressive muscle disease. Combining self-report and objective activity measures might improve the accuracy of physical activity assessment in this and other comparable populations.
Physical activity is a meaningful health outcome for adults with progressive muscle diseases, for whom precise activity quantification is important because of the potential for activity-related disease exacerbation.
The International Physical Activity Questionnaire (IPAQ) had unsatisfactory measurement properties compared to accelerometry; however, these were improved by adjunctive smart activity monitoring.
Objective or combined physical activity measurement is recommended over self-report alone for clinical assessment of physical activity as part of rehabilitation and self-management programmes.
Implications for Rehabilitation
Introduction
Physical activity is defined as “any bodily movement produced by skeletal muscles that requires energy expenditure”; insufficient physical activity has been linked to poor health outcomes [Citation1]. Measuring the physical activity of adults with progressive muscle diseases is important to inform clinical practice, activity recommendations and for outcomes, meaningful to participants, in clinical trials [Citation2]. In muscle disease, activity has been linked to improved muscle strength, cardiorespiratory fitness, fatigue management, quality of life and protection against comorbidities [Citation3–8].
However, concerns remain over potential activity-related exacerbations of muscle disease [Citation4,Citation9]. World Health Organisation (WHO) activity recommendations seem generally applicable and safe [Citation1] for many other neuromuscular diseases. For example, those originating from neuromuscular junction pathology, such as Myasthenia Gravis [Citation10], or nervous system pathologies, such as peripheral neuropathies [Citation11,Citation12] and Spinal and Bulbar Muscular Atrophy [Citation12]. However, there is less evidence regarding safe activity dosage for adults with muscle disease [Citation3]. The reciprocal relationship between activity and muscular gene expression [Citation13] means that, in muscle disease, greater understanding and precision is required for measurement, and evidence-based prescription, of physical activity throughout disease progression [Citation3,Citation14].
Progressive muscle diseases, including muscular dystrophies, myopathies and inclusion body myositis, are characterised by pathological changes in skeletal muscles [Citation15,Citation16], resulting in a clinical course of slowly progressive weakness [Citation17,Citation18]. Disease symptoms are variable, for some there is a mobility decline that may require wheelchair use [Citation19]. This functional heterogeneity makes activity measurement challenging. Multiple approaches have been used but there is insufficient evidence to recommend any activity measurement tool for use with adults who have progressive muscle diseases [Citation20,Citation21].
Despite not being designed originally for use in small samples for clinical studies [Citation22], IPAQ is more widely used in neuromuscular disease research than objective measures or questionnaires tailored for disabled populations [Citation21], such as the Physical Activity Scale for Individuals with Physical Disabilities [Citation23]. Disability and wheelchair-specific activity questionnaires have received criticisms relating to the questionable accuracy of self-report [Citation20] and may be suited to activity measurement only for those at later stages of disease progression. Continuity of activity measurement is preferrable using a single measure inclusive of early and late stages of disease progression.
The International Physical Activity Questionnaire (IPAQ) was developed to standardise population-level activity surveillance worldwide [Citation24,Citation25]. Modified versions of the IPAQ have also been designed to evaluate people with functional limitations [Citation26,Citation27]. It is an easy to administer, self-report, 7-day recall questionnaire (or interview). It collects information about time spent in vigorous, moderate, walking, and sedentary activities. The overall score estimates metabolic expenditure and was designed to categorise people into low, moderate, or high activity. Early studies reported satisfactory reliability and validity in the general population [Citation25,Citation28,Citation29]. However, subsequent systematic reviews indicated the IPAQ considerably overestimated activity and had only low to moderate validity when compared to objective measures [Citation30–32]. IPAQ overestimation might be attributed to social desirability and recall biases [Citation33] and systematic reviews in neuromuscular diseases have also questioned the accuracy of self-report physical activity measures [Citation20,Citation21].
However, if the IPAQ was valid for ambulant and non-ambulant adults with progressive muscle diseases, it would provide a suitable activity measurement tool to chart physical activity throughout the stages of muscle disease progression, confirm the accuracy of existing physical activity research in muscle disease, and allow comparisons with studies in the general population. To date, the IPAQ (modified or otherwise) has never been validated for people with progressive muscle diseases.
The objective of this study was to test the validity, reliability, and responsiveness of the IPAQ in adults with progressive muscle diseases. The secondary objective was to discover whether the measurement properties of the self-report questionnaire were improved by adjunctive smart activity monitoring. The hypothesis was that wearing a smart activity monitor would improve the accuracy of self-reported IPAQ physical activity data by reducing overestimation.
Materials and methods
Study design
Ethical approval for this longitudinal, measure evaluation study was granted by King’s College London Research Ethics Committee (LRS-18/19-10909). Recruitment started in April 2019. The setting was home-based as the study was conducted remotely. Baseline cross-sectional IPAQ and accelerometer data were collected from April to October 2019 and at longitudinal follow-up from February to August 2020. They were compared to evaluate validity, reliability, and responsiveness of the questionnaire. Measurement properties for the IPAQ alone were compared to those for IPAQ data collected after a week of adjunctive smart activity monitoring.
Participants
People with progressive muscle diseases were invited to participate via national muscle disease registries (The John Walton Muscular Dystrophy Research Centre, Newcastle University) and the charity, Muscular Dystrophy UK (https://www.musculardystrophyuk.org/) advertised via their website and newsletter. Participants were included if they were UK resident adults with a confirmed diagnosis of Inclusion Body Myositis, Myotonic Dystrophy or Muscular Dystrophy (including Facioscapulohumeral Dystrophy, Limb Girdle Muscular Dystrophy, Dysferlinopathy, Dystrophinopathy (including manifesting female carriers) or specific congenital myopathies lasting into adulthood). Participants were excluded if they were aged <18 years, cognitively impaired, unable to wear an accelerometer or did not have a confirmed diagnosis of muscle disease. After volunteers made contact with the research team, screening was carried out by telephone appointment. A physiotherapist (SRL) clinically assessed eligibility criteria and gathered supporting evidence (including diagnosis confirmation and cognitive history). Any eligibility discrepancies were resolved on a case-by-case basis by a consultant neurologist (MR). For eligible participants, informed consent and data were collected by email and post.
Outcomes
The primary outcome was concurrent validity of the IPAQ overall score correlated with overall activity intensity in mean accelerations per minute measured by accelerometer. Secondary outcomes included convergent, divergent, and discriminative validity according to demographic characteristics, test–retest reliability over 2 weeks and responsiveness to change from baseline to follow up. Measurement error of IPAQ activity minutes compared to weekly moderate and vigorous minutes measured by accelerometry was also tested. Each measurement property was also evaluated for the IPAQ plus adjunctive smart activity monitoring.
Measures
The self-report, short-form IPAQ [Citation25], consists of three questions about days and time of vigorous, moderate, and walking activity in bouts of ≥10 min, with one question about daily sedentary time in the preceding seven days. In this study, the question descriptors were modified to incorporate the range of functional ability in the sample; changes were similar to previous modified versions [Citation26,Citation27]. Modifications included extra vigorous and moderate activity examples, including wheelchair activities. The walking question was modified to include “time spent walking, self-propelled wheeling or equivalent light activities.” “Sedentary” time was changed to “inactive” time. The questionnaire was preceded by completion instructions and three questions about the types of vigorous, moderate, and light activity undertaken [Citation34]. It was followed by three questions about bedtime, waketime and sleep hours. Weekly activity times were calculated from days and minutes of vigorous, moderate, and walking/light activity. Total score in metabolic equivalents (METs) per week was calculated by multiplying weekly active time by intensity specific metabolic values as per IPAQ scoring instructions [Citation25].
Adjunctive smart activity monitoring was by Fitbit Inspire HR (Fitbit Inc., San Francisco, CA). It is a tri-axial accelerometer and continuous optical heart rate monitor. It syncs with the Fitbit smart phone app and yields physical activity metrics including daily active minutes, metabolic expenditure, steps, and sleep. In the adjunctive study, activity monitoring was used for a week preceding each IPAQ completion. Participants regularly synced the Fitbit with their smart phone app. Thus, participants had seen Fitbit activity metrics before completing the questionnaire at the end of the week.
The comparator measure was a GENEActiv tri-accelerometer (ActivInsights Ltd., Kimbolton, UK). It has been validated for wrist-wear [Citation35] and ankle-wear in adults with progressive muscle diseases [Citation36]. Participants wore it on their non-dominant wrists unless ankle-wear was indicated because of work or using crutches (which can interfere with wrist-worn activity monitors). The sampling frequency was 10 Hz. Data were processed using the GGIR package in R (version 3.6.0) [Citation37] in 1-min epochs of milli-gravitational units (milli-g) with gravitational correction [Citation38]. Weekly overall activity intensity was calculated by mean accelerations (milli-g) per minute. Minutes of sleep, inactivity, light activity, and bouts of ≥10 min of moderate and vigorous activity were calculated using the following cut-points: light ≥30 milli-g/min, moderate ≥100 milli-g/min and vigorous activity ≥400/milli-g/min [Citation39].
The moderate to vigorous physical activity (MVPA) threshold of 100 milli-g/min has been widely used for healthy adults and people with long-term conditions [Citation40,Citation41]. Lower thresholds were considered for this cohort, whose muscle weakness might mean greater exertion produces less activity compared to healthy controls [Citation36]. However, muscle strength is not a direct determinant of limb acceleration [Citation36] and a <100 milli-g/min threshold would have been misleading for more active individuals, including wheelchair users, whose activity and exertion are equivalent to people with other long-term conditions [Citation42].
Procedure
Before baseline, demographic information was collected; these data underpinned evaluation of IPAQ construct validity (discriminative, convergent, and divergent). Demographics included condition, age, gender, anthropometrics, handedness, employment status, mobility, quality of life and self-perception. Quality of life was measured using the Individualised Neurological Quality of Life (INQoL), a 45-item questionnaire designed and validated for people with neurological diseases [Citation43]. Items are Likert rated 0–6, yielding an indexed score of 0–100 from best to worst quality of life. Subscales include activities, independence, relationships, emotions, and appearance. Self-perceived activity level was measured using the Physical Self-Description Questionnaire activity subscale (PSDQ-activity) [Citation44]. The 40-item, Likert-rated PSDQ was validated for disabled adults with neuromuscular diseases [Citation45]. The PSDQ-activity subscale of four items yields a mean score of 1–6 from least to most activity.
At baseline weeks 1 and 2, test–retest reliability was tested by repeated administration of the IPAQ and testing for functional stability each week using the Health Assessment Questionnaire (HAQ) [Citation46]. The 24-item HAQ has been validated in electronic and paper format [Citation47]. Activities of daily living are rated 0–3 according to difficulty, yielding an indexed total score of 0–3 from least to most disabled. At baseline week 3, concurrent validity and measurement error were evaluated by comparison of IPAQ data with GENEActiv accelerometry data. The GENEActiv was posted to participants, who wore it for 7 days, only removing it to wash, then returned it by post. The third baseline week was used to ensure that accelerometry did not interfere with reliability testing of the questionnaire in baseline weeks 1 and 2.
At follow up, participants were sent the accelerometer to wear for another week and questionnaires were repeated. Responsiveness was examined by comparing changes in IPAQ and accelerometry over time.
In the adjunctive study, 3 months after baseline, participants were invited to wear a smart activity monitor and complete the IPAQ again. Those who opted in were sent a wrist-worn Fitbit. Participants used the Fitbit for 2 weeks at time points 1 and 2 and during the follow up week. They completed the IPAQ and HAQ each week to test adjunctive IPAQ test–retest reliability. Participants who wore their Fitbit at follow up provided data to test adjunctive IPAQ concurrent validity (compared to GENEActiv accelerometry) and construct validity (relationships with demographics re-tested at follow up) (see ).
Statistical analysis
A sample size of 100 (plus 10% to allow for attrition) was planned based on recommendations for evaluating measurement properties [Citation48] and a power calculation [Citation49]. Statistical analyses were carried out using R (version 3.6.0). Alpha was set at 0.05. Data were tested for normality using Shapiro–Wilk [Citation50] and nonparametric equivalents were used when data were not normally distributed. MVPA was summed moderate and vigorous activity time recorded using IPAQ/GENEActiv. Concurrent validity between IPAQ and GENEActiv data was tested by Spearman’s correlation; rho ≥0.70 was considered satisfactory [Citation48]. However, lower correlations of 0.09–0.39 [31], have been reported and ≥0.50 is sometimes used as a satisfactory correlation cut-point [Citation31]. Based on a predicted correlation of 0.50–0.70, the study had 99–100% power [Citation49]. Convergent and divergent validity were tested by correlation with related constructs (PSDQ-activity, INQoL, and HAQ) and unrelated constructs (height, handedness, and age) respectively. Satisfactory construct validity cut-points were ≥0.30 and ≤0.20, respectively [Citation48]. Discriminative validity tested the difference in IPAQ score between wheelchair users and ambulant participants by the Mann–Whitney U test. Measurement error of activity time reported in the IPAQ was examined by absolute measurement error and Bland–Altman plots. Reliability was tested by intra-class correlation coefficient (ICC) between repeated IPAQ scores one week apart. An ICC of ≥0.75 was considered satisfactory [Citation51]. Responsiveness of the IPAQ to change overtime was tested by area under the curve (AUC) analysis of receiver operating characteristic curves for GENEActiv change. An AUC of ≥0.70 was considered satisfactory [Citation48]. Questionnaires were scored using available items only; questionnaires with >10% of items missing were excluded from analyses. For accelerometry, missing data of ≤10 min were included in daily means, but days with <23 h monitored were excluded from analyses. Participants with missing datasets or lost to follow up were excluded from analyses.
Results
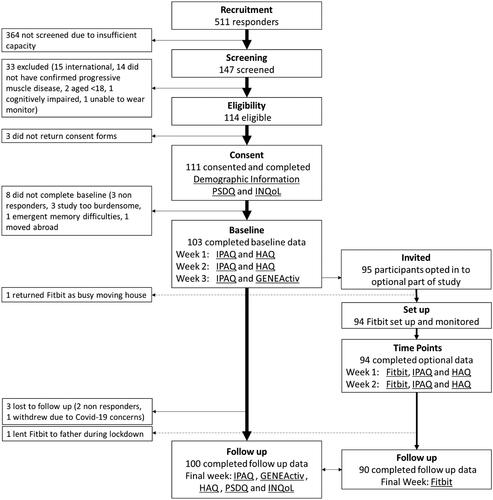
summarises recruitment. One hundred and three completed baseline data, 100 returned follow up data There was a mean of 9.4 months between baseline and follow up. Demographics at baseline are summarised in .
Table 1. Demographics and baseline information (N = 103).
The concurrent validity between IPAQ score and GENEActiv overall intensity did not meet acceptability criteria, although there was a significant moderate correlation (rho = 0.49, p < 0.0001, N = 103). Convergent validity of IPAQ score was demonstrated by significant low to moderate correlations with the HAQ Disability Index and PSDQ-Activity subscale (rho = −0.35 to 0.42). Divergent validity of IPAQ score was demonstrated by lack of significant correlation with height, handedness, employment status and age (see ). Discriminative validity was demonstrated by significant differences between wheelchair users and independently mobile participants for activity measured by IPAQ and GENEActiv (see and ). Test–retest reliability of the IPAQ was just below the acceptability threshold (ICC = 0.73, confidence interval = 0.66–0.81). There was no significant change in HAQ between reliability testing weeks. Responsiveness of the IPAQ to activity change over time was not satisfactory (AUC = 0.64). Using the IPAQ with the smart activity monitor (Fitbit) improved the validity and reliability of the questionnaire. However, criterion validity was still unsatisfactory (see ).
Table 2. Validity and reliability statistics for IPAQ total score.
The validity and test–retest reliability for activity time derivations of the IPAQ, including MVPA, light activity, inactivity, and sleep time were similarly unsatisfactory to that of IPAQ total score. However, like IPAQ total score, they showed slightly improved measurement properties for the IPAQ plus Fitbit compared to IPAQ alone (see ).
Table 3. Validity and reliability statistics for IPAQ activity, inactivity, and sleep time.
Measurement error was high for IPAQ derived MVPA time, compared to the GENEActiv accelerometer, as shown by the Bland–Altman plot (see ). There were wide limits of agreement, high proportional error (0.86) and considerable absolute and systematic errors indicating overestimation of MVPA using IPAQ alone (172 and 161 min per week, respectively). Those who reported fitness or transportation activities tended to overestimate less than respondents who reported only domestic activities. However, the types of activity reported were predominantly domestic (e.g., shopping, housework, self-care, house maintenance and caring responsibilities). Self-reported MVPA time showed the greatest overestimation error and inactivity time the greatest underestimation error. Whereas light activity and sleep time reporting had more random error (see ).
Figure 2. Bland–Altman plots of IPAQ measurement error in MVPA, light activity, inactive and sleep time. IPAQ: International Physical Activity Questionnaire; MVPA: moderate and vigorous activity.

IPAQ plus Fitbit MVPA showed the greatest improvement in self-report accuracy compared to IPAQ alone. MVPA measurement error reduced by 135 min per week and there was a 34% reduction in proportional bias (see ). The effect was similar, but smaller, for light activity (see ). However, there was little change in measurement error of inactivity or sleep time reporting between IPAQ alone and IPAQ plus Fitbit (see ).
Discussion
The IPAQ did not have satisfactory validity compared to accelerometry for the assessment of physical activity in adults with progressive muscle disease. Despite satisfactory construct validity (convergent, divergent, and discriminative), the IPAQ did not demonstrate satisfactory criterion validity, reliability, or responsiveness. IPAQ measurement error was unacceptably high with a strong tendency for overestimation of activity. However, measurement properties and reporting accuracy of the IPAQ were slightly improved when it was used in combination with a smart activity monitor.
Our findings are similar to other studies which have reported unsatisfactory validity and reliability of the IPAQ in healthy [Citation52] and clinical populations [Citation53]. Conversely, some earlier studies reported IPAQ had satisfactory reliability and validity, however, they used lower acceptability thresholds [Citation31]. Our findings of significant overestimations of IPAQ MVPA time are concordant with measurement error findings from other studies of self-reported physical activity measures [Citation31,Citation52,Citation53]. Measurement error might be explained by recall and social desirability bias (which leads people to overestimate self-reported activity) [Citation54].
Some researchers have suggested ways to improve the accuracy of self-report physical activity measurement [Citation55]. It is possible that self-report bias might be tempered by adjunctive objective monitoring. This might explain the improved IPAQ plus Fitbit measurement properties detected in this study. However, some experts recommend preferential reliance on objective activity measures, only using self-report tools, like the IPAQ, alone, if objective measurement is unfeasible [Citation52,Citation53,Citation56]. The IPAQ alone is unsuitable for clinical studies of adults with progressive muscle diseases, where activity represents a health outcome indicative of disease progression. Similarly, the IPAQ alone is unsuitable when the precise quantification of physical activity is crucial. For example, informing muscle disease activity prescription and guidelines, because inflated activity targets might lead to activity-related disease exacerbation [Citation9] or activity disengagement. Insufficient activity might increase the detrimental health sequelae associated with sedentarism [Citation57].
Self-report measures, like the IPAQ, are useful in population surveillance studies where activity categorisation, rather than exact activity quantification, is desirable [Citation22,Citation52]. Also, as additional activity outcomes to help interpret objective data and provide information about activity type and perceived intensity. Indeed, self-reported physical activity data provides insight into perceived activity intensity [Citation30]. Self-reported MVPA overestimation, compared to accelerometry, might be attributable to the increased energy cost of physical activity experienced by some people with progressive muscle diseases [Citation58,Citation59]. Accelerometry is unable to differentiate the level of effort required for limb acceleration and some experts recommend combined activity measurement approaches encompassing movement and associated exertion [Citation60].
In this study, the excessive activity overestimation of IPAQ alone might be explained by social desirability bias, recall bias and accelerometer insensitivity to exertion. Combined IPAQ plus adjunctive smart activity monitoring potentially represents a more accurate activity assessment, eliminating some self-report overestimation biases whilst also accounting for perceived energy cost of activity.
The improved accuracy of activity time reporting using the IPAQ plus smart activity monitoring might be accounted for because the monitoring app dashboard displayed active minutes. Thus, immediate availability of activity time information from smart activity monitoring might have facilitated more accurate MVPA (and light) activity self-report. However, self-report of inactivity time was consistently underestimated using IPAQ alone and unchanged with adjunctive smart activity monitoring. The monitoring app dashboard did not display inactivity data. This is a possible explanation for why smart activity monitoring did not improve awareness of inactivity. Thus, for assessment or interventions targeting inactivity, clinician-facilitated, or inactivity-focused, self-monitoring might be required to improve inactivity awareness and self-report.
The key strengths of this study were the thorough examination of multiple measurement properties and the remote design, allowing the inclusion of a wide range of participants from across England, Ireland, Scotland and Wales with varied functional ability and activity levels. Limitations include that the standard accelerometry cut points, used to differentiate vigorous, moderate, and light activity, might have been too severe for this population which included some highly disabled individuals. The measurement properties of the IPAQ may have been impacted by modifications to question descriptors or the administration method, because IPAQ was originally designed for telephone use which allows real-time response guidance (potentially reducing self-report errors) [Citation22]. Responsiveness might have been deflated by the lack of clinically important change in activity of the whole sample at follow up after less than a year. However, responsiveness might also have been inflated by the reduced overestimation linked to Fitbit use at follow up. The study would have been strengthened by a larger sample size allowing sub-group analyses of measurement properties by muscle disease diagnosis.
In conclusion, the IPAQ, compared to accelerometry, was not sufficiently valid, reliable, or responsive to measure physical activity in adults with progressive muscle diseases. Adjunctive objective activity monitoring improved the measurement properties and accuracy of IPAQ self-reported physical activity. For accurate quantification of physical activity, we recommend using objective or combined approaches to physical activity measurement in preference to self-report alone. Given our diverse sample, this recommendation is likely to be generalisable to activity measurement in other long-term conditions and, even, healthy adults.
Acknowledgements
The authors thank The John Walton Muscular Dystrophy Research Centre at Newcastle University and the Muscular Dystrophy UK charity for their help with advertising the study.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- WHO. Guidelines on physical activity and sedentary behaviour. Geneva: World Health Organization; 2020.
- Mercuri E, Mayhew A, Muntoni F, TREAT-NMD Neuromuscular Network, et al. Towards harmonisation of outcome measures for DMD and SMA within TREAT-NMD; report of three expert workshops: TREAT-NMD/ENMC workshop on outcome measures, 12th–13th May 2007, Naarden, The Netherlands; TREAT-NMD workshop on outcome measures in experimental trials for DMD, 30th June–1st July 2007, Naarden, The Netherlands; conjoint Institute of Myology TREAT-NMD meeting on physical activity monitoring in neuromuscular disorders, 11th July 2007, Paris, France. Neuromuscul Disord. 2008;18(11):894–903.
- Voet NB, van der Kooi EL, Riphagen II, et al. Strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev. 2019;12:CD003907.
- Reffin J, Dewar L, and the Adult Neuromuscular Physiotherapy Special Interest Group. Exercise advice for adults with muscle-wasting conditions. In: Campagn MD, editor. www.musculardystrophyuk.org. London: Muscular Dystrophy UK; 2014.
- Brady LI, MacNeil LG, Tarnopolsky MA. Impact of habitual exercise on the strength of individuals with myotonic dystrophy type 1. Am J Phys Med Rehabil. 2014;93(9):739–746. Quiz 747–748.
- Voet N, Bleijenberg G, Hendriks J, et al. Both aerobic exercise and cognitive-behavioral therapy reduce chronic fatigue in FSHD: an RCT. Neurology. 2014;83(21):1914–1922.
- Bankole LC, Millet GY, Temesi J, et al. Safety and efficacy of a 6-month home-based exercise program in patients with facioscapulohumeral muscular dystrophy: a randomized controlled trial. Medicine (Baltimore). 2016;95(31):e4497.
- Andersen G, Heje K, Buch AE, et al. High-intensity interval training in facioscapulohumeral muscular dystrophy type 1: a randomized clinical trial. J Neurol. 2017;264(6):1099–1106.
- Siciliano G, Simoncini C, Giannotti S, et al. Muscle exercise in limb girdle muscular dystrophies: pitfall and advantages. Acta Myol. 2015;34(1):3–8.
- O'Connor L, Westerberg E, Punga AR. Myasthenia gravis and physical exercise: a novel paradigm. Front Neurol. 2020;11:675.
- White CM, Hadden RD, Robert-Lewis SF, et al. Observer blind randomised controlled trial of a tailored home exercise programme versus usual care in people with stable inflammatory immune mediated neuropathy. BMC Neurol. 2015;15:147.
- Jones K, Hawke F, Newman J, et al. Interventions for promoting physical activity in people with neuromuscular disease. Cochrane Database Syst Rev. 2021;5:CD013544.
- Latouche C, Jowett JB, Carey AL, et al. Effects of breaking up prolonged sitting on skeletal muscle gene expression. J Appl Physiol (1985). 2013;114(4):453–460.
- Mercuri E, Mazzone E. Choosing the right clinical outcome measure: from the patient to the statistician and back. Neuromuscul Disord. 2011;21(1):16–19.
- Lovering RM, Porter NC, Bloch RJ. The muscular dystrophies: from genes to therapies. Phys Ther. 2005;85(12):1372–1388.
- Gang Q, Bettencourt C, Machado P, et al. Sporadic inclusion body myositis: the genetic contributions to the pathogenesis. Orphanet J Rare Dis. 2014;9:88.
- Emery AE. The muscular dystrophies. Lancet. 2002;359(9307):687–695.
- Mastaglia FL. Sporadic inclusion body myositis: variability in prevalence and phenotype and influence of the MHC. Acta Myol. 2009;28(2):66–71.
- Cassell J, Cassell R, Down F, et al. Wheelchair provision for children and adults with muscular dystrophy and other neuromuscular conditions. Best Practice Guidelines, Muscular Dystrophy Campaign. 2011;5:39–48.
- Jimenez-Moreno AC, Newman J, Charman SJ, et al. Measuring habitual physical activity in neuromuscular disorders: a systematic review. J Neuromuscul Dis. 2017;4(1):25–52.
- Roberts-Lewis SF, Rose MR, White CM, et al. Measurement of physical activity in adults with muscular dystrophy: a systematic review. J Phys Activity Res. 2019;4(1):1–25.
- Bauman A, Ainsworth BE, Bull F, et al. Progress and pitfalls in the use of the International Physical Activity Questionnaire (IPAQ) for adult physical activity surveillance. J Phys Act Health. 2009;6 Suppl 1:S5–S8.
- Washburn RA, Zhu W, McAuley E, et al. The physical activity scale for individuals with physical disabilities: development and evaluation. Arch Phys Med Rehabil. 2002;83(2):193–200.
- Hallal PC, Pratt M. Physical activity: moving from words to action. Lancet Glob Health. 2020;8(7):e867–e868.
- Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395.
- Hurtig-Wennlöf A, Hagströmer M, Olsson LA. The International Physical Activity Questionnaire modified for the elderly: aspects of validity and feasibility. Public Health Nutr. 2010;13(11):1847–1854.
- Rutkowska I, editor. Mobility in the Project – "Adapted Physical Activity – Theory and Practice". 4th International Conference: Adapted Physical Activity in Rehabilitation and Education; 2014. Warsaw: Josef Pilsudski University of Physical Education.
- Hagstromer M, Oja P, Sjostrom M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9(6):755–762.
- Ekelund U, Sepp H, Brage S, et al. Criterion-related validity of the last 7-day, short form of the International Physical Activity Questionnaire in Swedish adults. Public Health Nutr. 2006;9(2):258–265.
- Prince SA, Adamo KB, Hamel ME, et al. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56.
- Lee PH, Macfarlane DJ, Lam TH, et al. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8:115.
- Kowalski K, Rhodes R, Naylor PJ, et al. Direct and indirect measurement of physical activity in older adults: a systematic review of the literature. Int J Behav Nutr Phys Act. 2012;9:148.
- Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. 2003;37(3):197–206; discussion 206.
- Cleland C, Ferguson S, Ellis G, et al. Validity of the International Physical Activity Questionnaire (IPAQ) for assessing moderate-to-vigorous physical activity and sedentary behaviour of older adults in the United Kingdom. BMC Med Res Methodol. 2018;18(1):176.
- Esliger DW, Rowlands AV, Hurst TL, et al. Validation of the GENEA accelerometer. Med Sci Sports Exerc. 2011;43(6):1085–1093.
- Jimenez-Moreno AC, Charman SJ, Nikolenko N, et al. Analyzing walking speeds with ankle and wrist worn accelerometers in a cohort with myotonic dystrophy. Disabil Rehabil. 2019;41(24):2972–2977.
- van Hees VT, Sabia S, Anderson KN, et al. A novel, open access method to assess sleep duration using a wrist-worn accelerometer. PLoS One. 2015;10(11):e0142533.
- van Hees VT, Gorzelniak LD, Leon EC, et al. Separating movement and gravity components in an acceleration signal and implications for the assessment of human daily physical activity. PLoS One. 2013;8(4):e61691.
- van Hees VT, Fang Z, Langford J, et al. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: an evaluation on four continents. J Appl Physiol (1985). 2014;117(7):738–744.
- Harper L, Hewitt CA, Litchfield I, et al. Management of fatigue with physical activity and behavioural change support in vasculitis: a feasibility study. Rheumatology (Oxford). 2021;60(9):4130–4140.
- Chastin SF, Egerton T, Leask C, et al. Meta-analysis of the relationship between breaks in sedentary behavior and cardiometabolic health. Obesity (Silver Spring). 2015;23(9):1800–1810.
- Barfield JP, Newsome L, Malone LA. Exercise intensity during power wheelchair soccer. Arch Phys Med Rehabil. 2016;97(11):1938–1944.
- Vincent KA, Carr AJ, Walburn J, et al. Construction and validation of a quality of life questionnaire for neuromuscular disease (INQoL). Neurology. 2007;68(13):1051–1057.
- Marsh HW, Martin AJ, Jackson S. Introducing a short version of the physical self description questionnaire: new strategies, short-form evaluative criteria, and applications of factor analyses. J Sport Exerc Psychol. 2010;32(4):438–482.
- Martin JJ, Whalen L. Self-concept and physical activity in athletes with physical disabilities. Disabil Health J. 2012;5(3):197–200.
- Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: a review of its history, issues, progress, and documentation. The. J Rheumatol. 2003;30(1):167–178.
- Bruce B, Fries JF, editors. Internet versus mailed administration of the health assessment questionnaire disability index. Annual Scientific Meeting of the American College of Rheumatology and Association of Rheumatology Health Professionals; 2011. October 2017; Chicago, IL: Arthritis and Rheumatism; 2011.
- Mokkink LB, Prinsen CAC, Patrick DL, et al. COSMIN methodology for systematic reviews of Patient-Reported Outcome Measures (PROMs): user manual. In: Mokkink LB, editor. COnsensus‐based standards for the selection of health measurement instruments. Version 1.0 ed. Amsterdam: Amsterdam Public Health Research Institute; 2018.
- Mondal H, Mondal S. Sample size calculation to data analysis of a correlation study in Microsoft Excel: a hands on guide with example. Int J Clin Exp Physiol. 2016;3(4):180–189.
- Ghasemi A, Zahediasl S. Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab. 2012;10(2):486–489.
- Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163.
- Sember V, Meh K, Soric M. Validity and reliability of International Physical Activity Questionnaires for adults across EU countries: systematic review and meta analysis. Int J Environ Res Public Health. 2020;17(19):7161.
- Dowd KP, Szeklicki R, Minetto MA, et al. A systematic literature review of reviews on techniques for physical activity measurement in adults: a DEDIPAC study. Int J Behav Nutr Phys Act. 2018;15(1):15.
- Sylvia LG, Bernstein EE, Hubbard JL, et al. Practical guide to measuring physical activity. J Acad Nutr Diet. 2014;114(2):199–208.
- Ainsworth BE, Caspersen CJ, Matthews CE, et al. Recommendations to improve the accuracy of estimates of physical activity derived from self report. J Phys Act Health. 2012;9 (Suppl 1):S76–S84.
- Lavelle G, Noorkoiv M, Theis N, et al. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF) as a measure of physical activity (PA) in young people with cerebral palsy: a cross-sectional study. Physiotherapy. 2020;107:209–215.
- Dunstan DW, Dogra S, Carter SE, et al. Sit less and move more for cardiovascular health: emerging insights and opportunities. Nat Rev Cardiol. 2021;18(9):637–648.
- McCrory MA, Kim HR, Wright NC, et al. Energy expenditure, physical activity, and body composition of ambulatory adults with hereditary neuromuscular disease. Am J Clin Nutr. 1998;67(6):1162–1169.
- Ramdharry GM, Pollard AJ, Grant R, et al. A study of physical activity comparing people with Charcot-Marie-Tooth disease to normal control subjects. Disabil Rehabil. 2017;39(17):1753–1758.
- Nightingale TE, Rouse PC, Thompson D, et al. Measurement of physical activity and energy expenditure in wheelchair users: methods, considerations and future directions. Sports Med Open. 2017;3(1):10.