 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
To investigate the effect of two weeks of repetitive transcranial magnetic stimulation (rTMS) on the attention network in Parkinson’s disease (PD) patients.
Materials and methods
Sixty PD patients were randomly divided into equal-sized active- and sham-rTMS groups. Executive function was assessed by neuropsychological tests including the Trail-Making Test (TMT), word fluency test, digit span, Wisconsin Card Sorting Test (WCST) and Stroop test. The attention network was evaluated by the attention network test (ANT). rTMS (5 Hz) was applied over the left dorsolateral prefrontal cortex (DLPFC) in the active-rTMS group, and the sham-rTMS group underwent sham stimulation, both for two weeks. All tests were performed before and after rTMS.
Results
After active rTMS, nonparametric analysis revealed significant improvements in categories completed (CC) (p < 0.001) in the WCST and reaction times (RTs) in part 3 (p = 0.002) and the Stroop interference effect (SIE) (p < 0.001) in the Stroop test. Regarding the ANT, the RTs of the executive control network were significantly reduced (p < 0.001). There was no significant change after sham rTMS.
Conclusions
In the short term, in PD patients, rTMS improved the executive control network involved in resolving conflicting information. However, it showed milder effects on neuropsychological test outcomes assessing executive function, which may involve different neuromechanisms.
Cognitive impairment is common in patients with Parkinson’s disease (PD), and it is related to functional disability and reduced quality of life.
Attention is a main component of the cognitive system, and attention deficits are responsible for disability.
This study demonstrates that rTMS is beneficial for cognitive rehabilitation in PD, as patients showed improved performance on the attention network test and neuropsychological tests.
Implications for rehabilitation
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder that results in motor complications and nonmotor symptoms, including depression, autonomic disorder, and cognitive impairment. Attention is a main component of the cognitive system and is considered capable of allocating psychological resources [Citation1]. This process allows us to pursue goals against numerous unexpected distractors and is of great significance in individuals’ daily lives. Impairments in attention are common in PD patients, even those without dementia [Citation2]. Deficits in attention in the early stages of PD may not be clinically apparent but can be detected by certain neuropsychological tests [Citation3]. The attention network test (ANT) [Citation4] is an approach for evaluating attention networks using a flanker test. The ANT has been used in healthy participants alongside functional magnetic resonance imaging to confirm the anatomical locations of attention networks [Citation5]. The attentional system comprises the following three distinct networks: alerting, orienting, and executive control networks; these three networks work in coordination [Citation6]. Alerting refers to the ability to achieve and maintain an alert state sensitive to warning signals. Alerting is correlated with the cortices in the right hemisphere and the parietal region, which have been demonstrated to be activated by alert signals [Citation5]. Orienting refers to the ability to select information from sensory input. The relevant structures in the orienting network include the superior parietal lobe and temporal parietal lobe [Citation7]. Executive control refers to the ability to resolve conflict, make decisions, and control responses. The executive control network is associated with the prefrontal cortex and midline frontal regions [Citation5]. Aside from the ANT, there are other methods and tests to assess the executive control network. Resting-state functional connectivity within the executive control network and between it and other networks have been studied by functional magnetic resonance imaging [Citation8,Citation9]. The N-back task has also been used to assess the executive control network [Citation10]. However, the ANT is the most commonly used test for this purpose. Although each network performs processes independently, interactions among the three networks have been observed in ANT studies. A reduced signal in frontal areas was found during alerting tasks, demonstrating the inhibition of the executive network by the alerting network [Citation11]. The alerting network was associated with the locus coeruleus, which produces norepinephrine, and the locus coeruleus has extremely strong connections with posterior areas, which constitute an important part of the orienting network. Therefore, the alerting network leads to more efficient orientation towards stimuli [Citation12]. Additionally, the interaction effect of executive control on orienting validity was correlated with the activation of the pulvinar according to functional magnetic resonance imaging during ANT [Citation13]. PD patients show more abnormal activation of attention networks [Citation14]. Cristinzio et al. reported that the pattern of attention networks in PD patients is similar to that in normal individuals, except for there is a slower response [Citation3].
Repetitive transcranial magnetic stimulation (rTMS) has been considered an effective noninvasive treatment for the motor and nonmotor symptoms of PD in recent years [Citation15–17]. The prefrontal cortex is particularly important for cognitive control [Citation18] and is a central hub for neural processes [Citation18]. The dorsolateral prefrontal circuit may participate in cognition [Citation19] and has been considered a cognition loop. Koch et al. [Citation20] demonstrated that the underlying neural network may be a circuit comprising the basal ganglia and dorsolateral prefrontal cortex (DLPFC). Compared with controls, cortical atrophy was found in the left parietal lobe in PD patients with dementia, and areas of reduced grey matter were found in the left frontal lobe in PD patients with mild cognitive impairment [Citation21]. During ANT, PD patients showed more activation in the right frontal field, which is a region belonging to the dorsal attention and frontoparietal networks [Citation14]. Such dysregulated attention was due to decreased activity in the left DLPFC and was related to hyperactivation in the right frontal, parietal and subcortical regions [Citation22]. Therefore, we assumed that the activation of the right frontal field could be inhibited by high-frequency rTMS over the left DLPFC. In addition, the left DLPFC is more active in attentional selection in normal individuals [Citation23], and it has been demonstrated that active stimulation over the left DLPFC is beneficial for attention [Citation22,Citation24] and cognition in PD patients [Citation25]. Meanwhile, high-frequency rTMS could improve time processing [Citation20], resulting in the activation of the ventral tegmental area and influencing dopaminergic neurons, causing dopaminergic changes [Citation26]. Relevant studies have also investigated the effect of high-frequency rTMS on cognitive function [Citation27,Citation28]. Therefore, we assumed that high-frequency rTMS over the left DLPFC is effective in modulating the attention network on the basis of certain neuromechanisms.
Boggio et al. [Citation28] reported that rTMS improved the performance of PD patients in the Stroop and Wisconsin assessments. In Randver’s review, some studies suggested that rTMS had beneficial effects on cognition, such as executive function [Citation29]. In addition, rTMS over the motor cortex in PD patients improved their cognitive rating scores on the Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) and shortened the latency of P300 [Citation30]. However, although these studies reported the effects of rTMS on cognitive impairment in PD patients, to the best of our knowledge, this study is the first to examine the effects of rTMS on the attention network in PD patients. We combined the ANT for measuring attention with neuropsychological tests for assessing different aspects of executive function, including the Trail-Making Test (TMT), word fluency test, digit span test, Wisconsin Card Sorting Test (WCST), and Stroop test, to thoroughly assess the effects of rTMS on the attention network from the perspective of cognitive functioning.
Materials and methods
Ethical approval
This study was approved by the ethics committee of the Affiliated Hospital of Southwest Medical University and was registered in the Chinese Clinical Trial Registry (http://www.chictr.org.cn/). Written informed content was obtained from each participant before participating in the study.
Participants
Sixty PD patients were included in this study. The PD patients were selected from the Affiliated Hospital of Southwest Medical University. The demographic and clinical characteristics are shown in . The PD patients were diagnosed by experienced neurological physicians and met the UK Brain Bank criteria for PD [Citation31]. All PD patients had a positive response to dopaminergic medications. Patients with depression (Beck Depression Inventory II [Citation32] scores >14), cognitive impairment (MoCA scores <26), psychotic symptoms and other neurological diseases were excluded. The patients were randomly divided into the active-rTMS group (30) and sham-rTMS group (30). There were no significant differences in age, sex, education, disease duration, Hoehn–Yahr’s stage [Citation33], total Unified Parkinson’s Disease Rating Scale (UPDRS) score [Citation34], bradykinesia (upper limb) score [Citation35], or l-DOPA daily dose (LEDD) [Citation36] between the active- and sham-rTMS groups.
Table 1. Demographic and clinical variables of the PD patients.
Neuropsychological tests
The TMT, including both part A and part B, was applied [Citation37]. The word fluency test was carried out in accordance with the study by Garrido-Vásquez et al. [Citation38], but we permitted the participants to answer in Chinese because their mother language was Chinese. The test included the following three subtasks requiring the participants to generate as many words as possible in one minute: (1) generate words starting with “T”; (2) generate words starting with “S” and generate the names of countries alternately; and (3) generate active verbs. Digit span tests [Citation39] were carried out using forward and backward methods. The WCST was used to assess executive function [Citation40,Citation41]. The indices of the WCST, including categories completed (CC), perseverative error (PE), and nonperseverative error (NPE), were calculated. In the Stroop test [Citation42], the participants were instructed to complete the task on a computer by pressing the corresponding keys as fast as possible. Part 1 required the participants to read the names of the following four colours, which were presented in black: red, blue, yellow, and green. Part 2 required the participants to name the colour of the patches presented in one of the four colours. Part 3 required the participants to identify the colour of the colour words, and the colour and the meaning of the words were inconsistent. The reaction time (RT) of the Stroop interference effect (SIE) was calculated as the RT in part 3 minus that in part 2, and the accuracy of the SIE was calculated as correct responses in part 2 minus those in part 3 [Citation43].
Attention network test
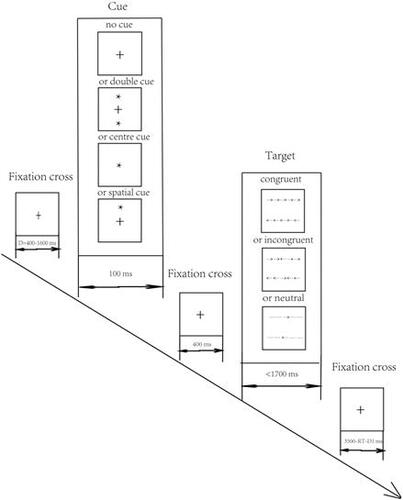
We carried out the ANT as described by Fan et al. [Citation4] and performed the test using a computer. First, a fixation cross was presented in the centre of the screen for a random time interval of 400–1600 ms. Then, the cue was presented for 100 ms. There were four possible cues as follows: (1) no asterisks were presented, and only a fixation cross was presented in the centre of the screen (no cue); (2) two asterisks were presented simultaneously with one above and one below the central fixation cross (double cue); (3) an asterisk was presented in the centre of the screen (centre cue); and (4) an asterisk was presented in the same position as the forthcoming target (spatial cue). Then, the fixation cross was presented again for 400 ms. Subsequently, the target stimulus was presented for a maximum period of 1700 ms. The target arrow may have been accompanied by flankers (arrows or lines) on both sides. There were three possible types of flankers as follows: (1) flanker arrows that were the same as the target arrow (congruent); (2) flanker arrows that differed from the target arrow (incongruent); and (3) flankers that appeared as lines (neutral). The participants were required to view the computer screen and judge the direction of the target arrow (“left” or “right”) by pressing the corresponding key as quickly as possible. The last fixation cross was presented for a period as an intertrial interval, and the duration of its presentation depended on the RT in response to the target arrow and the time of the first fixation cross intertrial interval=(3500 ms) – (RT) – (first fixation cross duration) (). The cues and targets were presented in pseudorandom sequences and were presented in 96 trials (4 cue types × 3 target types × 2 (up or down)×2 (left or right)×2 repetitions). Attention network function was assessed using the following calculations: alerting effect = no cue-double cue; orienting effect = centre cue-spatial cue; and executive effect = incongruent–congruent.
Figure 1. Schematic of the ANT. There were four types of cue conditions, namely, no cue, double cue, centre cue, and spatial cue. There were three types of targets, namely, congruent, incongruent, and neutral. After the presentation of the first fixation cross, one cue was shown. The presentation of the second fixation cross followed the cue, and one target was presented per trial.
The performance on all neuropsychological tests and the ANT was assessed before and after the rTMS treatment.
Repetitive transcranial magnetic stimulation
Five Hz rTMS was performed over the left DLPFC of the patients in the active-rTMS group using a system from YINGCHI (Shenzhen, China) with a figure-of-8-shaped coil. We collected the patients’ brain images by 3.0 T magnetic resonance imaging (Prisma, Siemens Medical Solutions, Erlangen, Germany), and we positioned the coil using a neuronavigation system. The spatial coordinates of the treatment location were recorded to ensure the accuracy of the coil placement on the left DLPFC. The resting motor threshold (RMT) was the lowest stimulus intensity that activated the right abductor pollicis brevis muscle when the coil was in the position of the left DLPFC, and the stimulus intensity was set as 110% of the RMT at 5 Hz in 24 trains of 50 pulses per train (1200 total pulses per session). The participants underwent two sessions of rTMS every consecutive weekday for 2 weeks. The two sessions were completed in the morning and afternoon, and the interval was 6 h. In the sham-rTMS group, the parameters were set to be the same as those in the active-rTMS group, but the coil was held at an inverted orientation. Both the active- and sham-rTMS groups were assessed with neuropsychological tests and the ANT before and after the stimulation.
Statistical analysis
SPSS (version 24.0, SPSS Inc., Chicago, IL) was used for the statistical analysis. The demographic, clinical and neuropsychological characteristics were compared by nonparametric tests because the data were nonnormally distributed. The differences in rates were assessed by chi-square tests. The neuropsychological tests were analysed by a repeated-measures analysis of variance (ANOVA) with the treatment (active or sham rTMS) and time (pre- or poststimulation) as factors. The ANT results were examined using a multivariate analysis of variance (MANOVA) to investigate the effect of rTMS. The variables that exhibited significant differences between the active- and sham-rTMS groups pre-rTMS were included as covariates. The comparisons between the groups were performed using a nonparametric test. In the ANT, because there were 12 variable combinations, including four cue conditions (no cue, double cue, centre cue, and spatial cue) and three target conditions (congruent, incongruent, and neutral), the p value of the nonparametric test was corrected to 0.0042 accordingly.
Results
There were no significant differences between the active- and sham-rTMS groups in bradykinesia after rTMS.
In the WCST, there were no differences in PEs or NPEs after rTMS or compared with the sham rTMS group. The CC were significantly increased (Z=–3.096, p = 0.002) after rTMS compared with the post-sham rTMS group (Z=–3.255, p = 0.001), and there was a significant rTMS × time interaction effect (F(1,29)=17.228, p < 0.001, =0.373) ().
Table 2. Neuropsychological test results of the PD patients before and after active or sham rTMS.
Regarding the RT in the Stroop test, there were significant rTMS × time interaction effects in part 3 (F(1,29)=11.148, p = 0.002, =0.278) and a significant SIE (RT for SIE) (F(1,29)=15.474, p < 0.001,
=0.348). Compared with the post-sham rTMS group, the post-active rTMS group showed a significant difference in RT in part 3 (Z=–3.980, p < 0.001) and RT for SIE (Z=–4.309, p < 0.001). There were four variables in the Stroop test, and the p value was corrected to 0.0125; thus, there was no significant difference in RT in part 2 (Z=–2.108, p = 0.035) after rTMS. Regarding accuracy, there were no differences in the Stroop test. There were no differences in the MoCA, TMT, word fluency, or digit span test scores after rTMS, and the performance on all neuropsychological tests did not differ before and after sham rTMS ().
In the attentional network test (ANT), the overall mean RT in the post-rTMS period was much shorter than that in the pre-rTMS period (Z=–4.782, p < 0.001) and post-sham rTMS (Z=–4.783, p < 0.001). The RTs for alerting (Z=–3.306, p = 0.001) and executive control (Z=–4.791, p < 0.001) in the active rTMS group were shorter than those before the stimulation, and the RT for orienting did not significantly differ (Z=–1.922, p = 0.055). However, the alerting network in the active-rTMS group did not significantly differ from that in the sham-rTMS group after the stimulation (Z=–1.047, p = 0.295), whereas the executive network was significantly reduced (Z = -5.145, p < 0.001), and the orienting network did not significantly differ (Z=–0.917, p = 0.359) (). In the comparison of the active- and sham-rTMS groups after the stimulation using a MANOVA including covariates, the RTs on all subtasks were reduced (p < 0.001), except for those with the centre cue (F(1,55)=3.744, p = 0.058, =0.064) and spatial cue (F(1,55)=2.150, p = 0.148,
=0.038) under the neutral condition. The nonparametric tests between the groups showed similar results (centre cue under the neutral condition: Z=–1.923, p = 0.054; spatial cue under the neutral condition: Z=–1.633, p = 0.103). After sham rTMS, there was no significant difference in RT compared with before sham rTMS (p value was corrected to 0.0042) (). Compared with the pre-rTMS accuracy, the accuracy significantly increased after rTMS only with spatial cues under the incongruent condition according to nonparametric testing (Z=–2.972, p = 0.003), but there were no significant differences between the active- and sham-rTMS groups after the stimulation (). Nevertheless, there was a significant difference with the centre cues under the congruent condition (F(1,56)=5.710, p = 0.020,
=0.093) between the post-active and sham rTMS groups as analysed by a MANOVA including covariates.
Table 3. ANT RT of the attention network and overall mean RT before and after active and sham rTMS.
Table 4. ANT RT under each condition before and after active and sham rTMS.
Table 5. ANT accuracy under each condition before and after active and sham rTMS.
Discussion
Executive functions refer to the ability to process novelty and goal setting [Citation44] and have been demonstrated to be related to social functioning [Citation45]. Executive dysfunction can be found even in the early stages of PD [Citation46] and predicts the later development of dementia [Citation47]. It has been suggested that a significant fraction of executive disorders may manifest as cognitive impairment [Citation48], and it appears to be a core symptom of cognitive impairment in PD [Citation49]. The WCST is a useful diagnostic tool as a supplement to the MoCA [Citation50] and is commonly used [Citation51] to evaluate executive function [Citation52]. Impairment in the WCST is a hallmark trait of executive function in PD patients [Citation52]. In the present study, there were improvements only in the CC of the WCST, while the PE and NPE scores did not improve after rTMS. The CC is used as a measurement of the performance on the overall WCST [Citation52]. As CC has been reported to be the factor for solving problems and has higher ecological validity than PE and NPE [Citation53], it may be more sensitive in reflecting the characteristics of WCST impairment and improvement. This finding is consistent with Huang et al.’s [Citation54] study. However, another study [Citation49] reported that CC improved after rTMS along with PE, which is not consistent with our present study. A probable reason may be the different rTMS treatments. The location of rTMS in our present study was the left DLPFC, whereas the PE scores of PD patients were associated with perfusion of the right prefrontal cortex [Citation55].
Before rTMS, the ability to engage in goal-directed behaviour and react to novel situations was lower, and behaviour was disinhibited and susceptible to distraction [Citation56] because executive function is impaired [Citation57] in PD patients. In the present study, the RT in part 1 and part 2 did not obviously change. This result suggests that simpler tasks may not reflect the efficacy of rTMS. The time taken in part 3 of the Stroop test significantly decreased after rTMS, suggesting that the processes of interference inhibition, conflict resolution and task-switching performance improved. Due to the reduction in RT in part 3, the RTs for the interference effect (SIE) showed significant improvement. Part 3 and the RTs for the SIE may better reflect the effect of rTMS on executive function. Although there was no improvement in accuracy, the results demonstrated better performance in the Stroop test as the RT decreased without the cost of a reduction in accuracy. The Stroop interference is considered an executive function controlled by the prefrontal cortex [Citation58,Citation59]. rTMS is an effective treatment that transsynaptically affects the prefrontal circuit and improves prefrontal cognitive function [Citation49].
The performance on certain parts of the WCST and Stroop test improved after rTMS, demonstrating that rTMS was beneficial for executive function in PD patients. As executive function behaviours are modulated by the dopaminergic system [Citation60,Citation61], executive function and dopamine may share a common neural mechanism. The loss of dopaminergic neurons in the posterior putamen is distinct in PD patients, and the posterior putamen is a structure of the basal ganglia that is specifically correlated with the execution of habitual behaviours [Citation62]. In addition, PD has pathological features involving dopaminergic depletion in the basal ganglia [Citation62]; thus, impairments in executive function are exhibited in PD patients. The basal ganglia and prefrontal cortex play a key role in executive function [Citation63]. As rTMS over the left DLPFC could modulate the dopaminergic system [Citation64], dopamine enhances frontal lobe function. In addition, the synapses in the frontostriatal circuit may be improved; thus, executive function is more efficient [Citation65]. It has been indicated that high-frequency rTMS over prefrontal areas is especially beneficial for cognitive dysfunction [Citation66], which is consistent with our study. Nevertheless, there were no significant differences in the TMT, word fluency test or digit span test, demonstrating that there are many aspects of executive function; thus, we should not be confined to several assessments of executive function.
Málly et al. [Citation67] reported that executive function improved after low-frequency rTMS over the DLPFC, while high-frequency rTMS had no effect. The results of our present study are partially consistent with this finding. We used high-frequency rTMS, and there was no significant difference in TMT performance between the pre- and post-rTMS periods. However, other tools evaluating executive function were used in our study, and participants with different durations and severities of disease were recruited in the study mentioned above. Furukawa et al. [Citation49] found that TMT performance improved after rTMS in PD patients, and Aleman and van't Wout [Citation68] reported that rTMS was beneficial for digit span performance. However, the performance on the TMT and digit span test did not show differences after rTMS in our study. The main reason for the inconsistency in our study may be the different rTMS frequencies and locations. Compared with high-frequency rTMS, the effect of low-frequency rTMS starts earlier and induces more neural stem cells [Citation69]. High-frequency rTMS over the left DLPFC may result in more selective improvement in executive functions.
In our study, regarding the ANT, although the RT on almost every subtask was reduced, the improvements in the three attention networks by rTMS were not identical. The reduction in RT under the incongruent condition was greater than that under the congruent condition. Therefore, the RTs based on the executive control network were significantly reduced. The ANT is a useful test for evaluating the executive control network by assessing elapsed time differences between tasks involving congruent and incongruent targets [Citation70]. The executive control network is related to addressing conflict produced by different stimuli [Citation5]. This network is impaired in PD probably because the executive control network is modulated by dopamine [Citation71], and there is abnormal activation in frontoparietal and dorsal attention networks during executive challenges in PD [Citation14]. The prefrontal cortex prompts more effective responses to conflicting stimuli and coordinates the executive control network [Citation72]. The prefrontal cortex is a common anatomical region shared by the executive control network and executive functions. However, although rTMS showed a selective effect on executive functions as assessed by the neuropsychological tests mentioned above, the ANT data showed definite effects of rTMS on the executive control network.
As the reduction in RT with no cues was greater than that with double cues after rTMS compared with before, it seems that the alerting network improved. The alerting network is related to parietal areas and includes the prefrontal cortex and thalamus [Citation6]. There are anatomical and functional connections among different brain areas; thus, the influence of rTMS may not be limited to the primary cortex being stimulated [Citation73]. Meanwhile, it has been suggested that there is functional overlap among the three attentional networks [Citation5]. However, the alerting network showed no significant difference between the active and sham rTMS groups after the stimulation, suggesting that the indirect effect of rTMS on the alerting network cannot be confirmed and deserves further investigation in our following study. Nevertheless, undoubtedly, the influence of rTMS on the alerting network was not as definitive as that on the executive control network.
The orienting network is relatively preserved in PD patients [Citation70], which may explain the lack of improvements in orienting. According to the MANOVA including covariates, there was improvement in the accuracy with the centre cues under the congruent condition. The centre cues did not provide spatial information during the ANT task; thus, these data illustrate better sustained attention after rTMS, i.e., the patients entered a more alert state to start orientating attention. Therefore, we do not exclude the possibility that rTMS could also be beneficial for the orienting network, and we should observe PD patients treated with rTMS for longer periods in the future. Furthermore, we found that the differences in accuracy on the ANT after rTMS were not as significant as those in RT. The reason may be that accuracy was close to normal and, therefore, difficult to improve because of a ceiling effect.
It has been suggested that rTMS could improve bradykinesia [Citation74]. We noticed that the overall mean RT on the ANT was significantly decreased after rTMS, but there were no differences in RTs in part 1 and part 2 of the Stroop test and no differences in performance on the TMT, word fluency test or digit span test. Meanwhile, there was no significant difference in the bradykinesia scores of the upper limb after rTMS, further demonstrating that the change in the mean ANT RTs was unlikely influenced by improvements in bradykinesia.
In summary, the present study shows that rTMS has a distinct effect on the executive control network and a selective effect on executive function. Although the executive control network and executive function are both correlated with the dopaminergic system and prefrontal cortex, rTMS has different effects on the performance of the executive control network and executive function. We speculate that different neuromechanisms underlie the executive control network and executive function even though they share common anatomical circuits. This hypothesis should be tested in further studies using neuroimaging. Further studies using noninvasive treatment methods over longer periods are also required, and an appropriate rTMS protocol should be identified to improve cognitive function in PD patients, increasing their quality of life and reducing the burden on their caregivers.
Acknowledgements
We are grateful to Ms. Rong Zhang for her expert advice regarding the statistical analysis.
Disclosure statement
The authors report no conflicts of interest.
Data availability statement
The data collected in this study are available from the corresponding authors upon request.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Anderson JR. Cognitive psychology and its implications. New York (NY): Worth Publishers; 2004.
- Aarsland D, Bronnick K, Williams-Gray C, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75(12):1062–1069.
- Cristinzio C, Bononi M, Piacentini S, et al. Attentional networks in Parkinson’s disease. Behav Neurol. 2013;27(4):495–500.
- Fan J, McCandliss BD, Sommer T, et al. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14(3):340–347.
- Fan J, McCandliss B, Fossella J, et al. The activation of attentional networks. Neuroimage. 2005;26(2):471–479.
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13(1):25–42.
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215.
- McHugh MJ, Gu H, Yang Y, et al. Executive control network connectivity strength protects against relapse to cocaine use. Addict Biol. 2017;22(6):1790–1801.
- Hobkirk AL, Bell RP, Utevsky AV, et al. Reward and executive control network resting-state functional connectivity is associated with impulsivity during reward-based decision making for cocaine users. Drug Alcohol Depend. 2019;194:32–39.
- Oyegbile TO, VanMeter JW, Motamedi G, et al. Executive dysfunction is associated with an altered executive control network in pediatric temporal lobe epilepsy. Epilepsy Behav. 2018;86:145–152.
- Cohen RM, Semple WE, Gross M, et al. Functional localization of sustained attention: comparison to sensory stimulation in the absence of instruction. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1(1):3–20.
- Callejas A, Lupiàñez J, Funes MJ, et al. Modulations among the alerting, orienting and executive control networks. Exp Brain Res. 2005;167(1):27–37.
- Xuan B, Mackie MA, Spagna A, et al. The activation of interactive attentional networks. Neuroimage. 2016;129:308–319.
- Boord P, Madhyastha TM, Askren MK, et al. Executive attention networks show altered relationship with default mode network in PD. Neuroimage Clin. 2017;13:1–8.
- Shukla AW, Shuster JJ, Chung JW, et al. Repetitive transcranial magnetic stimulation (rTMS) therapy in Parkinson disease: a meta-analysis. PM R. 2016;8(4):356–366.
- Aftanas LI, Brack IV, Kulikova KI, et al. Clinical and neurophysiological effects of dual-target high-frequency rTMS over the primary motor and prefrontal cortex in Parkinson's disease. Zh Nevrol Psikhiatr Im S S Korsakova. 2020;120(5):29–36.
- Brys M, Fox MD, Agarwal S, et al. Multifocal repetitive TMS for motor and mood symptoms of Parkinson disease: a randomized trial. Neurology. 2016;87(18):1907–1915.
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24(1):167–202.
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9(1):357–381.
- Koch G, Oliveri M, Brusa L, et al. High-frequency rTMS improves time perception in Parkinson disease. Neurology. 2004;63(12):2405–2406.
- Beyer MK, Janvin CC, Larsen JP, et al. A magnetic resonance imaging study of patients with Parkinson's disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78(3):254–259.
- Sanchez-Lopez A, De Raedt R, Puttevils L, et al. Combined effects of tDCS over the left DLPFC and gaze-contingent training on attention mechanisms of emotion regulation in low-resilient individuals. Prog Neuropsychopharmacol Biol Psychiatry. 2021;108:110177.
- Banich MT, Milham MP, Atchley RA, et al. Prefrontal regions play a predominant role in imposing an attentional 'set': evidence from fMRI. Brain Res Cogn Brain Res. 2000;10(1–2):1–9.
- Clarke PJ, Browning M, Hammond G, et al. The causal role of the dorsolateral prefrontal cortex in the modification of attentional bias: evidence from transcranial direct current stimulation. Biol Psychiatry. 2014;76(12):946–952.
- Trung J, Hanganu A, Jobert S, et al. Transcranial magnetic stimulation improves cognition over time in Parkinson's disease. Parkinsonism Relat Disord. 2019;66:3–8.
- Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLOS One. 2009;4(8):e6725.
- Srovnalova H, Marecek R, Kubikova R, et al. The role of the right dorsolateral prefrontal cortex in the Tower of London task performance: repetitive transcranial magnetic stimulation study in patients with Parkinson's disease. Exp Brain Res. 2012;223(2):251–257.
- Boggio PS, Fregni F, Bermpohl F, et al. Effect of repetitive TMS and fluoxetine on cognitive function in patients with Parkinson's disease and concurrent depression. Mov Disord. 2005;20(9):1178–1184.
- Randver R. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex to alleviate depression and cognitive impairment associated with Parkinson's disease: a review and clinical implications. J Neurol Sci. 2018;393:88–99.
- Khedr EM, Mohamed KO, Ali AM, et al. The effect of repetitive transcranial magnetic stimulation on cognitive impairment in Parkinson's disease with dementia: pilot study. Restor Neurol Neurosci. 2020;38(1):55–66.
- Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184.
- Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4(6):561–571.
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–427.
- Fahn S, Elton R, Members of the UPDRS development committee. Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne DB, editors. Recent developments in Parkinson’s disease. Florham Park (NJ): Macmillan Health Care Information; 1987. p. 153–163.
- Ridgel AL, Peacock CA, Fickes EJ, et al. Active-assisted cycling improves tremor and bradykinesia in Parkinson's disease. Arch Phys Med Rehabil. 2012;93(11):2049–2054.
- MacDonald PA, MacDonald AA, Seergobin KN, et al. The effect of dopamine therapy on ventral and dorsal striatum-mediated cognition in Parkinson's disease: support from functional MRI. Brain. 2011;134(Pt 5):1447–1463.
- Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Motor Skills. 1958;8(3):271–276.
- Garrido-Vásquez P, Pell MD, Paulmann S, et al. Impaired neural processing of dynamic faces in left-onset Parkinson's disease. Neuropsychologia. 2016;82:123–133.
- Wechsler DW. Adult intelligence scale-III. New York (NY): Psychological Corporation; 1997.
- Nelson HE. A modified card sorting test sensitive to frontal lobe defects. Cortex. 1976;12(4):313–324.
- Parker SM, Erin JR, Pryor RR, et al. The effect of prolonged light intensity exercise in the heat on executive function. Wilderness Environ Med. 2013;24(3):203–210.
- Stroop JR. Studies of interference in serial verbal reactions (reprinted from Journal of Experimental Psychology, Vol 18, 643–662, 1935). J Exp Psychol. 1992;121(1):15–23.
- Wang C, An Y, Yu H, et al. Association between exposure to the Chinese famine in different stages of early life and decline in cognitive functioning in adulthood. Front Behav Neurosci. 2016;10:146.
- Kudlicka A, Clare L, Hindle JV. Executive functions in Parkinson's disease: systematic review and meta-analysis. Mov Disord. 2011;26(13):2305–2315.
- Hurtado MM, Triviño M, Arnedo M, et al. Are executive functions related to emotional intelligence? A correlational study in schizophrenia and borderline personality disorder. Psychiatry Res. 2016;246:84–88.
- Li S, Ou R, Yuan X, et al. Executive dysfunctions and behavioral changes in early drug-naïve patients with Parkinson's disease. J Affect Disord. 2019;243:525–530.
- Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson's disease: a review. J Neuropsychol. 2013;7(2):193–224.
- Stuss DT, Alexander MP. Executive functions and the frontal lobes: a conceptual view. Psychol Res. 2000;63(3–4):289–298.
- Furukawa T, Izumi S, Toyokura M, et al. Effects of low-frequency repetitive transcranial magnetic stimulation in Parkinson's disease. Tokai J Exp Clin Med. 2009;34(3):63–71.
- Yoshii F, Onaka H, Kohara S, et al. Early detection of cognitive impairment in Parkinson's disease with the use of the Wisconsin Card Sorting Test: correlations with Montreal Cognitive Assessment and Smell Identification Test. J Neural Transm. 2019;126(11):1447–1454.
- Gardizi E, King JP, McNeely HE, et al. Comparability of the WCST and WCST-64 in the assessment of first-episode psychosis. Psychol Assess. 2019;31(2):271–276.
- Lange F, Brückner C, Knebel A, et al. Executive dysfunction in Parkinson's disease: a meta-analysis on the Wisconsin Card Sorting Test literature. Neurosci Biobehav Rev. 2018;93:38–56.
- Chiu EC, Wu WC, Hung JW, et al. Validity of the Wisconsin Card Sorting Test in patients with stroke. Disabil Rehabil. 2018;40(16):1967–1971.
- Huang ML, Luo BY, Hu JB, et al. Repetitive transcranial magnetic stimulation in combination with citalopram in young patients with first-episode major depressive disorder: a double-blind, randomized, sham-controlled trial. Aust N Z J Psychiatry. 2012;46(3):257–264.
- Matsui H, Nishinaka K, Oda M, et al. Wisconsin Card Sorting Test and brain perfusion imaging in Parkinson's disease. Parkinsonism Relat Disord. 2006;12(5):273–278.
- Elliott R. Executive functions and their disorders. Br Med Bull. 2003;65(1):49–59.
- Friedman NP, Miyake A. Unity and diversity of executive functions: individual differences as a window on cognitive structure. Cortex. 2017;86:186–204.
- Williams JMG, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychol Bull. 1996;120(1):3–24.
- Ozonoff S, Pennington BF, Rogers SJ. Executive function deficits in high-functioning autistic individuals: relationship to theory of mind. J Child Psychol Psychiatry. 1991;32(7):1081–1105.
- Logue SF, Gould TJ. The neural and genetic basis of executive function: attention, cognitive flexibility, and response inhibition. Pharmacol Biochem Behav. 2014;123:45–54.
- Leroi I, Barraclough M, McKie S, et al. Dopaminergic influences on executive function and impulsive behaviour in impulse control disorders in Parkinson's disease. J Neuropsychol. 2013;7(2):306–325.
- Redgrave P, Rodriguez M, Smith Y, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nat Rev Neurosci. 2010;11(11):760–772.
- Hazy TE, Frank MJ, O'Reilly RC. Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philos Trans R Soc Lond B Biol Sci. 2007;362(1485):1601–1613.
- Seminowicz DA, de Martino E, Schabrun SM, et al. Left dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation reduces the development of long-term muscle pain. Pain. 2018;159(12):2486–2492.
- Egan MF, Goldberg TE, Kolachana BS, et al. Effect of COMT Val108/158 met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98(12):6917–6922.
- Jiang Y, Guo Z, McClure MA, et al. Effect of rTMS on Parkinson's cognitive function: a systematic review and meta-analysis. BMC Neurol. 2020;20(1):377.
- Málly J, Geisz N, Dinya E. Follow up study: the influence of rTMS with high and low frequency stimulation on motor and executive function in Parkinson’s disease. Brain Res Bull. 2017;135:98–104.
- Aleman A, van't Wout M. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex disrupts digit span task performance. Neuropsychobiology. 2008;57(1–2):44–48.
- Abbasnia K, Ghanbari A, Abedian M, et al. The effects of repetitive transcranial magnetic stimulation on proliferation and differentiation of neural stem cells. Anat Cell Biol. 2015;48(2):104–113.
- Pauletti C, Mannarelli D, Locuratolo N, et al. Attention in Parkinson's disease with fatigue: evidence from the attention network test. J Neural Transm. 2017;124(3):335–345.
- Posner MI. Imaging attention networks. Neuroimage. 2012;61(2):450–456.
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302(5648):1181–1185.
- Esser SK, Huber R, Massimini M, et al. A direct demonstration of cortical LTP in humans: a combined TMS/EEG study. Brain Res Bull. 2006;69(1):86–94.
- González-García N, Armony JL, Soto J, et al. Effects of rTMS on Parkinson's disease: a longitudinal fMRI study. J Neurol. 2011;258(7):1268–1280.
