Abstract
Purpose
International multi-professional expert consensus was sought to develop best practice recommendations for clinical management of patients following cervical spinal cord injury with oropharyngeal dysphagia and associated complications. Additionally, risk factors for dysphagia were identified to support the development of a screening tool.
Materials and Methods
A two-round Delphi study was undertaken with a 27-member panel of expert professionals in cervical spinal cord injury and complex dysphagia. They rated 85 statements across seven topic areas in round one, using a five-point Likert scale with a consensus set at 70%. Statements not achieving consensus were revised for the second round. Comparative group and individual feedback were provided at the end of each round.
Results
Consensus was achieved for 50 (59%) statements in round one and a further 12 (48%) statements in round two. Recommendations for best practice were agreed for management of swallowing, respiratory function, communication, nutrition and oral care. Twelve risk factors for dysphagia were identified for components of a screening tool.
Conclusions
Best practice recommendations support wider clinical management to prevent complications and direct specialist care. Screening for risk factors allows early dysphagia identification with the potential to improve clinical outcomes. Further evaluation of the impact of these recommendations is needed.
Dysphagia is an added complication following cervical spinal cord injury (cSCI) affecting morbidity, mortality and quality of life.
Early identification of dysphagia risk allows focused interventions that reduce associated nutritional and respiratory impairments.
Best practice recommendations based on expert consensus provide a baseline of appropriate interventions, in the absence of empirical evidence.
A multi-professional approach to rehabilitation encourages a consistent and coordinated approach to care across acute and rehabilitation settings.
Implications for Rehabilitation
Introduction
The prevalence of spinal cord injury (SCI) across the world is estimated to be 1:1000 [Citation1] with a recent increase in the number of injuries to the cervical spinal cord resulting in tetraplegia and requiring ventilator support [Citation2,Citation3]. With good trauma care, survival has improved globally especially in the post-acute phase [Citation4]. An ageing demographic with additional co-morbidities increases the complexity of clinical needs [Citation3]. Individuals with tetraplegia have an increased risk of mortality associated with respiratory complications especially pneumonia [Citation5,Citation6]. Access to specialist spinal care is not always universal or timely, for example in the UK 60% of SCI do not access specialist provision [Citation7]. Those with high cervical level injuries are more likely to need a tracheostomy and acute respiratory support, which itself has been strongly associated with dysphagia [Citation8,Citation9]. The trauma care pathway in the UK directs primary admission to a major trauma centre to stabilise the injury and cardiorespiratory function before transfer to a specialised Spinal Injury Unit (SIU) [Citation10]. Limited SIU bed capacity for those requiring ongoing respiratory support means that transfer is often delayed for months [Citation11] with individuals being admitted to non-specialised units whilst awaiting SIU admission [Citation12]. Variations in practice have been identified in the acute and rehabilitation management of those with SCI in non-specialised units [Citation13], with better outcomes reported for patients admitted early to specialised Spinal Injury Unit (SIU), as they benefit from specialist multi-disciplinary interventions and discharge planning [Citation14,Citation15]. The lifetime cost of SCI care in the UK reports expected costs for those with tetraplegia (ASIA Impairment Scale A, B and C [Citation16]) to be £1.87milion compared to £0.47 million for those with a grade D [Citation17]. This excludes additional costs due to complications and hospital re-admissions that may occur with dysphagia. By reducing the risk of complications for those ventilated with cSCI, there are likely to be personal and economic healthcare benefits.
Oropharyngeal dysphagia has been recognised as a complication in Cervical Spinal Cord Injury (cSCI), with a reported incidence of between 8 and 80% [Citation18–20], and associated increased risks of pneumonia, morbidity and mortality [Citation21]. The pathophysiology is not clearly understood but studies report the involvement of multiple factors including neurological, mechanical and respiratory disruption [Citation21]. Commonly reported dysphagia characteristics are aspiration, reduced or absent hyolaryngeal elevation, reduced or absent pharyngeal movement and pharyngeal residue as identified using instrumental assessment [Citation22]. Despite identifying contributing factors such as the presence of a tracheostomy, surgery, gender, age, level and severity of injury [Citation9,Citation20,Citation21], no standard protocols or care pathways are in place to reduce the impact of dysphagia.
Studies reporting on dysphagia are often retrospective and vary in their definition, methods of evaluation and often report small cohorts in specialist rehabilitation settings, making it difficult to generalise practice in the acute setting. Clinically dysphagia in cSCI is poorly identified due to the high incidence of silent aspiration and poor airway protection which obscures motor signs of coughing, which are traditionally associated with dysphagia [Citation23]. The first symptomatic signs are chest infection, pyrexia or pneumonitis [Citation24]. Associated with dysphagia are a number of secondary clinical complications such as reduced nutritional intake [Citation25] and increased need for respiratory interventions including ventilation via tracheostomy [Citation26]. This can have an impact on oral hygiene due to secretion-drying medication and the need for oxygen [Citation27], as well as limiting the ability to communicate because of closed-circuit ventilation. These challenges have been reported by people with cSCI during their care [Citation28] and highlight the importance of staff having specialist clinical knowledge and skills to recognise and manage these issues.
As with other neurological conditions that impair swallowing function, such as stroke, the best practice aims to identify risks for oropharyngeal dysphagia in advance of developing clinical signs and symptoms to prevent additional complications [Citation29,Citation30] and reduce the need for additional interventions that prolong the length of stay [Citation31]. The management of oropharyngeal dysphagia is an area of specialism for speech and language therapists, however, the small workforce means that they rely on referrals from the team based on information from a swallow screening process undertaken by nurses and other healthcare professionals [Citation32]. Currently, there are no agreed screening tools or standards of care for the management of oropharyngeal dysphagia in cSCI patients and associated clinical issues.
In the absence of empirical evidence, an expert consensus has been employed to establish guidance for care in previous SCI studies. These have helped to set goals and best practice guidance in other clinical areas, such as testing for balance [Citation33], an ICF core set [Citation34], nursing intervention goals [Citation35], and management of acute thoracolumbar spinal cord injury [Citation36]. Due to the multidisciplinary team involved in the acute care of individuals with cSCI and dysphagia, a multi-professional expert panel was required to enable findings to translate into clinical practice. This study uses the CREDES reporting guidelines [Citation37].
The primary aim of the study was to gather expert consensus on best practices for the acute clinical management of cSCI patients with dysphagia and associated complications. A secondary aim was to identify risk factors for dysphagia that could be developed as a screening tool for early identification of swallowing difficulties.
Materials and methods
Study design
A Delphi study requires the recruitment of an expert panel, development of consensus statements and a rating scheme using a system that allows anonymity of group members to encourage free opinion. An online portal was used to coordinate all Delphi invitations and responses, which ensured the anonymity of participant responses. Qualitative and quantitative controlled feedback was provided to all participants at the end of each round, to provide an overview of the group’s opinion compared to their own. A multi-professional steering group was set up to provide oversight of the process and ensure rigour in evaluating the responses.
Expert panel
Participants from five professional groups were recruited for the expert panel through a direct invitation to international clinical experts in the field of SCI identified through publications and professional networks. The professions were chosen to represent multi-disciplinary teams, specifically doctors, nurses, physiotherapists (PT), dietitians and speech and language therapists (SLT). The criteria for inclusion as an expert necessitated at least 3 years’ experience working with acute SCI patients and managing complex dysphagia in this population. Consent to participate was provided through the online system for those who fulfilled the criteria.
Statement development
Statements were developed following a review of the literature on clinical factors linked to oropharyngeal dysphagia in cSCI. MeSh search terms were used to generate papers through PubMed (Supplemental Data 1), specifically on dysphagia identification and management, nutritional management, respiratory impairment, mouth care and associated clinical complications in acute SCI. A total of seven topic areas were generated related to the clinical pathway of dysphagia care post-SCI, namely co-morbid status, the definition of dysphagia, screening for dysphagia, assessment, identification, management and therapeutic intervention. Each topic had further subcategories resulting in a total of 90 statements for inclusion in the Delphi questionnaire. The steering group reviewed the statements for content and construct and merged similar items reducing the questionnaire to 85 statements ().
Table 1. Topic areas and sub-categories with statement selection by the steering group.
In preparation for the Delphi, each statement had a similar sentence structure to aid interpretation and limit bias (Supplemental Data 2). A five-point Likert scale was used to rank each statement ranging from ‘disagree strongly’, ‘disagree’, ‘neutral’, ‘agree’ and ‘agree strongly’. A free-text box allowed any additional comments to be added for each statement.
Steering group
A steering group, made up of five healthcare professionals working in an acute SCI, was established to ensure content validity and reduce bias. The steering group was provided with details of their role, estimated time scale, and frequency of meetings, which would take place at the start and end of each round. The steering group included an SLT, two doctors, a PT and a specialist nurse, with information shared by email and face-to-face discussions. Before the Delphi commenced, the steering group reviewed all statements online indicating their individual recommendation to keep, discard or rephrase each statement and adding free-text comments. Each of the responses was anonymised and statements were selected based on majority agreement. Any statements with equal votes were reviewed and modified using the comments. Members of the steering group were also invited to participate as expert participants, allowing them to submit their own professional opinion on each statement. This differed from their role in the steering group and as there were no correct or incorrect answers and all responses were anonymised and unseen by other participants.
Ethics
Ethics approval was granted by the Royal National Orthopaedic Hospital NHS Trust’s Research and Development Committee (R&D ID: 15.016) with adherence to the Trust and Research Governance Framework.
Data analysis
The level of consensus was set at ≥70% for a cumulative score for ‘disagree strongly’ and ‘disagree’ rankings or ‘agree’ and ‘agree strongly’ rankings. Any statements with less than 70% consensus or over 50% neutral rankings were reviewed with the steering group alongside the free-text comments and considered for rephrasing or discarding prior to the next round. Due to the heterogeneous membership of the expert panel, statements were deemed to have majority agreement if they were ranked by 55% to 70% of the participants [Citation38].
Results from each round were analysed through SPSS Version 22 generating descriptive statistics to show percentage agreement, mean, median and mode of rankings and variance per statement for each round [Citation39]. Statements that gained less than 55% ranking were analysed in terms of measures of central tendency and level of dispersion to identify any change in voting between rounds one and two. The median score demonstrated the group opinion and the inter-quartile range (IQR) showed the spread of opinion. Qualitative comments were collated for each statement to qualify responses. The decision to end the Delphi after two rounds were reached alongside the steering group when no further changes in rankings were generated for statements not achieving consensus, so further changes were unlikely to be elicited in another round [Citation40].
Data collection
Each expert panel member was asked to complete the questionnaires via a dedicated online portal, which collated individual responses under a personal log-in. The completion deadline was three weeks, with email reminders sent after 10 days. If the survey was not completed by the deadline, individuals were contacted directly by email to check if there were technical issues that restricted their participation. Where necessary, technical support was provided and the deadline extended by a few days to ensure participation.
At the end of each round, participants were sent feedback on their responses with quantitative and qualitative data comparing it to the group response per statement with free-text comments. For the first round, participants were asked to rank 85 statements, grouped under topic headings, using the Likert scale with the option of adding free-text comments.
For the second round, participants ranked the revised statements, which were presented in randomised order to reduce selection bias and ensure judgements were independent of the first-round sequence of statements.
The Delphi was ended after the second round and a report was sent to participants, with a summary of the items achieving consensus for dysphagia risk factors and best practice recommendations for the management of dysphagia and associated complications. A summary of the Delphi study flow chart can be seen in .
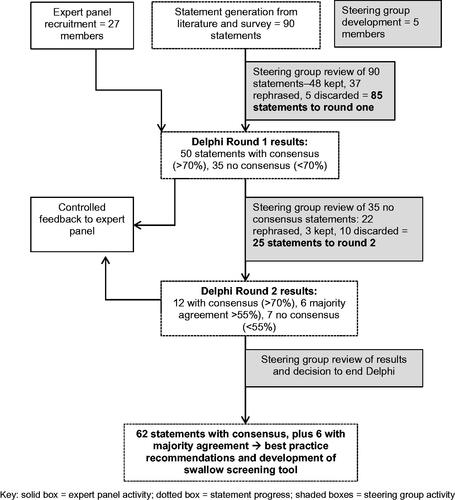
Figure 1. caption: Delphi study flow chart.
A flow chart explains the process of the Delphi study. An expert panel of 27 members was recruited, who participated in two rounds of the Delphi with controlled feedback after each round. A steering group of five members reviewed the results of each process. Ninety statements were initially generated and reviewed by the steering group and modified to 85 statements. These were distributed to the expert panel for round one. At the end of round, one 50 statements had consensus and 35 had no consensus. After steering group review 25 were submitted for round two. At the end of round two, a further 12 had consensus, six had majority agreement and seven had no consensus. After steering group review a decision was made to end the Delphi process. A total of 62 statements achieved consensus and six had majority agreement. These results formed the best practice recommendations and development of a swallow screening tool.
Results
Expert panel participant demographics
Email invitations with details of the study were sent to 55 healthcare practitioners identified as working in spinal injury units based in English-speaking countries, namely the UK, Australia, New Zealand, USA, Canada and members of the UK Respiratory SCI network (RISCI). Thirty-nine people expressed interest in being involved, eight did not meet the inclusion criteria leaving 31 participants who were invited to participate in a Delphi study that may include up to four rounds of consensus. Consent and demographic details were received from 27 participants with representation from each of the five professional groups ().
Table 2. Professionals invited and consented as expert panellists.
Participants reported a high level of clinical experience working with SCI patients, primarily gained in SIU (88.9%) and ICU (81.5%) with less than half reporting experience from working in Major Trauma Centres (44.4%) (). Overall, members of the expert panel had an average of 21.3 working years, (range 6–42 years) with an average of 13.2 years specifically in SCI (range 3–29 years). Over three-quarters of the group were female (77.8%). In terms of geographical representation most participants were based in the UK (63.0%) including six doctors, seven PTs, two nurses and two SLTs; six (22.2%) participants were from Australia including two SLTs, two dietitians, one PT and one nurse; two SLTs (7.4%) were from New Zealand and the USA and Ireland each had representation from an SLT.
Table 3. Demographic details of expert panel participants.
Delphi first-round results
There was a 100% participant response rate in the first round, with 50 (58.8%) out of 85 statements achieving 70% or more consensus, and 35 (41.1%) statements did not achieve this threshold of consensus (). Levels of consensus varied for each topic area ().
Figure 2. caption: Number of statements per topic achieving consensus in Delphi round one.
A bar graph shows the results of the first Delphi round with the numbers of statements per topic with and without consensus. ‘Co-morbid status’ has seven statements with consensus and five with no consensus, ‘definition’ has nine with consensus and six with no consensus, ‘screening’ has eight with consensus and nine with no consensus, ‘assessment’ has two with consensus and three with no consensus, ‘identification’ has four with consensus and two with no consensus, ‘management’ has 13 with consensus and 10 with no consensus and ‘therapeutic management’ has seven with consensus and none with no consensus.
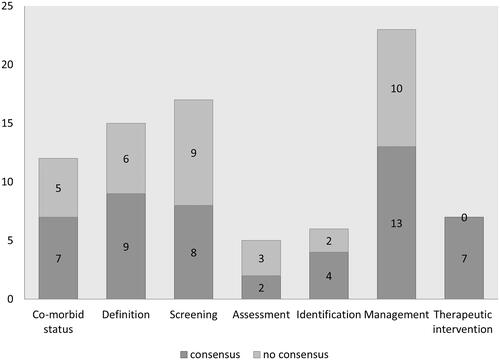
Table 4. Descriptive statistics and levels of consensus for round one statements.
Co-morbid status (12 statements)
Seven (58%) statements achieved consensus on being contributing factors for dysphagia, which included upper and lower cervical spinal injury, brain injury, cognitive impairment, anterior cervical spine surgery. There was no consensus on the impact of age, posterior spinal surgery, thoracic level injury and severity of the injury.
Definition of dysphagia (15 statements)
Nine (60%) statements achieved consensus on the defining features of dysphagia included wet sounding voice, aspiration, impaired laryngeal movement, impaired laryngeal sensation, ineffective cough and absent cough. Two statements had consensus to disagree that lip and facial weakness were features of dysphagia in cSCI.
Screening for risk of dysphagia (17 statements)
Eight (47.1%) statements achieved consensus. Statements on the presence of a tracheostomy tube, need for invasive ventilation and prolonged intubation having an inflated tracheostomy cuff, issues with saliva management and respiratory function achieved consensus of agreement. Evaluation of laryngeal elevation and sensation achieved consensus as screening methods whilst voice, oromotor and respiratory measures did not. The use of swallow trials with blue dye, water and yoghurt did not gain consensus and were rephrased for round 2.
Assessment (five statements)
Only two (40%) statements achieved consensus of disagreement that clinical bedside assessment and signs of aspiration are the methods of assessing for the presence of dysphagia. None of the gold standard instrumental assessments achieved consensus and these statements were rephrased for round 2.
Identification (six statements)
Four statements (66.7%) achieved consensus on the clinical signs of dysphagia, which included the food or fluid appearing on suction, chest infection, increased need for oral suction and spiking pyrexia. Nutritional measures achieved a high neutral ranking and were rephrased for the second round.
Management (23 statements)
Thirteen (56.5%) statements on clinical management achieved consensus. The use of nasogastric (NG) feeding gained unanimous agreement, whilst Percutaneous Endoscopic Gastrostomy (PEG) feeding was supported for longer-term nutritional needs as was remaining nil by mouth (NBM) to resolve a paralytic ileus. There was consensus for disagreeing with the statements on restricted oral intake when ventilated and when positioned in upright with consensus for semi-recumbent positioning rather than supine. Mouthcare issues achieved consensus on the risk of VAP due to supine positioning as did dry mouth from polypharmacy that benefits from moisturisation, however, the use of regular sips of water did not attract consensus. There was agreement that patients should be enabled to communicate using their own voice rather than communication aids.
Therapeutic interventions (seven statements)
All seven (100%) statements about therapeutic interventions achieved consensus. There was a unanimous disagreement that dysphagia is unchanging in cSCI and high disagreement that patients should remain nil by mouth and with tracheostomy cuff inflated to prevent aspiration. There was a high consensus for daily therapy interventions to facilitate a return to oral intake, support verbal communication and self-ventilation.
At the end of round one, feedback was sent to each participant with a summary of the results and the group response to each statement compared to their own responses. In preparation for round two, the 35 statements that did not achieve consensus were reviewed by the steering group alongside free text comments that had been submitted. Twenty-two statements were rephrased, ten were either discarded or merged with another statement if they had similar themes; only three statements were re-submitted for the second round unchanged. This resulted in a total of 25 statements submitted for round two.
Delphi second round
In the second round, there was a 96% response rate from the expert panel. Twelve (48%) statements achieved 70% or more consensus, with a further six (24%) gaining majority agreement of 55% or over and seven (28%) not achieving any level of consensus (). Each topic area achieved further consensus ().
Figure 3. caption: Number of statements per topic achieving consensus, majority agreement in Delphi round two.
A bar graph shows the results of the second Delphi round with the numbers of statements per topic with consensus, majority agreement or no consensus. ‘Co-morbid status’ has two statements with consensus, one with majority agreement and two with no consensus, ‘definition’ has four statements with consensus, none with majority agreement and two with no consensus, ‘screening’ has two with consensus, two with majority agreement and none with no consensus, ‘assessment’ has one with consensus, one with majority agreement and none with no consensus, ‘identification’ has none with consensus and one with no consensus, ‘management’ has three with consensus, two with majority agreement and two with no consensus and ‘therapeutic management’ has no statements in this round.
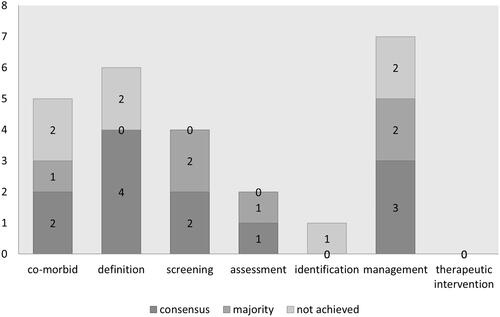
Table 5. Descriptive statistics for round 2 statements.
Co-Morbid status (five statements)
Two (40%) statements achieved consensus, supporting the need for a swallow assessment for anyone with a complete or incomplete level of injury. There was majority agreement on the possible impact of posterior cervical spine surgery on swallowing function. Advanced age and thoracic level injury did not gain consensus on being factors for dysphagia.
Definition (six statements)
Four (66.7%) statements achieved consensus, with a weak voice, coughing on oral intake, food leaking from tracheostomy and delayed swallowing being features of dysphagia. Neither tongue or velopharyngeal weakness was supported as typical features.
Screening (four statements)
Two (50%) statements achieved consensus for screening, with oral trials being useful to evaluate swallowing disruption and that non-invasive ventilation may be disruptive. Statements detailing the use of oromotor assessment and monitoring deteriorating forced vital capacity (FVC) gained majority agreement.
Assessment (two statements)
One (50%) statement achieved consensus for assessment of the patient in an upright position, where possible, whilst the preferred use of Fibreoptic Endoscopic Evaluation of Swallowing (FEES) for evaluating dysphagia achieved majority agreement.
Identification (one statements)
The use of serum albumin did not achieve any consensus on the basis that this measure was not a reliable indicator of dysphagia or associated malnutrition.
Management (seven statements)
Three (42.9%) statements achieved consensus with the agreement of the importance of oral hygiene to reduce VAP and that coughing at meals was suggestive of dysphagia and disagreement that patients should be allowed to commence oral intake prior to having their swallow assessed. Utilising cuff deflation for eating and allowing position at mealtimes to be adjusted both achieved majority agreement. The use of thickened fluids and artificial saliva to relieve dry mouth did not achieve consensus.
Non-consensus statements
For those 13 statements that did not achieve a group consensus of ≥ 70%, an analysis of descriptive statistics identified a change in rankings between round one and round two (). A move towards a consensus score of 4 (agree) or 2 (disagree) was seen for all statements in the mean, median and mode of ranking, except for serum albumin, which remained neutral. The mode shows a shift of two levels from disagree to agree for tongue weakness and the use of FVC, whilst tracheostomy showed strong agreement in the mode but a mean that was just below agreement. These variations are likely to be due to the heterogenous panel which had different levels of representation.
Table 6. Descriptive statistics for change in non-consensus statements between round 1 and round 2.
As no further change in consensus was achievable with a further round, the Delphi was ended after round two and a summary report was sent to the participants. A total of 62 (72.9%) of the 85 statements achieved consensus and a further six had majority agreement. The statements with consensus were utilised to develop best practice recommendations and a checklist for screening for dysphagia risk.
Best practice recommendations
This Delphi study generated consensus on best practice recommendations for the management of secondary complications in cSCI () and risk factors for dysphagia (). The best practice recommendations have been applied to six key clinical areas – swallowing, respiratory function, communication, nutrition, and oral care to provide a clear outline for healthcare professionals who may be working together to manage one or more of these areas.
Table 7. Best practice recommendations for acute cSCI management of dysphagia and associated complications.
Table 8. Domains, category and sub-category of swallow risk screening tool.
The swallow risk screening tool that was generated from the Delphi items, is divided into three domains (). The first two domains list risks that occur as a direct result of the injury or are associated with the clinical interventions required to manage acute symptoms, such as ventilation. The third domain identifies the acute symptoms that may be associated with or contribute to dysphagia and require urgency to alter clinical management. The presence of an injury or clinical risk factor indicates a risk for dysphagia and a referral to a Speech and Language Therapist is recommended prior to any oral trials, to minimise silent aspiration and development of pneumonia. If symptoms of urgency are evident, the team are strongly encouraged to review current clinical management, identify the cause and engage with SLT guidance. The utility of the swallow risk screening tool was evaluated in a two-site feasibility study and is reported separately.
Discussion
This Delphi study aimed to achieve consensus from a multi-professional group of experts working in the field of cSCI, to provide a co-ordinated multidisciplinary approach to the management of dysphagia. Several areas of contention or uncertainty were resolved by this process of consensus helping to provide recommendations for best practices to support a more consistent approach to clinical management. Despite the variations in professional role and geography, few topic areas remained without consensus, which helped to highlight that much of our clinical practice is based on experience rather than robust evidence. It is hoped that this set of recommendations engages all members of the team and forms a benchmark against the wish to define the clinical needs of cSCI patients through further data gathering and research. Each of the five key areas of clinical management will be discussed separately.
Swallowing
There was little agreement amongst the expert panel on the mechanisms of screening and assessment of dysphagia, suggesting that current tools may be inadequate or inappropriate for this. The group was able to identify specific factors that were likely to contribute to dysphagia and may help to provide early identification of modifiable or fixed factors. Currently, there is a reliance on standard dysphagia assessments, which are developed for other aetiologies and rely on intact laryngeal motor and sensory functions. Posillico, Golob [Citation41] proposed a screening tool for evaluating dysphagia in those with traumatic cervical injuries, that is based on the Yale swallow protocol [Citation42]. This is a 2-stage nurse assessment, whereby in the first stage, a brief cognitive assessment is undertaken followed by a review of any signs of cranial nerve impairment such as slurred speech or facial asymmetry. In the second stage, a patient is given 3 oz of water to drink whilst monitoring for any signs of coughing, voice change or swallowing difficulty. If signs of neurological impairment or overt swallowing difficulties are evident, a patient would be kept nil by mouth and await a videofluoroscopy assessment by SLT. Unfortunately, the validation of this protocol excluded those who are intubated or critically ill with the authors suggesting that they remain nil by mouth and tube-fed [Citation41]. This fails to support those with SCI who remains in intensive care, who have intact swallowing function and may be able to commence oral intake. This approach to screening also fails to identify the nature of the swallowing impairment or contributing factor to direct the selection of a suitable intervention. Cranial nerve impairments are not a common feature of cSCI, yet silent aspiration is [Citation19] and this method of screening would not be sensitive to detect this. Instead, the use of a checklist of known risk factors, as agreed by the expert panel, would allow a consistent approach to screening for risks of dysphagia in cSCI patients. This would focus on presenting symptoms without compromising the patient’s status with oral trials of food or fluids. If risk factors are identified, patients who require dysphagia assessments will be referred onto SLT services. To ensure validity and predictive value, the screening tool requires further validation against current methods of assessment.
Once the risk of dysphagia is identified, it is important that appropriate interventions are delivered to minimise or reduce the risk of complications. The routine use of thickened fluids, blue dye and yoghurt has been reported by healthcare staff in a survey of clinical practice with cSCI patients [Citation12]. The expert panel provided consensus against routine use of these textures in recognition of the challenge for pharyngeal clearance, known to be a specific component of dysphagia impairment in cSCI patients [Citation21,Citation43]. By supporting the use of instrumental assessment in the identification of dysphagia, more suitable interventions can be developed to improve dysphagia management and outcomes in line with other acquired conditions [Citation44].
Respiratory
The optimal management of respiratory impairment is vital in cSCI patients who often have a little reserve. In spinal units in the UK, respiratory weaning of cSCI follows a step-wise approach to ventilator-free breathing, developed as a national protocol by expert clinicians [Citation45]. In contrast, less than 5% of staff in non-specialised units reported using this national weaning protocol [Citation12]. With evidence that cSCI patients with ventilatory needs, experience prolonged stays in non-specialised units [Citation7,Citation46], the use of a patient-specific protocol ensures consistency in care and an effective method of weaning from ventilation. The expert panel supported the use of respiratory measurements, such as vital capacity, as outcome measures to indicate respiratory muscle fatigue which help to modify the weaning programme.
The practice of tracheostomy cuff deflation can be contentious in terms of balancing risk and benefit [Citation47,Citation48]. The expert panel agreed that it was beneficial to facilitate both speech and swallowing function in cSCI patients and should be a rehabilitation goal. These help to provide clinicians with direction to establish suitable interventions alongside existing cSCI respiratory management techniques [Citation49,Citation50]. By improving respiratory and laryngeal function, will enhance patients’ quality of life during their acute care, which is of great importance to them and their families [Citation28,Citation51].
Communication
The expert panel agreed that early options for communication were important to enable greater patient involvement in care decisions. Limited patient communication in the ICU, especially whilst being ventilated, has a huge detrimental impact on engagement with staff and one’s own treatment [Citation52,Citation53]. This is a key area of frustration for cSCI patients during their acute care who report ongoing issues with staff attitude when they are speechless [Citation28]. Although a number of alternative communication aids are increasingly available, such as picture charts, alphabet boards or electronic devices [Citation54,Citation55], these rely on intact upper limb movement, which makes them inaccessible for those with upper limb paralysis as experienced by those with cSCI. Speech is seen as the preferred option for direct quick and effective communication. For ventilated patients, this can be achieved through manipulation of the tracheostomy and ventilator, although requires a team approach with specialist clinical knowledge, skills and close monitoring to ensure safety [Citation56,Citation57]. For those who are unable to achieve speech, they should have access to a range of communication aids and individual assessments for suitability by the team.
Nutrition
The risk of malnutrition in SCI has been identified in the specialist literature [Citation58–60], although this is not always linked to dysphagia. Nutritional requirements of cSCI are often high post-injury and the challenges of gastric absorption, altered taste and appetite as well as inability to feed oneself affect levels of intake. High incidences of malnutrition have been reported in those transferring to specialist spinal injury units [Citation25] with a need for nutritional supplementation to address these issues [Citation61].
The expert panel identified the detrimental consequences of being nil by mouth and reduced oral intake due to dysphagia, especially during the acute recovery phase. There was unanimous agreement that nutritional support via NG feeding should be provided for any cSCI patient with dysphagia to ensure consistent nutrition. For those needing ongoing nutritional support, staff should consider longer-term tube placement, which staff in ICU are more reluctant to undertake [Citation12]. These recommendations will help to support recovery and participation in rehabilitation.
Mouthcare
Poor oral care and the experience of dry mouth add to patient discomfort with impact on eating, swallowing and speaking [Citation28,Citation62]. Causes are linked to the side effects of medication and ventilation that are routinely required in ICU [Citation63] as well as autonomic dysfunction seen in SCI [Citation27]. For individuals with cSCI in acute care, this is a significantly negative experience, especially as care is dependent on others to provide relief [Citation28]. Long-term issues with oral hygiene and dental health in those with SCI cause pain and have an impact on quality of life with an increased need for dental treatment [Citation64,Citation65]. Preventative measures and education are important to reduce this, whilst expert consensus supported twice-daily oral care as routine. The use of artificial saliva to relieve dry mouth is often used for long-term conditions such as Sjorgen’s Syndrome [Citation66] and Head and Neck radiotherapy [Citation67] but did not attract consensus by the expert panel. This may be because these products are not universally available or currently used in acute settings. Further research into the contribution of artificial saliva for those experiencing dry mouth due to polypharmacy would be beneficial as these offer simple options for self-management to bring relief.
Despite the availability of specialised spinal injury units in the UK, access for those with high-level CSCI requiring ventilatory support is restricted due to limited bed capacity [Citation7,Citation11]. As a result, acute CSCI patients remain in non-specialised units for ongoing care, with staff reporting variable knowledge and skills in the management of cSCI patients with dysphagia and respiratory dysfunction [Citation12]. This is known to affect clinical outcomes [Citation14,Citation68,Citation69] and affect the experiences of those surviving this devastating injury [Citation28,Citation70]. To overcome this variation, all healthcare staff would benefit from training on delivering specialist SCI care alongside the use of best practice recommendations for the clinical management of cSCI patients, as proposed in this study.
Limitations and strengths
There are a number of limitations to the study, that are worth considering. Using a heterogenous group of professionals may have led to reduced consensus for some items, due to varying roles and practices, however, it was considered of greater value to align the study to the reality of clinical practice, which has multi-professional stakeholders involved in the management of patients with cSCI and dysphagia. It is recognised that in some countries Occupational Therapists are involved in the assessment and management of dysphagia and feeding and were not included in this study. This should be a consideration for future research.
Setting the level of consensus at 70% was intended to reflect this heterogenous group, although in the second round, statements that achieved over 55% but not 70% consensus, were considered to have majority agreement. These may have reflected the opinions of a single professional group and would be of significance to warrant further exploration at a later stage. Recruiting only English-speaking representatives on the expert panel is a limitation of this study and it would be of great value to extend this work to non-English speaking countries to establish an international focus on best practices.
There is a risk of bias in the results of a Delphi study which are overseen by the lead researcher. To reduce bias in the evaluation of responses, a steering group was recruited who oversaw the results from each round and approved any actions. Similarly, to limit researcher bias, all consensus data was collected through an online site, which then generated individualised feedback to each participant without modification by the researcher.
A strength of the study was the value in gathering details of clinical practices in different countries that were very similar, which may reflect the lack of specific acute cSCI guidance. This provides an opportunity to share these best practice recommendations with international colleagues and establish a network of experts for future research studies. A further strength is the representation and involvement of multiple professions to demonstrate that clinical decision-making needs to be made as a part of a team as dysphagia intersects multiple other systems and functions such as respiratory and nutrition.
The acute management of acute cSCI patients is known to be complex for multi-disciplinary teams. There is clinical value in early screening for dysphagia risk and coordinating dysphagia care to reduce associated complications linked to respiratory function, nutrition and the ability to communicate. In the absence of evidence, an international multi-professional expert panel achieved consensus on 73% of statements to generate best practice recommendations in these areas. These aim to support staff to deliver cSCI specific interventions and improve clinical outcomes. Further research is needed to evaluate the contribution of these recommendations in settings caring for acute cSCI patients. A number of statements generated for the Delphi study () could be considered as hypotheses to be tested through research and compared to the findings of the expert consensus.
Supplemental_file.pdf
Download PDF (219.6 KB)Acknowledgements
This publication presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. The authors wish to thank Dr Hugh Boardman and Ian Redsell of the Delphi Process Research Unit for their support and expertise, and members of the Delphi steering group and expert panel for their time and willingness to participate and contribute to this study: Kathy Castillo, Rees Collins, Cecilia Daly, Brooke Duggan, Anton Emmanuel, Aram Farad, Rik Fox, Maggie Garvey, Ram Hariharan, Matt Henley, Louise Kelly, Anna Miles, Sarah Morgan, Rachael Moses, Vivien Mulgrew, Sophie Nawarski, Clare Park, Lee Pryor, Pauline Robertson, Helena Rodi, Jack Ross, Jacqui Ross, Claire Salisbury, Fiona Stephenson.
Disclosure statement
Jackie McRae was funded for a Clinical Doctoral Research Fellowship award by the National Institute for Health Research (NIHR) and Health Education England (HEE) (Grant Reference Number CDRF 2013–04-024). Anton Emmanuel was supported by grants through the UCL Biomedical Research Centre.
Data availability statement
All research data are stored securely in the Research Innovation Centre at the Royal National Orthopaedic Hospital ([email protected]).
Additional information
Funding
References
- Thietje R, Hirschfeld S. Epidemiology of spinal cord injury. In: Weidner N, Rupp R, Tansey KE, editors. Neurological aspects of spinal cord injury. Cham: Springer International Publishing; 2017. p. 3–17.
- Lee BB, Cripps RA, Fitzharris M, et al. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2014;52(2):110–116.
- McCaughey EJ, Purcell M, McLean AN, et al. Changing demographics of spinal cord injury over a 20-year period: a longitudinal population-based study in Scotland. Spinal Cord. 2016;54(4):270–276.
- Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50(5):365–372.
- van den Berg ME, Castellote JM, de Pedro-Cuesta J, et al. Survival after spinal cord injury: a systematic review. J Neurotrauma. 2010;27(8):1517–1528.
- Chamberlain JD, Meier S, Mader L, et al. Mortality and longevity after a spinal cord injury: systematic review and meta-analysis. Neuroepidemiology. 2015;44(3):182–198.
- NHS England. Specialised Spinal Cord Injury Services Annual Statement 2018. [updated 19 2019; cited 2020 Nov 20]. Available from: www.nscisb.nhs.uk/docs.aspx?section=Annual%20Reports
- Skoretz SA, Riopelle SJ, Wellman L, et al. Investigating swallowing and tracheostomy following critical Illness: a scoping review. Crit Care Med. 2020;48(2):e141–e151.
- Iruthayarajah J, McIntyre A, Mirkowski M, et al. Risk factors for dysphagia after a spinal cord injury: a systematic review and meta-analysis. Spinal Cord. 2018;56(12):1116–1123.
- NHS England. Service Specification: Spinal Cord Injury Services (all ages); 2019. [cited 2019 May 1]. Available from: https://www.england.nhs.uk/wp-content/uploads/2019/04/service-spec-spinal-cord-injury-services-all-ages.pdf
- Spinal Injuries Association, All-Party Parliamentary Group. A Paralysed System? London; 2015. [cited 2016 Oct 1]. Available from: https://spinal.co.uk/wp-content/uploads/2015/11/SIA-APP-Paralysed-System-Report-FINAL-lo-res.pdf.
- McRae J, Smith C, Beeke S, et al. Oropharyngeal dysphagia management in cervical spinal cord injury patients: an exploratory survey of variations to care across specialised and non-specialised units. Spinal Cord Ser Cases. 2019;5(1):31–40.
- Sharwood L, Dhaliwal S, Ball J, et al. Emergency and acute care management of traumatic spinal cord injury: a survey of current practice among senior clinicians across Australia. BMC Emerg Med. 2018;18(1):57.
- Maharaj MM, Stanford RE, Lee BB, et al. The effects of early or direct admission to a specialised spinal injury unit on outcomes after acute traumatic spinal cord injury. Spinal Cord. 2017;55(5):518–524.
- Parent S, Barchi S, LeBreton M, et al. The impact of specialized centers of care for spinal cord injury on length of stay, complications, and mortality: a systematic review of the literature. J Neurotrauma. 2011;28(8):1363–1370.
- Kirshblum SC, Burns SP, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011;34(6):535–546.
- Mcdaid D, Park A-L, Gall A, et al. Understanding and modelling the economic impact of spinal cord injuries in the United Kingdom. Spinal Cord. 2019;57(9):778–788.
- Wolf C, Meiners TH. Dysphagia in patients with acute cervical spinal cord injury. Spinal Cord. 2003;41(6):347–353.
- Shin JC, Yoo JH, Lee YS, et al. Dysphagia in cervical spinal cord injury. Spinal Cord. 2011;49(9):1008–1013.
- Shem K, Wong J, Dirlikov B, et al. Pharyngeal dysphagia in individuals with cervical spinal cord injury: a prospective observational cohort study. Top Spinal Cord Inj Rehabil. 2019;25(4):322–330.
- Hayashi T, Fujiwara Y, Ariji Y, et al. Mechanism of dysphagia after acute traumatic cervical spinal cord injury. J Neurotrauma. 2020;37(21):2315–2319.
- Valenzano TJ, Waito AA, Steele CM. A review of dysphagia presentation and intervention following traumatic spinal injury: an understudied population. Dysphagia. 2016;31(5):598–609.
- Ihalainen T, Rinta-Kiikka I, Luoto TM, et al. Risk factors for laryngeal penetration-aspiration in patients with acute traumatic cervical spinal cord injury. Spine J. 2018;18(1):81–87.
- Chaw E, Shem K, Castillo K, et al. Dysphagia and associated respiratory considerations in cervical spinal cord injury. Top Spinal Cord Inj Rehabil. 2012;18(4):291–299.
- Wong S, Derry F, Jamous A, et al. The prevalence of malnutrition in spinal cord injuries patients: a UK multicentre study. Br J Nutr. 2012;108(5):918–923.
- Berney S, Bragge P, Granger C, et al. The acute respiratory management of cervical spinal cord injury in the first 6 weeks after injury: a systematic review. Spinal Cord. 2011;49(1):17–29.
- McRae J. Dry mouth in spinal cord injury: causes and treatment. Dental Nursing. 2011;7(8):446–449.
- McRae J, Smith C, Emmanuel A, et al. The experiences of individuals with cervical spinal cord injury and their family during post-injury care in non-specialised and specialised units in UK. BMC Health Serv Res. 2020;20(1):783.
- Benfield J, Michou E. Dysphagia screening and assessment in the stroke unit. BJNN. 2016;12(Suppl. 2):S24–S28.
- Palli C, Fandler S, Doppelhofer K, et al. Early dysphagia screening by trained nurses reduces pneumonia rate in stroke patients: a clinical intervention study. Stroke; a Journal of Cerebral Circulation. 2017;48(9):2583–2585.
- Attrill S, White S, Murray J, et al. Impact of oropharyngeal dysphagia on healthcare cost and length of stay in hospital: a systematic review. BMC Health Serv Res. 2018;18(1):594–611.
- Cichero JAY, Heaton S, Bassett L. Triaging dysphagia: nurse screening for dysphagia in an acute hospital. J Clin Nurs. 2009;18(11):1649–1659.
- Ardolino EM, Hutchinson KJ, Pinto Zipp G, et al. The ABLE scale: the development and psychometric properties of an outcome measure for the spinal cord injury population. Phys Ther. 2012;92(8):1046–1054.
- Chen HC, Taiwan ICF Team, Yen TH, Chang KH, et al. Developing an ICF core set for Sub-acute stages of spinal cord injury in Taiwan: a preliminary study. Disabil Rehabil. 2015;37(1):51–55.
- Boldt C, Velstra IM, Brach M, et al. Nurses' intervention goal categories for persons with spinal cord injury based on the international classification of functioning, disability and health: an international Delphi survey. J Adv Nurs. 2013;69(5):1109–1124.
- Zhang Z, Li F, Sun T. An expert consensus on the evaluation and treatment of acute thoracolumbar spine and spinal cord injury in China. Neural Regener Res. 2013;8(33):3077–3086.
- Jünger S, Payne SA, Brine J, et al. Guidance on conducting and Reporting Delphi Studies (CREDES) in palliative care: recommendations based on a methodological systematic review. Palliat Med. 2017;31(8):684–706.
- Rankin G, Rushton A, Olver P, et al. Chartered society of physiotherapy's identification of national research priorities for physiotherapy using a modified Delphi technique. Physiotherapy. 2012;98(3):260–272.
- von der Gracht HA. Consensus measurement in Delphi studies review and implications for future quality assurance. Technol Forecasting Social Change. 2012;79(8):1525–1536.
- Keeney S, McKenna H, Hasson F. The Delphi technique in nursing and health research. Oxford: Wiley-Blackwell; 2010.
- Posillico SE, Golob JF, Rinker AD, et al. Bedside dysphagia screens in patients with traumatic cervical injuries: an ideal tool for an under-recognized problem. J Trauma Acute Care Surg. 2018;85(4):697–703.
- Suiter DM, Leder SB. Clinical utility of the 3-ounce water swallow test. Dysphagia. 2008;23(3):244–250.
- Brady S, Miserendino R, Statkus D, et al. Predictors to dysphagia and recovery after cervical spinal cord injury during acute rehabilitation. J Appl Res. 2004;4(1):1–11.
- Ward EC, Morgan AT. Dysphagia post trauma. San Diego (CA): Plural Publishing Inc.; 2009.
- Respiratory Information for Spinal Cord Injury. Weaning Guidelines for Spinal Cord Injury Patients in Critical Care Units 2012. [cited 2015 Aug 4]. Available from: http://risci.org.uk/weaning-guidelines-for-spinal-cord-injured-patients-in-critical-care-units/
- Spinal Injuries Association. A Paralysed System?; 2015.
- Goff D, Patterson J. Eating and drinking with an inflated tracheostomy cuff: a systematic review of the aspiration risk. Int J Lang Commun Disord. 2019;54(1):30–40.
- Pryor LN, Ward EC, Cornwell PL, et al. Clinical indicators associated with successful tracheostomy cuff deflation. Aust Crit Care. 2016;29(3):132–137.
- Berlowitz DJ, Wadsworth B, Ross J. Respiratory problems and management in people with spinal cord injury. Breathe. 2016;12(4):328–340.
- Galeiras Vazquez R, Rascado Sedes P, Mourelo FM, et al. Respiratory management in the patient with spinal cord injury. Biomed Res Int. 2013;2013:168757.
- Freeman-Sanderson AL, Togher L, Elkins MR, et al. Return of voice for ventilated tracheostomy patients in ICU: a randomized controlled trial of early-targeted intervention. Crit Care Med. 2016;44(6):1075–1081.
- Karlsson V, Bergbom I, Forsberg A. The lived experiences of adult intensive care patients who were conscious during mechanical ventilation: a phenomenological-hermeneutic study. Intensive Crit Care Nurs. 2012;28(1):6–15.
- Guttormson JL, Bremer KL, Jones RM. "Not being able to talk was horrid": a descriptive, correlational study of communication during mechanical ventilation. Intensive Crit Care Nurs. 2015;31(3):179–186.
- Grossbach I, Stranberg S, Chlan L. Promoting effective communication for patients receiving mechanical ventilation. Crit Care Nurse. 2011;31(3):46–60.
- Patak L, Gawlinski A, Fung NI, et al. Communication boards in critical care: patients' views. Appl Nurs Res. 2006;19(4):182–190.
- Pryor LN, Ward EC, Cornwell PL, et al. Establishing phonation using the Blom® tracheostomy tube system: a report of three cases post cervical spinal cord injury. Speech Lang Hearing. 2016;19(4):227–237.
- Sutt AL, Caruana LR, Dunster KR, et al. Speaking valves in tracheostomised ICU patients weaning off mechanical ventilation–do they facilitate lung recruitment? Crit Care. 2016;20(1):91.
- Thibault-Halman G, Casha S, Singer S, et al. Acute management of nutritional demands after spinal cord injury. J Neurotrauma. 2011;28(8):1497–1507.
- Dionyssiotis Y. Malnutrition in spinal cord injury: more than nutritional deficiency. J Clin Med Res. 2012;4(4):227–236.
- Chen X, Liu Z, Sun T, et al. Relationship between nutritional status and mortality during the first 2 weeks following treatment for cervical spinal cord injury. J Spinal Cord Med. 2014;37(1):72–78.
- Wong S, Graham A, Green D, et al. Nutritional supplement usage in patients admitted to a spinal cord injury center. J Spinal Cord Med. 2013;36(6):645–651.
- Thomson WM. Dry mouth and older people. Aust Dent J. 2015;60 (Suppl. 1):54–63.
- Kjeldsen CL, Hansen MS, Jensen K, et al. Patients' experience of thirst while being conscious and mechanically ventilated in the intensive care unit. Nurs Crit Care. 2018;23(2):75–81.
- Pakpour AH, Kumar S, Scheerman JF, et al. Oral health-related quality of life in Iranian patients with spinal cord injury: a case-control study. Injury. 2016;47(6):1345–1352.
- Yuen HK, Shotwell MS, Magruder KM, et al. Factors associated with oral problems among adults with spinal cord injury. J Spinal Cord Med. 2009;32(4):408–415.
- Shirlaw PJ, Khan A. Oral dryness and Sjögren's: an update. Br Dent J. 2017;223(9):649–654.
- Hahnel S, Behr M, Handel G, et al. Saliva substitutes for the treatment of radiation-induced xerostomia-a review. Support Care Cancer. 2009;17(11):1331–1343.
- Middleton JM, Sharwood LN, Cameron P, et al. Right care, right time, right place: improving outcomes for people with spinal cord injury through early access to intervention and improved access to specialised care: study protocol. BMC Health Serv Res. 2014;14(1):600.
- Donovan W, Carter R, Bedbrook G, et al. Incidence of medical complications in spinal cord injury: patients in specialised, compared with non-specialised centres. Paraplegia. 1984;22(5):282–290.
- Scheel-Sailer A, Post MW, Michel F, et al. Patients' views on their decision making during inpatient rehabilitation after newly acquired spinal cord injury-A qualitative interview-based study. Health Expect. 2017;20(5):1133–1142.
