Abstract
Purpose
This exploratory case-controlled study examined whether the same amount of effort leads to similar feelings of fatigue and whether feelings of fatigue decline at the same rate in people with traumatic brain injury (pwTBI) compared to controls.
Methods
Twenty pwTBI and 20 healthy controls (HC) completed an adaptive n-back task to induce fatigue and reported mental effort upon task completion and state-fatigue pre-task and several times during 30-minutes rest-period post-task. Task difficulty adapted to performance allowing both groups to invest substantial amounts of mental effort.
Results
Fatigue and effort levels were higher in pwTBI compared to controls. Multiple linear regression analyses showed that effort was positively related to post-task fatigue and this relationship did not differ between groups. Pre-task fatigue was the only predictor of post-task fatigue. Multilevel models showed no significant difference in decline of fatigue over the rest-period between groups.
Conclusions
Excessive feelings of fatigue following TBI could not be explained by a higher vulnerability to the fatigue-inducing effects of mental effort needed to perform a specific task. In pwTBI pre-task fatigue levels might be more related to the complex demands of everyday life. Future studies should investigate recovery of fatigue and applications of this knowledge to rehabilitation interventions.
People with TBI experience long-term fatigue as one of the most frequent and disabling symptoms and this long-term fatigue is a risk factor for development of secondary psychiatric symptoms such as depression or anxiety.
Since people with TBI did not show a higher vulnerability to the fatigue-inducing effects of mental effort, fatigue following TBI might be better explained by the complex demands of everyday life such as external (environment) and internal (emotions) factors.
Rehabilitation programs should be directed to this complex and highly individual interplay of fatigue in relation to other factors.
Implications for rehabilitation
Introduction
Many people with traumatic brain injury (TBI) experience cognitive problems and report excessive feelings of fatigue [Citation1]. These feelings of fatigue are associated with poorer social, physical, cognitive, and general functioning [Citation2]. Furthermore, fatigue is a risk factor for emotional difficulties in the long term and has been associated with sleep disturbances [Citation3]. In addition, some studies report relationships between fatigue and injury-related factors (such as time since injury, injury severity), psychosocial factors (such as age, employment), or lifestyle (caffeine use, exercise) [Citation1,Citation2]. However, these relationships are not consistently found [Citation4]. Especially, mental fatigue, which is described as insufficient energy reserves to perform everyday mental activities, is a dominating factor that limits people with TBI to lead a normal life including work and social activities [Citation5,Citation6].
Mechanisms underlying fatigue following TBI are still poorly understood. Existing theories assume that fatigue following TBI is directly or indirectly related to increased effort needed to perform the task. Van Zomeren et al. were the first to suggest that fatigue following TBI might be related to effort to overcome cognitive impairment [Citation7]: people with brain injury need more effort to perform tasks compared to pre-injury and people without brain injury [Citation7–9]. Recent neuroimaging studies propose that fatigue following TBI is related to a dysfunction in the corticostriatal pathway involved in motivational processing [Citation10–13]. Previous studies have demonstrated significant relationships between performance and effort [Citation14,Citation15], and between performance and fatigue in people with TBI [Citation8,Citation9,Citation16,Citation17]. The one study that did directly examining the relationship between effort and fatigue found a positive correlation between effort and trait fatigue in people with TBI [Citation8].However, this study did not examine state fatigue, which is the experience of fatigue at a particular moment. Nor did they compare this relationship between effort and fatigue in people with TBI to that of healthy controls (HC) to examine whether similar levels of effort would result in similar or higher levels of fatigue in individuals with TBI compared to HC.
Fatigue following TBI could also be due to slowness in recovery following a mentally exhausting task resulting in longer duration and accumulation of fatigue [Citation5,Citation18]. People with TBI estimated hours to several days to recover fully from mental exhaustion contrary to HC who reported one hour or less [Citation18,Citation19]. This suggests that the rate of alleviation of post-task fatigue is slower in people with TBI compared to HC. This effect has been found in other neurological disorders such as, multiple sclerosis [Citation20]. However, to the authors’ knowledge, there have been no studies examining the recovery curve of fatigue following mental exhaustion in people with TBI.
This exploratory study therefore examined the relationship between subjective mental effort and state fatigue, and whether feelings of fatigue resolve at the same rate in people with TBI and controls. To explore our hypotheses, we needed a task that allows people with TBI and controls to invest substantial amounts of effort, and that induces fatigue in both groups. This could be achieved by a task that adjusts workload or difficulty to the performance of the person, such as an adaptive n-back task [Citation21]. This working memory task adjusts difficulty (n-back level) to accuracy of performance of the participant. We hypothesized that the same amount of effort in people with TBI and HC would lead to similar levels of fatigue in both groups, indicating a similar relationship between effort and fatigue in both groups. In addition, we expected that task-induced fatigue would decline at a slower rate in people with TBI compared to HC.
Methods
Participants
Participants with TBI were recruited from an outpatient rehabilitation unit at Zuyderland Medical Center and via an institutional register of prior study participants who gave permission to be contacted again for research. A medical doctor referred participants to the study and confirmed the TBI. The Mayo classification system was used to classify TBI as mild or moderate–severe using imaging data and/or injury characteristics including loss of consciousness, post-traumatic amnesia, and behavioral symptoms [Citation22]. Inclusion criteria for the TBI group were a history of TBI, age between 18 and 75 years and completion of treatment for consequences of the TBI. The HC group consisted of healthy age, gender, and education matched participants. For both groups exclusion criteria were: history of a neurological disorder (for TBI group other than TBI), history of a medical disorder that could account for current fatigue (such as cancer or sleep disorder), and a current diagnosed mental disorder (such as depression or schizophrenia) based on clinical judgment and self-report. Participants were recruited between November 2017 and November 2019.
Procedure
The study protocol received university ethics approval (ERCPN-185_04_11_2017). All participants provided written informed consent. Participation consisted of one visit of 1.5–2 h at the hospital or the university. During this visit, participants completed questionnaires measuring their trait fatigue, sleep quality, and mood, since fatigue is often associated with these symptoms. In addition, participants performed four cognitive tasks that are particularly sensitive to cognitive impairments following TBI [Citation23]. Next, the n-back was practiced. This was followed by the adaptive n-back after which participants rated the amount of effort invested in this task using the Rating Scale for Mental Effort (RSME). Participants completed a Visual Analog Scale (VAS) to measure their state fatigue immediately before (VASpre), immediately after (VASpost) and at 5, 10, 15, 20, and 30 min following the adaptive n-back task since we expected a fast decline in fatigue due to the shortness of the task. During these 30 min, participants performed an activity of their choice that they experienced as relaxing. Participants were informed before the visit about this resting period and could bring a book, music, or something else of their liking. Participants were allowed to take breaks when necessary. The assessment visit was scheduled at participants’ convenience between 9 am and 5 pm on a weekday. Participants were requested to be rested and to consume maximally two cups of coffee at breakfast on the testing day, to minimize the effect of caffeine on task-performance but also prevent withdrawal effects [Citation24].
Sample description
Subjective sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI) [Citation25], trait fatigue with the Fatigue Severity Scale (FSS) [Citation26], and mood with the Hospital Anxiety and Depression Scale (HADS) which consist of a subscale for anxiety and depression [Citation27]. Standard scoring of the questionnaires was used leading to mean or sum scores. Cognitive performance was measured with four tasks [Citation23] including the STROOP [Citation28], Symbol Digit Modalities Test (SDMT) [Citation29], Controlled Word Association Task (COWAT) [Citation30], and Digit Span forward and backward [Citation31]. Scores on the cognitive tasks were adjusted for age and education of participants and transformed to T-scores.
Main outcome measures
Visual Analog Scale for fatigue
The VAS for fatigue (VAS-f) was used to measure state fatigue (momentary fatigue) [Citation32]. The VAS-f consists of a 100 mm horizontal line with the left end representing “absolutely no fatigue” and the right end “most severe fatigue imaginable” with no intermediate divisions or descriptive terms. Scores range from 0 to 100, with higher scores indicating more fatigue. To mask the purpose of the study, five additional VAS scales including frustration, pain, tension, happiness, and anger, were administered together with the VAS-f. Participants were instructed to rate the intensity of these feelings at that specific moment. The VAS-f has been used in previous studies with TBI participants and was found to be valid and reliable [Citation11,Citation12,Citation32].
Rating Scale Mental Effort
Subjective mental effort invested in the n-back task was assessed with the RSME [Citation33]. The RSME is a 150-mm vertical VAS containing nine anchor points with descriptive labels ranging from “absolutely no effort” (near 0 mm on the scale), through “rather much effort” (at 57 mm on the scale) to “extreme effort” (at 112 mm on the scale). Participants are requested to mark the line at the point corresponding to the amount of mental effort it took to them to complete a task. This point is also the score of the RSME. The RSME is a reliable and valid scale and has been used in previous studies including people with TBI [Citation14,Citation33,Citation34].
Adaptive n-back
The adaptive n-back task is a working memory task measuring accuracy and reaction time. Participants were shown a sequence of letters, one at a time on a computer screen, and had to respond with their middle finger each time the current letter was identical to the one presented n positions back in the sequences and with their index finger if the letter was not identical, using a button box. The 1-, 2-, and 3-back levels were included in the task. The adaptive n-back was adjusted from a dual-n-back task of Jaeggi et al. [Citation35] and consisted of 15 blocks and each block consisted of 20+n letters and contained six targets and 14+n non-targets (n stands for the n-back level). After each block, the accuracy of the participant’s performance in that block (percentage correct responses) was evaluated to set the n-back level of the following block. When the participant had an accuracy of 90% or more, the level increased by one (to a maximum 3-back). When accuracy was 75% or less the level was decreased by one (to a minimum of 1-back). In all other cases, the level stayed the same. Task duration was approximately 25 min. Participants were not informed that changes in n-back levels depended on their performance. By adjusting the difficulty of the task based on performance of the participants, we aimed to have high and similar amounts of effort invested by all participants and thereby induce fatigue. The mean reaction time and the percentage of trials spent in each n-back level, and the overall accuracy over the complete task were calculated to compare performance between the TBI and HC group.
Statistics
Based on a multiple regression analysis with a medium to large effect size (f2=0.30), an alpha of 0.05, power level of 0.8 and 3 predictors (group, effort and the interaction between group and effort) the required sample size is 41 participants, so 21 participants per group [Citation36].
Differences between the TBI and control group in demographic characteristics, questionnaire scores and performance on cognitive tasks and adaptive n-back were examined using independent samples t-tests and Chi-square tests or Welch’s t-tests in case of unequal variance.
The associations between effort and fatigue at each time point, and task-induced fatigue (VASpost–VASpre) for the TBI and control group were assessed using Pearson’s correlation coefficients (r, two-tailed). To examine whether the association between effort (RSME) and fatigue immediately after the task (VASpost) is the same in people with TBI and HC, a multiple linear regression analysis was used including VASpost as dependent variable and group (TBI and HC), effort (RSME) and the interaction between group and effort as predictors. According to our hypothesis, only effort would be a significant predictor of fatigue since the same amount of effort would lead to similar levels of fatigue in people with TBI and HC. Since pre-task fatigue (VASpre) was different between the groups, the analysis for VASpost was repeated adding the VASpre as predictor, to control for the effects of fatigue before the task.
The recovery from the task-induced fatigue was analyzed using a multilevel linear model with restricted maximum likelihood estimation method and an autoregressive error variance structure to take into account that consecutive fatigue observations might be correlated more highly than non-consecutive observations [Citation37]. This model included group and time as predictors and the interaction between group and time to assess group difference in the rate of change in fatigue (VAS-f) over time.
Statistical analyses were performed with IBM SPSS version 25 (SPSS Inc., Chicago, IL) and statistical significance was set at 0.05.
Results
Participant characteristics
One control participant was excluded from the analysis due to self-reported caffeine withdrawal symptoms because limited caffeine consumption was request for the experiment. Participant characteristics and scores on the questionnaires and cognitive tasks are presented in . Of the participants with TBI, 70% reported severe levels of fatigue (FSS ≥4). There was no significant difference between the groups in age, gender distribution, education, and living situation. Participants with TBI reported worse sleep quality, more fatigue, and more emotional symptoms compared to controls. In the TBI group, the causes of injury were motor vehicle accidents (n = 17) and falls (n = 3). Based on the Mayo classification, 25% experienced a mild TBI (n = 5) and 75% a moderate–severe TBI (n = 15) [Citation22]. The mean time since injury was 7 years (SD = 11.5 years) and ranged from 7 months to 43 years.
Table 1. Demographics, subjective symptomatology, and neuropsychological functioning in traumatic brain injury and healthy control groups.
Neuropsychological functioning in participants with TBI was impaired. They performed worse on the SDMT, STROOP, and Digit Span forward and their average cognitive task score was lower compared to controls.
Performance adaptive n-back
In line with their impaired cognitive functions, the adaptive nature of the n-back task resulted in a higher proportion of trials on the less demanding 1-back level (p = 0.033) in the TBI group compared to controls. Conversely, for controls, it resulted also in a higher proportion of trials on the more demanding 3-back level (p = 0.047) compared to the TBI group. Overall percentage correct responses in the n-back was similar between groups, but mean response times of participants with TBI were longer than controls. The difference was significant on the 1-back level (p = 0.035) but not on the 2-back or 3-back levels (Supplementary materials Table 1). There was a negative correlation between accuracy score on the adaptive n-back and mental effort (r= −0.5, p = 0.03) and between accuracy and fatigue directly after the task (VASpost; r= −0.5, p = 0.02) in people with TBI.
Relationship between effort and fatigue
Participants with TBI reported significantly higher levels of fatigue (VAS-f) at each time point and reported to invest significantly more mental effort (RSME) compared to controls (). There was no difference between the groups in task-induced fatigue (VASpost–VASpre). Within the TBI group, there were strong positive correlations between RSME and VAS-f at each time point (; ), so more mental effort was related to higher levels of fatigue. There was a trend toward a positive association between RSME and VAS-f directly after the task in the control group (; ). Thus, in both groups, more mental effort was related to higher levels of fatigue directly after the task, as can be seen in . No correlations between RSME and VAS-f were found for the other time points in controls. There was no correlation between effort and task-induced fatigue in the TBI or control group ().
Figure 1. Relationship between effort and fatigue following the task for TBI participants (black circles) and controls (white triangles), including the 95% confidence intervals. VASpost: Visual Analog Scale fatigue immediately after the task; RSME: Rating Scale Mental Effort.
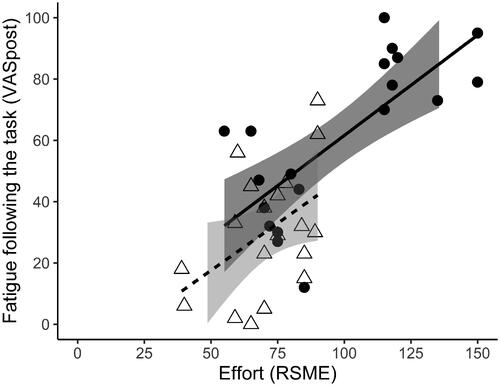
Table 2. Effort score and fatigue scores at the different time points for the traumatic brain injury and healthy control groups.
Table 3. Correlations between effort and fatigue scores for the traumatic brain injury and healthy control group.
Results of hierarchical multiple linear regression analysis examining the association between effort (RSME) and fatigue directly after the task (VASpost), showed that only mental effort was a significant predictor of VAS-f directly after the task (VASpost; ). As expected, group and the interaction between group and effort were not a significant predictors of VASpost, indicating that fatigue was not higher in people with TBI compared to HC when controlling for effort and that there was no difference between groups in the relationship between effort and VASpost. The complete model explained 56% of the variance in VASpost score.
Table 4. Results of hierarchical multiple linear regression analysis of the relationship between effort and fatigue, including the effect of group (traumatic brain injury and healthy controls) and the interaction between effort and group.
When adding the VAS-f before the task (VASpre) to the model, to control for fatigue levels before the task, only the VASpre was a significant predictors of VASpost (). Effort, group, and the interaction between group and effort were not significant predictors of VASpost. The complete model explained 62% of the variance in VASpost. This indicates that VASpre was associated with VASpost levels.
Recovery from fatigue
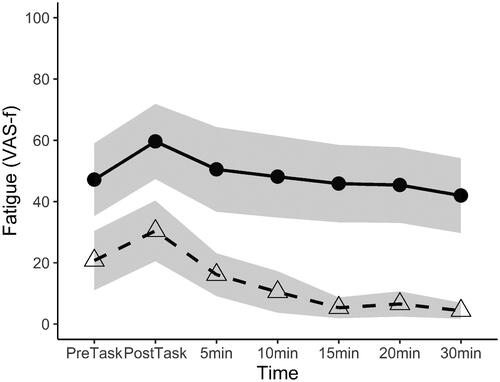
Fatigue scores from one control participant at 10, 15, 20, and 30 min after the task were missing since this participant had to leave for reasons unrelated to the study. The multilevel model examining the recovery of fatigue, using the VAS-f scores at the different time points, showed a significant effect of time (β(95% CI): −0.76 (–1.07 to 0.44), p < 0.001) and group (β(95% CI): 31.32 (17.51 to 45.13), p < 0.001). These results indicate that fatigue declined over time and that fatigue scores were higher in participants with TBI compared to controls (). However, no significant interaction between time and group was found (β(95% CI): 0.22 (–0.22 to 0.66), p = 0.32). Thus, no significant difference was found in the rate of decline in fatigue in the 30-min rest period post-task between groups, which is displayed by similar slopes of the lines of .
Figure 2. Levels of fatigue before and following the task, showing the task-induced increase and decline in fatigue during the 30-min rest-period for participants with TBI (circles, solid line) and controls (triangles, dashed line) with 95% confidence intervals (grey area). VAS-f: Visual Analog Scale fatigue; PreTask: before the task; PostTask: immediately after the task.
Discussion
This exploratory study examined the relationship between momentary fatigue and mental effort, and the recovery from task-induced fatigue in individuals with TBI. Mental effort was a significant predictor of fatigue directly following the task, independent of group. This is in line with our hypothesis that more mental effort invested in the task was associated with higher levels of fatigue following the task in both groups. In addition, it implies a similar association between effort and fatigue in people with TBI and HC. Furthermore, fatigue levels before the task were a significant predictor of fatigue levels directly after the task. There was no significant difference in the decline of fatigue and in both groups fatigue scores declined in the 30-min rest period post-task. However, levels of fatigue were higher at the start of the task and remained higher in the 30-min following the task in participants with TBI compared to controls.
More mental effort was associated with higher fatigue levels directly after the task. This relationship between effort and fatigue following the task is in line with our hypothesis and with existing theories about fatigue following TBI [Citation7]. The moderate negative relationship between the accuracy score on the adaptive n-back and mental effort and accuracy and fatigue in people with TBI, supports the assumption that fatigue following TBI is related to decreased cognitive capacity and increased effort needed to perform a task. The relationship between effort and fatigue did not differ between groups, since there was no interaction between effort and group. This could be interpreted as no higher vulnerability to the fatigue inducing effects of effort in people with TBI.
When adding fatigue before the task to the model, this was the only significant predictor of fatigue directly after the task. In people with TBI the level of fatigue before the task was strongly related with the amount of mental effort needed to perform the task, while this relationship was absent in controls. This is in accordance with previous research showing that higher baseline fatigue was associated with higher mental effort in people with TBI [Citation8,Citation38]. This might indicate that due to the high fatigue already present before the task, the cognitive resources to perform the task are limited leading to an increased experience of mental effort and high levels of fatigue in the TBI group. Another possibility is that people with TBI estimate the effort needed to perform the task differently or that the task is more demanding for them due to cognitive impairments. Overall, these high baseline levels of fatigue following TBI might be best explained with a biopsychosocial model, to capture the complex demands of everyday life, including external (environment, multitasking) and internal (emotions, rumination) factors.
There was no significant difference in the decline of fatigue following the task between people with TBI and controls. Therefore, in contrast to the expectations, there seems to be no slower recovery from fatigue in the 30-min following the task in people with TBI. However, fatigue levels were higher at each time point and there was more variation in the fatigue scores in TBI participants compared to controls. Despite the fact that fatigue is a common complaint after TBI, treatment options are still limited [Citation5]. The current findings may inform clinicians in adapting their rehabilitation programs: managing fatigue during the day and in different circumstances can be more targeted when incorporating knowledge about (personal) recovery rates. At the end of the 30 min recovery period, fatigue in the control group had almost completely resolved, while people with TBI still reported medium levels of fatigue, suggesting they may have needed more time for full recovery, which is seen as a characteristic of mental fatigue following TBI [Citation39]. To confirm that the decrease of fatigue during the rest period is similar in people with TBI and controls it would be necessary to study it when both groups are equally fatigued, and over a longer recovery period with pre-defined relaxation options. A task of longer duration and higher complexity such as dual-task, might be needed to increase feelings of mental effort and fatigue in HC.
Levels of fatigue and mental effort were significantly higher in participants with TBI compared to controls. Increased levels of fatigue in people with TBI are consistent with previous research [Citation6]. By having, participants perform an adaptive n-back task, we aimed to have task difficulty similar for both groups. Accuracy scores indeed indicated that task difficulty was comparable between groups and the increase of fatigue due to the task was similar. However, TBI participants reported significantly higher levels of mental effort compared to controls. Nonetheless, this might be related to the higher fatigue levels before the task instead of the task manipulation. This is in line with research in healthy participants indicating that higher baseline levels of fatigue increase effort perception [Citation40]. Simple everyday tasks such as vacuuming can be harder [Citation41] and subsequently take more effort following a TBI. This might therefore lead to increased “baseline” feelings of fatigue.
Previous research adapting the task to performance of the participant did find equal ratings of mental effort between participants with TBI and controls [Citation14]. Their control group consisted of a trauma control group, which might explain why the task was more effortful for them as well [Citation14]. Studies using the same task for both groups found mixed results with some reporting equal amounts of efforts [Citation8], while others reported higher effort ratings in people with TBI compared to controls [Citation42]. This could be related to the task used. In addition, this may support the often-reported heterogeneity in the TBI population, which was also indicated in this study by a high variability in the fatigue and effort scores in this group. This is in accordance with previous research that found that some of the people with TBI showed a greater increase in fatigue during task performance while others had a similar increase as the control group [Citation9].
Subjective fatigue following TBI often reflects more than solely symptoms of fatigue and a strong emotional component is often involved in the experience of fatigue following TBI [Citation43]. There were indeed moderate to high correlations between HADS depression scores and mental effort, general fatigue (FSS), and state fatigue at every time point in the TBI group. While, these relationships were absent in HC. However, adding depressive mood as a predictor of fatigue in the model did not change the results (Supplementary materials Table 2). Therefore, higher levels of depression did not explain the relationship between fatigue and effort in people with TBI.
There were several limitations to the present study. The adaptive n-back was meant to have both groups invest comparable amounts of effort and induce fatigue in both groups. Controls reported less mental effort compared to people with TBI; therefore, this could mean that the manipulation was not completely successful. However, task-induced fatigue was comparable between the groups. Furthermore, as already mention, the TBI group might have perceived the task as more effortful due to higher fatigue levels before the task, instead of unsuccessful task manipulation. Second, since this was an exploratory study to examine the task manipulation, the sample was small and heterogeneous with respect to age and injury characteristics. Moreover, due to the adaptive nature of the task, performance could not be compared to earlier research that also employed a n-back task in people with TBI or between the TBI and control group [Citation11,Citation44]. Future research could examine differences in brain activation when the subjective experience of fatigue or effort is comparable between people with TBI and controls to further examine the underlying mechanisms of fatigue following TBI. In addition, future studies could explore the built up of fatigue during task performance by adding measures of fatigue, such as the VAS-f, during the task.
In conclusion, this study could not explain high levels of fatigue in people with TBI by a higher vulnerability to the fatigue inducing effects of mental effort. There was no slower decline in fatigue in the 30-min following the task in people with TBI compared to HC. However, in people with TBI baseline fatigue levels seemed to play a large role for mental effort needed and feelings of fatigue following the task. From a clinical perspective, this is important information for people with TBI who refrain from activities requiring mental effort because they assume that their fatigue will exponentially increase. Further research on the recovery of fatigue and the underlying mechanisms of excessive fatigue following TBI are needed to understand these experiences of fatigue and how interventions can be targeted.
SupplementaryTable_EffortFatigueTBI.pdf
Download PDF (379 KB)Acknowledgements
We would like to thank S. Koch, I. Bras, M. Smeets, L. Tummers, and C. Voorter for data collection.
Disclosure statement
The authors report no conflicts of interest.
Data availability statement
Data can be obtained via the Dutch Dataverse Network upon request: https://doi.org/10.34894/FA2XYR.
References
- Mollayeva T, Kendzerska T, Mollayeva S, et al. A systematic review of fatigue in patients with traumatic brain injury: the course, predictors and consequences. Neurosci Biobehav Rev. 2014;47:684–716.
- Ponsford JL, Sinclair KL. Sleep and fatigue following traumatic brain injury. Psychiatr Clin North Am. 2014;37(1):77–89.
- Schönberger M, Herrberg M, Ponsford J. Fatigue as a cause, not a consequence of depression and daytime sleepiness: a cross-lagged analysis. J Head Trauma Rehabil. 2014;29(5):427–431.
- Ponsford JL, Ziino C, Parcell DL, et al. Fatigue and sleep disturbance following traumatic brain injury—their nature, causes, and potential treatments. J Head Trauma Rehabil. 2012;27(3):224–233.
- Johansson B, Rönnbäck L. Long-lasting mental fatigue after traumatic brain injury – a major problem most often neglected diagnostic criteria, assessment, relation to emotional and cognitive problems, cellular background, and aspects on treatment. In: Sadaka F, editor. Traumatic brain injury. London: IntechOpen; 2014.
- Ouellet M-C, Morin CM. Fatigue following traumatic brain injury: frequency, characteristics, and associated factors. Rehabil Psychol. 2006;51(2):140–149.
- Van Zomeren A, Brouwer W, Deelman B. Attentional deficits: the riddles of selectivity, speed and alertness. In: Brooks N, editor. Closed head injury: psychological, social and family consequence. Oxford (UK): Oxford University Press; 1984.
- Belmont A, Agar N, Azouvi P. Subjective fatigue, mental effort, and attention deficits after severe traumatic brain injury. Neurorehabil Neural Repair. 2009;23(9):939–944.
- Ziino C, Ponsford J. Selective attention deficits and subjective fatigue following traumatic brain injury. Neuropsychology. 2006;20(3):383–390.
- Dobryakova E, Genova HM, DeLuca J, et al. The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Front Neurol. 2015;6:52.
- Wylie GR, Dobryakova E, DeLuca J, et al. Cognitive fatigue in individuals with traumatic brain injury is associated with caudate activation. Sci Rep. 2017;7(1):8973.
- Dobryakova E, Genova H, Schneider V, et al. Reward presentation reduces on-task fatigue in traumatic brain injury. Cortex. 2020;126:16–25.
- Dobryakova E, DeLuca J, Genova HM, et al. Neural correlates of cognitive fatigue: cortico-striatal circuitry and effort-reward imbalance. J Int Neuropsychol Soc. 2013;19(8):849–853.
- Riese H, Hoedemaeker M, Brouwer W, et al. Mental fatigue after very severe closed head injury: sustained performance, mental effort, and distress at two levels of workload in a driving simulator. Neuropsychol Rehabil. 1999;9(2):189–205.
- Ashman TA, Cantor JB, Gordon WA, et al. Objective measurement of fatigue following traumatic brain injury. J Head Trauma Rehabil. 2008;23(1):33–40.
- Ziino C, Ponsford J. Vigilance and fatigue following traumatic brain injury. J Int Neuropsychol Soc. 2006;12(1):100–110.
- Johansson B, Berglund P, Rönnbäck L. Mental fatigue and impaired information processing after mild and moderate traumatic brain injury. Brain Inj. 2009;23(13–14):1027–1040.
- Johansson B, Starmark A, Berglund P, et al. A self-assessment questionnaire for mental fatigue and related symptoms after neurological disorders and injuries. Brain Inj. 2010;24(1):2–12.
- Johansson B, Ronnback L. Evaluation of the mental fatigue scale and its relation to cognitive and emotional functioning after traumatic brain injury or stroke. Int J Phys Med Rehabil. 2014;2:182.
- Neumann M, Sterr A, Claros-Salinas D, et al. Modulation of alertness by sustained cognitive demand in MS as surrogate measure of fatigue and fatigability. J Neurol Sci. 2014;340(1–2):178–182.
- Jaeggi SM, Studer-Luethi B, Buschkuehl M, et al. The relationship between n-back performance and matrix reasoning — implications for training and transfer. Intelligence. 2010;38(6):625–635.
- Malec JF, Brown AW, Leibson CL, et al. The Mayo classification system for traumatic brain injury severity. J Neurotrauma. 2007;24(9):1417–1424.
- Wilde EA, Whiteneck GG, Bogner J, et al. Recommendations for the use of common outcome measures in traumatic brain injury research. Arch Phys Med Rehabil. 2010;91(11):1650–1660.
- van Duinen H, Lorist MM, Zijdewind I. The effect of caffeine on cognitive task performance and motor fatigue. Psychopharmacology. 2005;180(3):539–547.
- Buysse DJ, Reynolds CF 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213.
- Krupp LB, LaRocca NG, Muir-Nash J, et al. The Fatigue Severity Scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370.
- Golden CJ, Freshwater SM. Stroop color and word test. Chicago (IL): Stoelting; 1978.
- Smith A. Symbol digit modalities test. Los Angeles: Western Psychological Services; 1973.
- Schmand B, Groenink S, Van den Dungen M. Letter fluency: psychometric properties and Dutch normative data. Tijdschr Gerontol Geriatr. 2008;39(2):64–74.
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale. New York: Psychological Corp; 1955.
- LaChapelle DL, Finlayson MAJ. An evaluation of subjective and objective measures of fatigue in patients with brain injury and healthy controls. Brain Inj. 1998;12(8):649–659.
- Zijlstra FRH. Efficiency in work behaviour: a design approach for modern tools. Delft: Delft University Press; 1995.
- Widyanti A, Johnson A, de Waard D. Adaptation of the Rating Scale Mental Effort (RSME) for use in Indonesia. Int J Ind Ergon. 2013;43(1):70–76.
- Jaeggi SM, Buschkuehl M, Jonides J, et al. Improving fluid intelligence with training on working memory. Proc Natl Acad Sci USA. 2008;105(19):6829–6833.
- Cohen J, Cohen P, West SG, et al. Applied multiple regression/correlation analysis for the behavioral sciences. New York: Routledge; 2013.
- Lee S, Crain TL, McHale SM, et al. Daily antecedents and consequences of nightly sleep. J Sleep Res. 2017;26(4):498–509.
- Ramage AE, Tate DF, New AB, et al. Effort and fatigue-related functional connectivity in mild traumatic brain injury. Front Neurol. 2018;9:1165.
- Johansson B, Rönnbäck L. Mental fatigue; a common long term consequence after a brain injury. In: Agrawal A, editor. Brain injury – functional aspects, rehabilitation and prevention. London: IntechOpen; 2009.
- Iodice P, Calluso C, Barca L, et al. Fatigue increases the perception of future effort during decision making. Psychol Sport Exerc. 2017;33:150–160.
- Fisher AG. The assessment of IADL motor skills: an application of many-faceted Rasch analysis. Am J Occup Ther. 1993;47(4):319–329.
- Azouvi P, Couillet J, Leclercq M, et al. Divided attention and mental effort after severe traumatic brain injury. Neuropsychologia. 2004;42(9):1260–1268.
- Chiou KS, Chiaravalloti ND, Wylie GR, et al. Awareness of subjective fatigue after moderate to severe traumatic brain injury. J Head Trauma Rehabil. 2016;31(3):E60–E68.
- Medaglia JD, Chiou KS, Slocomb J, et al. The less BOLD, the wiser: support for the latent resource hypothesis after traumatic brain injury. Hum Brain Mapp. 2012;33(4):979–993.
