Abstract
Purpose
To assess attainment of individual treatment goals one year after intrathecal baclofen (ITB) pump implantation in individuals with dyskinetic cerebral palsy (CP).
Materials and methods
A multi-center prospective cohort study was conducted including 34 non-walking individuals with severe dyskinetic CP, classified as Gross Motor Function Classification System (GMFCS) IV/V, aged 4–24 years, 12 months after pump implantation. The main outcome measure was Goal Attainment Scaling (GAS). Predictors of GAS results were analyzed. Complications were registered systematically.
Results
Seventy-one percent of individuals with dyskinetic CP fully achieved one or more treatment goals. One or more treatment goals were partially achieved in 97% of individuals. Two factors were found to be associated with attainment of goals: Dyskinesia Impairment Scale (DIS) score at baseline and the difference in pain score between baseline and follow-up. These two variables explain 30% of the variance in the outcome.
Conclusions
Intrathecal baclofen is effective in achieving individual treatment goals in children and young adults with dyskinetic CP after nine to 12 months of ITB treatment. A positive outcome on treatment goals is, for a small part, associated with higher severity of dystonia at baseline and with improvement of pain during treatment.
Clinical trial registration number
Dutch Trial Register, number NTR3642.
Intrathecal baclofen treatment is effective in attainment of personal treatment goals, one year after pump implantation in patients with dyskinetic cerebral palsy.
A positive outcome on treatment goals is, for a small part, related to higher severity of dystonia at the start and on improvement of pain during treatment.
Implications for rehabilitation
Cerebral palsy (CP) is defined as a group of developmental disorders of movement and posture, attributed to non-progressive disturbances that have occurred in the developing fetal or infant brain [Citation1]. Dyskinetic CP is the second most common type of CP with a prevalence of 0.12–0.3 per 1000 live births in Europe [Citation1–3]. Dyskinesia in CP can be subdivided into dystonia and choreo-athetosis. Dystonia is featured by twisting, repetitive movements and abnormal postures caused by involuntary sustained or intermittent muscle contractions. Tone is easily increased (hypertonia) but can fluctuate [Citation4]. Choreo-athetosis is characterized by hyperkinetic movements and tone fluctuation (mainly hypotonia) [Citation4]. Dystonia is usually the prominent feature; however, in dyskinetic CP, dystonia and choreo-athetosis are frequently present simultaneously [Citation5]. It has been reported that most individuals (59–80%) with dyskinetic CP have no walking ability and are classified as Gross Motor Function Classification System (GMFCS) level IV or V (using a wheelchair for mobilization: level IV can do this independently, level V are dependent on others to mobilize them) [Citation6,Citation7]. Treatment goals for these individuals can be on different components of the International Classification of Functioning, Disability and Health for Children and Youth (ICF-CY) and are mostly to decrease pain and improve comfort, to promote mobility (including sitting position and transfers), and to facilitate care giving by others [Citation8–10]. Oral medication, for example, baclofen, trihexyphenidyl, or benzodiazepines, can be used before considering neurosurgical intervention [Citation5]. The neurosurgical interventions include intrathecal baclofen (ITB) treatment and deep brain stimulation (DBS) [Citation5,Citation11].
Our previous trial, the Intrathecal baclofen in DYSkinetic cerebral palsy (IDYS) trial, provided level II evidence for the beneficial effect of ITB in individuals with severe dyskinetic CP (GMFCS level IV and V) on attainment of individual treatment goals, after three months of blinded treatment [Citation8]. However, high level evidence on the longer term for attainment of individual treatment goals is still lacking. The primary aim of the current study was therefore to evaluate the effect on attainment of individual goals in daily life one year after pump implantation in children and adolescents with dyskinetic CP. Secondary aims were: (1) to address the effects on secondary outcome measures (dystonia, choreoathetosis, spasticity, pain, comfort, functional skills, and range of motion (ROM)); (2) to identify factors that are associated with the attainment of individual treatment goals; (3) to report complications within the first year after pump implantation.
Methods
This study is a multi-center prospective cohort study, conducted at the Amsterdam University Medical Center (Amsterdam UMC, location VUmc), Amsterdam, and the Maastricht University Medical Center (MUMC), Maastricht, The Netherlands. The study was approved by institutional review boards at both sites and by the Medical Ethical Review Committee of the VU University (Amsterdam) (IRB number: IRB00002991). The trial is registered with the Dutch Trial Register, number NTR3642.
Participants
Previously, a trial on the effect of ITB in dyskinetic CP, the IDYS trial, was conducted. The IDYS trial was a multi-center, randomized, double-blind, placebo-controlled trial. Included individuals were randomized to receive either ITB or placebo, both given for three months, through an implanted micro-infusion pump. For this current prospective follow up cohort study, individuals were included from the cohort of individuals who previously participated in the IDYS trial in either the intervention or the placebo group. Inclusion criteria for this current study were: a diagnosis of dyskinetic CP, GFMCS level IV or V, aged 4–24 years, effectively receiving ITB via an implanted micro-infusion pump (Medtronic Synchromed® II pump, Minneapolis, MN) and an intrathecal catheter (Ascenda® catheter, model 8780, Medtronic Inc., Minneapolis, MN).
Patient characteristics including age, sex, height, weight, GMFCS level, Manual Ability Classification System (MACS) level [Citation12], level of comprehension of spoken language (determined with the Computer Based instrument for Low motor Language Testing (C-BiLLT)) [Citation13], were registered [Citation14]. The severity of brain magnetic resonance imaging (MRI) abnormalities was scored by two child neurologists as mild, moderate, or severe on a scale adapted from Cioni et al. [Citation15] for grey matter structures (cortex and basal ganglia) and white matter (loss of white matter and gliosis) [Citation14]. Any discrepancies were discussed until consensus.
Intervention
Outcome measures were assessed before pump implantation and after nine or 12 months of ITB treatment (with a range from nine to fifteen months). Treatment characteristics were registered, including catheter tip position, ITB dose at time of follow-up, use of flexible dosing with periodical boluses of ITB throughout the day, and change of oral anti-spastic medication since baseline [Citation8,Citation14]. During the first three months after pump implantation, individuals participated in the IDYS trial and received either ITB or placebo [Citation8]. Therefore, at follow-up of 12 months, individuals previously in the placebo groups received nine months ITB, and individuals previously in the ITB group received 12 months ITB [Citation8].
Outcome measures
The primary outcome measure was Goal Attainment Scaling (GAS) to assess achievement of individual treatment goals [Citation14]. Two or three individual goals, which could be on any of the components of the ICF-CY were identified prior to pump implantation by questioning parents and included individuals. Two or three most important individual goals, which could be on any of the components of the ICF-CY were identified prior to pump implantation by questioning parents and patients. To identify these goals, caregivers were asked to name two or three main problems in daily life (baseline situation). In collaboration with the professional team, these problems were transformed into specific, measurable, attainable, realistic, and timely (SMART) treatment goals (desired outcome). All goals were considered equally important. Consequently, for GAS, all possible outcomes on a goal were defined on a six-point scale by the researcher. This scale consists of scores for deterioration (score −3), no change compared to baseline (score −2), partial achievement of the goal (score −1), achievement of the desired goal (score 0), somewhat more (score 1) and much more (score 2) than achievement of the desired goal [Citation14]. This individual GAS set prior to ITB was used by the researcher to score the situation, together with the caregivers, at the 12 month follow-up. By combining scores for separate goals, a single aggregated T-score is produced using a standardized mathematic formula, which can be used for statistical analysis [Citation14,Citation16]. Clinical significance was defined as achievement of at least one goal on the GAS [Citation17].
Secondary outcome measures were: (1) dystonia and choreoathetosis (Barry-Albright Dystonia Scale (BADS) [Citation18] and Dyskinesia Impairment Scale (DIS) [Citation19]; (2) passive ROM for hip abduction, knee flexion, popliteal angle, ankle dorsiflexion (with flexed and extended knee), elbow flexion and elbow extension [Citation14,Citation20]; (3) Spasticity (a) clinical: spasticity test (SPAT) by which spasticity in lower extremity muscles is scored to be present or not. Spasticity is present when a catch is established during a fast stretch of the muscle [Citation14,Citation21], (b) electrophysiological: ratio of the soleus Hoffmann reflex (H reflex) to M wave (H/M ratio) [Citation14,Citation22]; (4) pain and comfort (both with a visual analogue scale (VAS) ranging from 0 (no pain or discomfort) to 10 (very severe pain or discomfort) (VAS)) [Citation14]; (5) capability in mobility, self-care, and social function (Pediatric Evaluation of Disability Inventory (PEDI)) [Citation14,Citation23]. Measurements were conducted by the same research assistants and physicians in both centers, who all had the same training in the study protocol. Scoring of the DIS was done by two research assistants who were well trained and who had experience with individuals with dyskinetic CP.
Complications
Complications were derived from the (Serious) Adverse events (S) (AE)s, which were systematically reported, and from a specific subscale of the Pediatric Sleep Questionnaire, recording symptoms related to sleep-related breathing disorders (SRBDs) [Citation14,Citation24].
Statistical analysis
A linear mixed-model analysis was used to determine the development over time for the primary outcome measure, the GAS T-score. A model was built with time as a categorical variable. The same analyses were used to determine the development over time for secondary outcome measures. All analyses were adjusted for possible covariates (age, sex, center, and randomization group at baseline). For all models, the normal distribution of the residuals was visually inspected. Effect size (ES) Cohen's d was calculated for all outcome measures, by dividing the estimated mean difference between baseline and 12-month follow-up by the total standard deviation (SD). In addition, differences for GAS T-score between the previous IDYS trial groups (ITB and placebo) at 12 months follow up were assessed using a linear mixed-model analysis with time, group and the interaction between time and group. The baseline GAS T-score was included as covariate.
To assess which factors are possibly associated with GAS T-score at 12-month follow-up, we first performed a univariable linear regression analysis for each potential variable (list of variables available as Supplement). Factors showing a p value of ≤0.20 were selected for input in a final model in which a multiple regression analysis using backward stepwise selection was performed to identify the predicting factors.
Data were analyzed in SPSS 26.0 (SPSS, Inc., Chicago, IL). A p value < 0.05 was considered statistically significant. Correction for multiple testing was done by the Holm correction of p values.
Results
Participants
From the 36 eligible individuals, 34 were included in this study for follow up and analysis. Two individuals were not included because one individual never got a pump implanted because of severe illness after inclusion but prior to implantation, and in the second individual the pump was removed due to pump infection and was not reimplanted at a later moment in time. Two other individuals also required explantation due to pump infection; however, they received a new pump several months later and were included in the analyses one year after renewed implantation. In the included individuals, measurements were done between 9 and 15 months after initiation of ITB. For one individual with catheter related complications, measurements were postponed to 10 months after catheter revision. Patient characteristics are presented in . An overview of all treatment characteristics is presented in .
Table 1. Patient characteristics at baseline (n = 34).
Table 2. Treatment characteristics.
Attainment of individual goals
The mixed-model analysis of the GAS T-scores shows a significant improvement between baseline and 12-month after implantation of 19.41 (95% CI 14.33–24.48, p < 0.001, ES 1.39) (). As some individuals were receiving nine months of ITB and others 12 months, we also compared if there was a difference between those two groups. They showed no difference in outcome on GAS T-scores (p = 0.973). The covariates used in the statistical model (age, sex, center, and randomization group at baseline) were not significant. Outcomes with and without covariates were similar. Reported outcomes are with the use of covariates in the model.
Table 3. Outcomes at baseline and 12 month follow-up.
shows the outcomes on GAS. Twenty-four out of 34 individuals (70.6%) fully achieved one or more treatment goals. Nine individuals more (total 33 out of 34; e.g., 97%) at least partially achieved a treatment goal (e.g., improvement compared to baseline but no full achievement of preset goal). Nine individuals (26.5%) showed deterioration of one (n = 6) or two (n = 3) goals. Four of these individuals showed at least partial achievement on the two other goals, and four others on one other goal. Only one individual showed overall deterioration and no improvement.
Figure 1. Patient outcomes on Goal Attainment Scaling. For the outcome on the Goal Attainment Scale (GAS), improvement is defined as goal being partially achieved, achieved, more than achieved or much more than achieved. Other possible GAS outcomes are goal being unchanged or deterioration.
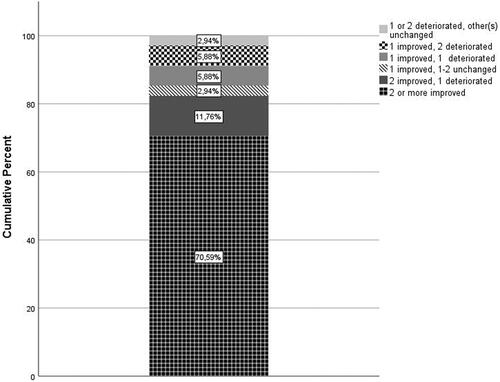
The different domains on which goals were set, and goal attainment on the different domains is shown in . Deterioration in the nine individuals occurred on different components of the ICF-CY. A cross table analysis and Kruskal–Wallis test were done to assess a difference between the achievement of different type of goals. There was no significant difference between the different ICF-CY components (Cross table analysis, p = 0.28; Kruskal–Wallis test, p = 0.26).
Table 4. Goal attainment at 12 month follow-up.
Secondary outcome measures
Results for the secondary outcome measures are provided in . DIS total dystonia score, DIS total choreoathetosis score and the DIS total score significantly decreased after nine to 12 months follow-up after ITB initiation. Also the BADS significantly decreased.
The H/M ratio decreased significantly, while no difference was found on clinically assessed spasticity (SPAT score). ROM measures did not change. For the PEDI, a significant improvement in social functioning was found with a mean difference of 1.3 points on the raw score. This change however, is not of clinical significance. Pain score and comfort scores both showed no significant change for the total sample, though the eight individuals who had pain as a personal goal, all achieved at least partial improvement of their goal concerning pain.
Factors associated with GAS T-score
Of all factors that were hypothesized to possibly be associated with outcome on GAS T-score (Supplement 1), the DIS total baseline score (B = 41.74, p = 0.053) and the change in pain scores (Δ pain) between baseline and 12-month follow-up (B=–2.30, p = 0.004) were included in the final model. The variables in this model explained 30% of the variance in the outcome. With post hoc analysis, we did not find baseline DIS score to be associated with which type of goals was chosen. There was no significant difference in the DIS total baseline score for goals on the different ICF components (p=.798), or goals in the different subcategories (p=.918).
Complications
Fourteen SAEs occurred in 11 of 34 individuals (32%) (). Eight individuals experienced one SAE, three experienced two SAEs. Of the 14 SAEs, there were three catheter related complications, two pump infections, and two temporary cerebrospinal fluid (CSF) leaks. The catheter related complications and pump infections needed surgical intervention. Based on the sleep related questionnaires, seven of 34 individuals (19.4%) showed an increased risk for SRBD compared to baseline, while four of 34 individuals (11.1%) showed a decreased risk for SRBD.
Discussion
This study is the first prospective cohort study on the effect of nine to 12 months of ITB treatment in dyskinetic CP using attainment of pre-implantation set individualized goals as an outcome measure (level 2 evidence). Previous studies are of lower level of evidence, or show the effects after a short follow up period [Citation25–28]. In this multi-center study, 71% of the 34 included individuals achieved at least one treatment goal, which was considered to be clinically significant. Nine individuals more (total 33 out of 34 individuals (97%)) at least partially achieved a treatment goal (e.g., improvement compared to baseline but no full achievement of preset goal).
Previous studies report improvement of caregiving [Citation25–28], quality of life (including comfort, mood, and pain) [Citation25–27], mobility (including upper and lower limb functioning, sitting, and transfers) [Citation25–27], and communication (including speech) [Citation25,Citation26]. In our population, treatment goals were mostly set and achieved on the ICF-CY component of environmental factors (care giving), body functions and structures (such as pain and comfort), and mobility (including transfers and sitting). Goals set in the component of communication (e.g., using a communication device) were least likely to improve. Only one patient showed overall deterioration, eight others showed improvement on one or two goals and deterioration on the other goal(s). Deterioration was reported on various components of the ICF-CY (). The GAS unfortunately does not provide information on the reasons why a patient deteriorated on a specific treatment goal.
Only two factors, baseline DIS score and change in pain scores, were found to be associated with attainment of individual treatment goals, and they only accounted for 30% of the variance. The first association found, between higher DIS scores at baseline (e.g., more severe dystonia) and better goal attainment, is in line with the expectation in general that more severely affected patients will benefit more from treatment. Theoretically, another explanation for the relationship between DIS score and outcome could be that goals set in more severely affected patients were more modest, and so more obtainable. However, we did not find a difference between baseline DIS and type of goal set. The other association was between a decrease in pain score and a better goal attainment. Pain occurs in 14–76% of children with CP and often goes unrecognized [Citation29–32]. In our population, eight individuals (24%) reported decrease of pain as one of their personal treatment goals on the GAS and in all improvement was reported by their parents. Pain negatively influences activities of everyday life and quality of life [Citation29,Citation31]. Decrease of pain could subsequently lead to improvement of daily functioning and in this study achievement of treatment goals. Our analysis could possibly have missed factors associated with goal attainment because we looked at factors associated with all treatment goals, while certain factors could possibly only influence only specific type of treatment goal. Unfortunately, current data size is not sufficient to look into the separate goals.
Temporary CSF leakage, infections, and catheter related problems were the most frequent complications in our study. The number of complications found in previous studies varies, but are generally in line with our findings [Citation33–35]. Other adverse events such as pneumonia, gastro-intestinal infection, obstipation, apnea, and seizures also occurred. These complications are probably not directly related to ITB treatment but likely correlate to the overall health status of the severely affected children included in our trial [Citation6,Citation36]. Adequate management of nutritional status and general health should be considered before pump implantation to prevent related complications.
The risk of SRBDs was separately evaluated and some individuals show an increased risk of SRBD with ITB while others showed a decreased risk with ITB. Further examination and testing of these individuals is necessary to confirm if they have actual SRBD or not. Future studies should address this important topic further, since little is known in current literature.
Study limitations
There are several limitations to this study. The sample size was too small for regression analysis, especially considering factors with a small sample within a category. This was the case for the height of the catheter tip in which most were placed at Th1 or higher (77.5%) and only few at Th4 or lower. A similar problem occurred for SAEs in which we were not able to differentiate for the effect of the different types of SAE due to the small number for each SAE type. Therefore, results of the regression analysis should be interpreted carefully.
Another limitation lies in the use of the PEDI as an outcome measure to assess skills in mobility, self-care, and social function. This scale is developed for children from six month to seven and half years of age. It can be used for older children if their functional capabilities are expected to be below the level of a seven and half year old; however, the PEDI is not validated for older children with disabilities. Furthermore, the individuals in our study were severely affected and, despite their age, were still on the bottom of the PEDI scores.
Last, the primary outcome measure, GAS, has some limitations. Setting goals, establishing the scale and scoring can be subjectively influenced by patients, parents, and assessors. The magnitude of goals can differ between individuals. Goals of one individual can be easier to achieve than goals of another. Furthermore, assessment of goal attainment can be hampered if the scale is poorly constructed and when goals are not clear (SMART). To minimize these influences, the same assessors performed the GAS during the study, and formulated SMART goals. They were all previously trained and had experience with GAS.
Conclusions
One year after pump implantation and nine to 12 months of ITB treatment, achievement of individual, daily life goals in children and young adults with severe dyskinetic CP was observed. Mainly goals on the ICF-CY components of environmental factors (care giving), body functions and structures (including comfort and pain), and mobility were achieved.
A positive outcome on treatment goals was, for a small part, associated with higher severity of dystonia before the start of the treatment and with improvement of pain. Studies with a longer follow up period and a larger cohort are needed to gain knowledge on other factors influencing outcome, which can also be used for better patient selection.
Supplement_1.docx
Download MS Word (15.4 KB)Disclosure statement
The authors have no conflicts of interest relevant to this article to disclose.
Additional information
Funding
References
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109(Suppl.):8–14.
- Graham HK, Rosenbaum P, Paneth N, et al. Cerebral palsy. Nat Rev Dis Primers. 2016;2:15082.
- Himmelmann K, Uvebrant P. The panorama of cerebral palsy in Sweden part XII shows that patterns changed in the birth years 2007–2010. Acta Paediatr. 2018;107(3):462–468.
- Krägeloh-Mann I, Petruch U, Weber PM. SCPE reference and training manual (R&TM); 2005. Available from: http://www.scpenetwork.eu
- Monbaliu E, Himmelman K, Lin JP, et al. Clinical presentation and management of dyskinetic cerebral palsy. Lancet Neurol. 2017;16(9):741–749.
- Himmelmann K, Hagberg G, Wiklund LM, et al. Dyskinetic cerebral palsy: a population-based study of children born between 1991 and 1998. Dev Med Child Neurol. 2007;49(4):246–251.
- Himmelmann K, McManus V, Hagberg G, et al. Dyskinetic cerebral palsy in Europe: trends in prevalence and severity. Arch Dis Child. 2009;94(12):921–926.
- Bonouvrie LA, Becher JG, Vles JSH, et al. The effect of Intrathecal baclofen in DYSkinetic cerebral palsy: the IDYS trial. Ann Neurol. 2019;86(1):79–90.
- Campbell WM, McLaughlin JF, Grant GA, et al. Long-term safety and efficacy of continuous intrathecal baclofen. Dev Med Child Neurol. 2007;44(10):660–665.
- Liew PY, Stewart K, Khan D, et al. Intrathecal baclofen therapy in children: an analysis of individualized goals. Dev Med Child Neurol. 2018;60(4):367–373.
- Fehlings D, Brown L, Harvey A, et al. Pharmacological and neurosurgical interventions for managing dystonia in cerebral palsy: a systematic review. Dev Med Child Neurol. 2018;60(4):356–366.
- Eliasson AC, Krumlinde-Sundholm L, Rösblad B, et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48(7):549–554.
- Geytenbeek JJ, Mokkink LB, Knol DL, et al. Reliability and validity of the C-BiLLT: a new instrument to assess comprehension of spoken language in young children with cerebral palsy and complex communication needs. Augment Altern Commun. 2014;30(3):252–266.
- Bonouvrié LA, Becher JG, Vles JSH, et al. Intrathecal baclofen treatment in dystonic cerebral palsy: a randomized clinical trial: the IDYS trial. BMC Pediatr. 2013;13:175–183.
- Cioni G, Di Paoo MC, Bertuccelli B, et al. MRI findings and sensorimotor development in infants with bilateral spastic cerebral palsy. Brain Dev. 1997;19(4):245–253.
- Turner-Stokes L. Goal Attainment Scaling (GAS) in rehabilitation: a practical guide. Clin Rehabil. 2009;23(4):362–370.
- Kakooza-Mwesige A, Andrews C, Peterson S, et al. Prevalence of cerebral palsy in Uganda: a population-based study. Lancet Glob Health. 2017;5(12):e1275–e1282.
- Barry MJ, VanSwearingen JM, Albright AL. Reliability and responsiveness of the Barry-Albright Dystonia Scale. Dev Med Child Neurol. 1999;41(6):404–411.
- Monbaliu E, Ortibus E, de Cat J, et al. The Dyskinesia Impairment Scale: a new instrument to measure dystonia and choreoathetosis in dyskinetic cerebral palsy. Dev Med Child Neurol. 2012;54(3):278–283.
- Becher JG, Doorenbosch CA, Folmer K, et al. Handbook standard physical examination in children with Central motor paresis [Handleiding standaard lichamelijk onderzoek bij kinderen met een centraal motorische parese] (Dutch). Houten: Springer Media B.V.; 2011.
- Scholtes VA, Dallmeijer AJ, Becher JG. The spasticity test: a clinical instrument to measure spasticity in children with cerebral palsy. The effectiveness of multilevel botulinum toxin type A and comprehensive rehabilitation in children with cerebral palsy. Amsterdam (The Netherlands): VU University Medical Center; 2007. p. 65–86.
- Hoving MA, van Kranen-Mastenbroek VHJM, van Raak EPM, et al. Placebo controlled utility and feasibility study of the H-reflex and flexor reflex in spastic children treated with intrathecal baclofen. Clin Neurophysiol. 2006;117(7):1508–1517.
- Feldman AB, Haley SM, Coryell J. Concurrent and construct validity of the Pediatric Evaluation of Disability Inventory. Phys Ther. 1990;70(10):602–610.
- Chervin RD, Weatherly RA, Garetz SL, et al. Pediatric Sleep Questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg. 2007;133(3):216–222.
- Albright AL, Barry MJ, Shagron DH, et al. Intrathecal baclofen for generalized dystonia. Dev Med Child Neurol. 2001;43:652–657.
- Bonouvrie L, Becher J, Soudant D, et al. The effect of intrathecal baclofen treatment on activities of daily life in children and young adults with cerebral palsy and progressive neurological disorders. Eur J Paediatr Neurol. 2016;20(4):538–544.
- Motta F, Stignani C, Antonello CE. Effects of intrathecal baclofen on dystonia in children with cerebral palsy and the use of functional scales. J Pediatr Orthop. 2008;28:213–217.
- Woon K, Tsegaye M, Vloeberghs M. The role of intrathecal baclofen in the management of primary and secondary dystonia in children. Br J Neurosurg. 2007;21(4):355–358.
- Tedroff K, Gyllensvärd M, Löwing K. Prevalence, identification, and interference of pain in young children with cerebral palsy: a population-based study. Disabil Rehabil. 2019;43(9):1292–1298.
- Penner M, Xie WY, Binepal N, et al. Characteristics of pain in children and youth with cerebral palsy. Pediatrics. 2013;132(2):e407–e413.
- McKinnon CT, Meehan EM, Harvey AR, et al. Prevalence and characteristics of pain in children and young adults with cerebral palsy: a systematic review. Dev Med Child Neurol. 2019;61(3):305–314.
- Westbom L, Rimstedt A, Nordmark E. Assessments of pain in children and adolescents with cerebral palsy: a retrospective population-based registry study. Dev Med Child Neurol. 2017;59(8):858–869.
- Motta F, Buonaguro V, Stignani C. The use of intrathecal baclofen pump implants in children and adolescents: safety and complications in 200 consecutive cases. J Neurosurg. 2007;107(1 Suppl.):32–35.
- Ward A, Hayden S, Dexter M, et al. Continuous intrathecal baclofen for children with spasticity and/or dystonia: goal attainment and complications associated with treatment. J Paediatr Child Health. 2009;45:720–726.
- Murphy NA, Nicole Irwin MC, Hoff C. Intrathecal baclofen therapy in children with cerebral palsy: efficacy and complications. Arch Phys Med Rehabil. 2002;83:1721–1725.
- Jesus A, Stevenson RD. Optimizing nutrition and bone health in children with cerebral palsy. Phys Med Rehabil Clin N Am. 2020;31(1):25–37.
Appendix 1
The members of the IDYS study group are, from the Amsterdam UMC, Vrije Universiteit, Amsterdam, the Netherlands: Laura A. Bonouvrié MD, Prof Jules G. Becher MD, Annemieke I. Buizer MD, Karin Boeschoten, Johanna J.M. Geytenbeek PhD, Prof Vincent de Groot MD (Rehabilitation Medicine), Laura A. van de Pol MD (Child Neurology), Willem J.R. van Ouwerkerk MD, K M Slot MD, Prof S. M. Peerdeman MD (Neurosurgery), Rob L.M. Strijers MD (Clinical Neurophysiology), Elisabeth M.J. Foncke MD (Neurology), Jos W.R. Twisk PhD, Peter van de Ven PhD (Epidemiology and Biostatistics), and from Maastricht University Medical Center, Maastricht, the Netherlands: Prof R. Jeroen Vermeulen MD, Prof Johan S.H. Vles MD, Dan Soudant MANP, Sabine Fleuren (Neurology) and Onno P. Teernstra MD (Neurosurgery).
