Abstract
Purpose
To guide better prevention and treatment and to develop research priorities, this study aims to create an overview of facilitators and barriers for the development and persistence of musculoskeletal complaints (MSCs) in individuals with upper limb absence (ULA).
Methods
Exploratory mixed methods design. A focus group (FG) was organized with individuals with ULA about MSCs and associated factors. An inductive approach was employed to the transcript and the studies. A scoping review was performed to systematically identify barriers and facilitators. The International Classification of Functioning, Disability, and Health was used to create an integrated overview of the results.
Results
Eleven participants participated in the FG, eight of them currently sustained or had sustained MSCs in the last year. Ten studies were included in the scoping review. The final overview consisted of 67 associated factors. Participants of the FG predominantly mentioned psychosocial factors, whereas the literature dominantly reported biomechanical factors.
Conclusions
The extensive overview of 67 factors showed that facilitators and barriers for MSCs are heterogeneous and aids in a better understanding of the complex nature of MSCs. Several biomechanical and psychosocial factors contribute to MSCs, but the association with a prosthesis remains unclear.
Musculoskeletal complaints (MSCs) are highly prevalent in the population with upper limb absence (ULA) and the overview of 67 factors could help in the prevention and treatment of MSCs.
Psychosocial factors in the development and persistence of MSCs are underreported in literature, but are important contributors to MSCs according to patients.
Wearing a prosthesis does not seem to be protective for the development or persistence of MSCs.
Social support, especially from significant others and employers, is essential to help protect MSCs in those with ULA.
Implications for rehabilitation
Introduction
Musculoskeletal complaints (MSCs) include many disorders associated with repetitive movements, awkward postures, and force [Citation1,Citation2], among which are complaints of arm, neck, and/or shoulder (CANS) that are not caused by acute trauma or any systemic disease [Citation3]. Complaints often include pain, but other sensations can also be present [Citation3]. MSCs are highly prevalent among the general population [Citation4–7], but (functionally) single-handed individuals are even more prone to MSCs [Citation6–8]. Single-handedness can be caused by upper limb absence (ULA), due to a congenital reduction deficiency (RD) or an acquired amputation (AA). The year prevalence of MSCs in Dutch individuals with ULA is nearly twice as high compared to their two-handed peers (65% versus 35%) [Citation6]. MSCs in ULA are often chronic and observed in the residual limb, unaffected limb, neck, and back [Citation6,Citation7]. Their presence may affect physical functioning and quality of life and may result in higher disability [Citation7]. The presence of MSCs in single-handed individuals may even result in dual-disability: disability due to MSCs, and disability due to single-handedness [Citation9].
The International Classification of Functioning, Disability, and Health (ICF) is a conceptual framework for the categorization of health and disability [Citation10]. Using ICF as a framework for MSCs (“health condition”) could highlight several factors that may contribute to the development and persistence of MSCs. There is ample evidence on external and personal factors influencing MSCs in the general population [Citation1]. Repetitive movements, awkward postures, sustained force, high workload, stress, and low (co-worker) support are associated with the development and persistence of MSCs [Citation1,Citation2,Citation11–14]. Additionally, sex, age, marital status, employment status, and educational level are described as factors associated with MSCs [Citation1,Citation2,Citation6,Citation15–17]. Factors associated with MSCs specific for patients with ULA have only been scarcely investigated [Citation6–8,Citation18]. Individuals with ULA need to compensate for the loss of a limb, resulting in alterations in biomechanical functioning, categorized as body functions and structures in ICF. Some factors are similar to the two-handed population, such as age and educational level, but additional psychosocial factors may arise. Individuals with ULA, especially with an amputation, may lose their job or need to change jobs and hobbies because they are no longer able to execute these properly due to limb loss or its consequences. Furthermore, limb loss may also affect relationships and mental health [Citation19]. Factors influencing MSCs are classified as facilitators and barriers, both being commonly used in clinical practice and research. A barrier is a factor that negatively influences and increases the risk of the development or persistence of MSCs. Terms as “risk factors, predictors, increasing factors, and negative effects” will be called barriers in this study. A facilitator is a factor that contributes to protection from MSCs and reduces the risk on the development or persistence of MSCs. Terms as “benefits, protective factors, reducing factors, and positive effects” will be called facilitators in this study.
The high prevalence of MSCs in individuals with ULA and its effects on daily functioning emphasize the high personal impact of this problem. It is warranted to gain more knowledge about ULA-specific facilitators and barriers in the development and persistence of MSCs. Studies [Citation6,Citation7,Citation20] only investigated quantitative data, even though experiences and factors mentioned by individuals with ULA could give additional insight and could highlight the importance of specific factors. Therefore, this study uses an exploratory sequential mixed methods study [Citation21] and combines a focus group (FG) with a scoping review to identify quantitatively and qualitatively derived facilitators and barriers associated with MSCs in individuals with ULA and to create an overview of all these factors. This knowledge may help in the better understanding of MSCs in individuals with ULA. Furthermore, it may help to synthesize research priorities and ultimately help in the development of new or better interventions to prevent and treat MSCs in ULA. The aim of the FG was to explore the experiences and the opinions of individuals with ULA on the factors influencing the development and persistence of MSCs, and to create a preliminary overview of these factors. The aim of the scoping review was to add any remaining factors and to create a final overview of facilitators and barriers influencing the development and persistence of MSCs in the target group. Finally, with the mixed methods design, we aimed to reveal similarities and differences between the results of the two approaches.
Methods
Part 1: focus group
The Medical Ethics Review Board of the University Medical Center Groningen (METc UMCG) concluded that formal approval of the study was not necessary (METc 2019/228). All participants signed an informed consent before the start of the study. The “Consolidated criteria for Reporting Qualitative research” (COREQ) were applied [Citation22].
Participants
A convenience sample of eligible patients (≥18 years old) was identified from clinical records, with ULA due to an AA or a congenital transversal deficiency, with different levels of ULA and different types of prostheses. All potential participants received an information letter. Additionally, an advertisement was published in the magazine of the Dutch patient association.
Data collection
The FG took place in April 2019 at the UMCG, the Netherlands. The moderator of the 60 min-FG was an independent female researcher with experience in qualitative research and the moderation of FGs (SvT). A female doctoral student (AAP) and a female resident in rehabilitation medicine and researcher (SGP) assisted the moderator. One additional female doctoral student was present during the FG to assist with the logistic aspects. Beforehand, there was no connection between the researchers and the participants.
At the start of the FG, the participants answered a short questionnaire on socio-demographic details (age, sex, origin of limb loss, level of limb loss, job, and prosthesis use). Furthermore, details about MSCs were requested; i.e., presence, duration, intensity, nature, and location of the complaints. The FG was audio-recorded and transcribed verbatim. The FG started with the introduction of the topic and the purpose of this study. Subsequently, a conversation guide with predefined topics and questions was used to structure the FG until data sufficiency, i.e., no new topics were introduced, was reached. The first two questions introduced the topic of MSCs: (1) who is familiar with MSCs, and if so what type of complaints have been experienced? (2) Who is not familiar with MSCs, and how can that be explained? The main topic of the FG, i.e., the associated factors for MSCs, was addressed in the following question: (3) What are or could be the causes of MSCs? The participants did not receive the transcript or the results of the FG for feedback or comments.
Part 2: scoping review
The scoping review was registered in the “Open Science Framework” (OSF) [Citation23]. The “Preferred Reporting Items for Systematic Reviews and Meta-Analyses: Extension for Scoping Reviews” (PRISMA-ScR) was used as reporting guideline [Citation24]. Additionally, the reviewer’s manual of the Joanna Briggs Institute on scoping reviews was used [Citation25].
Study selection
Scientific literature was searched in the following electronic bibliographic databases: PubMed, Web of Science, CINAHL, Cochrane Library, PsycINFO, and EMBASE (search date 10 June 2021). The search strategy was built together with a specialized librarian using the PCC approach: Population, Concept, and Context (Supplementary Appendix 1) [Citation25]. Studies were eligible if they investigated MSCs according to the following definition: CANS not caused by acute trauma or by any systemic disease [Citation3]. This definition comprises several synonyms, such as overuse complaints or repetitive strain injuries, and the manifestation of these complaints, e.g., pain and tingling [Citation1–3]. Studies investigating adult individuals with ULA, either due to an AA or congenital deficiency, were considered eligible. This review was conducted to investigate all factors associated with MSCs; therefore, both qualitative and quantitative content was considered for inclusion. There were no limits to a certain type of study. Exclusion criteria were: (1) studies investigating solely phantom limb pain and sensations; (2) studies applying interventions; (3) the text was not available in English or Dutch; (4) the studied population did not fit the target population and the target population was not separately analyzed; and (5) the study was a summary or review. Studies about residual limb pain (RLP) were excluded if the definition only reflected stump pain. If the definition also encompassed the residual arm as a whole and complaints in that region, the study was considered for inclusion. There were no restrictions on the publication date of the articles. The reference lists of the included articles for full text were searched for additional articles.
The retrieved articles from all databases were merged and duplicates were removed. Two reviewers (AAP, MAdB) screened the studies on title and abstract. Possible relevant studies were selected and screened on full text by the same two reviewers, to confirm eligibility criteria. Any inconsistency between the reviewers at each step was discussed until consensus was reached. A third reviewer (SGP) was consulted if disagreements could not be solved. Cohen’s kappa was used to calculate the agreement between reviewers.
Quality assessment
The “Appraisal tool for cross-sectional studies” (AXIS tool) was used to assess the quality of the studies, which all had a cross-sectional design [Citation26]. The two reviewers (AAP, MAdB) independently conducted the quality assessment. Any disagreement was resolved through discussion, and a third reviewer (SGP) was consulted if necessary. Cohen’s kappa score was calculated to express agreement. The quality assessment was not used to further exclude articles.
Data extraction
Descriptive information was extracted from the included studies by one reviewer (MAdB): study design, sample size, participant demographics, and MSCs outcome parameters and prevalence. A second reviewer (AAP) checked the extracted information on consistency and completeness.
Part 3: associated factors
Focus group
The audio recordings were transcribed verbatim. An iterative process similar to a thematic framework approach was performed [Citation27,Citation28]. The first step to analyze the data was familiarization of the data. The data were independently read by two researchers (AAP, SGP), and an initial set of codes was created. The second step for the framework approach was to identify a thematic framework. The domains of the ICF (body functions and structures, activities, participation, environmental factors, and personal factors) were used as main categories for the overview of the influencing factors for MSCs [Citation10]. The two assessors (AAP, SGP) independently checked the overview and added (sub)categories if necessary. These (sub)categories were discussed and the data were reassessed until consensus was met and a final set of (sub)categories was established (step 3: indexing). In the next step (step 4: charting), the transcript was entered in the Atlas.ti software. Both assessors identified sections of the transcript that corresponded to a particular (sub)category or code. The selected information was qualified as level of evidence 5 (LOE 5) [Citation29]. The results were analyzed and sections that were used to illustrate the findings were translated into English (AAP) and checked by the co-authors (step 5: mapping and interpretation). The quotes were not assigned to specific participants, since identification from the audio recordings was not feasible.
Scoping review
The overview of MSCs-associated factors, composed after the FG, was supplemented by the results of the scoping review. Out of the included articles, the text under the headings “results”, including the information from figures and tables, and “discussion and conclusion” was entered into the Atlas.ti software. Quantitative results that were supported with statistical analyses were classified as LOE 4, quantitative results without statistical analyses, qualitative results, references, or expert opinions were classified as LOE 5 [Citation29]. The data analysis was performed independently by two reviewers (AAP, MAdB). Discussion took place in case of disagreements until consensus was reached. If necessary, a third reviewer (SGP) was consulted.
Data synthesis
In the final overview, all factors associated with MSCs were divided into barriers, facilitators, both barriers and facilitators, or associations. This categorization was performed after the initial analysis of the FG and scoping review, and was used to incorporate contradicting results and to capture the distinction between facilitators and barriers used in the articles from the scoping review. Factors were categorized as a barrier if the FG or the studies mentioned, hypothesized, or analyzed that the factor increases MSCs. Terms as “risk factors, predictors, increasing factors, and negative effects” were also categorized as barriers in this study. Factors that reduced MSCs were categorized as facilitators. Terms as “benefits, protective factors, reducing factors, and positive effects” were called facilitators in this study. If there were contradicting results between the FG or studies, the factor was classified as both. The remaining associated factors consist of factors of which the association was undefined in the FG and all studies. The results for factors with LOE 4, including statistical measures, and p values, are presented in Supplementary Appendix 2. The last column shows the three different types of relations (barrier, facilitator, or association) and whether the factor was statistically significant (proven) or analyzed but not significant (expected). The reviewers synthesized the results independently.
Results
Part 1: focus group
Patient characteristics
Eleven out of 22 eligible participants responded and agreed to participate (). Four additional potential participants expressed their interest via the patient association, however, due to large travel distances or unavailability at the scheduled time, none of them were able to participate. Eight participants currently sustained or had sustained MSCs during the past year. Four mentioned the shoulders as a problem area (affected and non-affected sides). The neck and the back were the second most mentioned locations. The other locations were on the non-affected side.
Table 1. Characteristics of focus group participants (n = 11).
Table 2. Study characteristics (n = 10).
Part 2: scoping review
Study selection
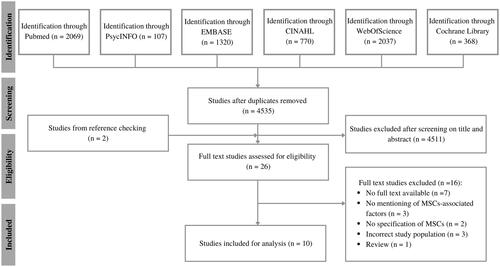
After screening 6688 articles, nine articles were included in the scoping review. One article was added based on reference checks (). The agreement on title and abstract screening and full text assessment was substantial (k = 0.69 and k = 0.70, respectively).
Figure 1. Flowchart for the selection of studies according to the PRISMA guidelines. MSCs: musculoskeletal complaints.
Quality assessment
All studies were cross-sectional. One study [Citation30] reported three case reports, aside from their cross-sectional study, for which no quality assessment was performed (Supplementary Appendix 3). The agreement between the reviewers was substantial (k = 0.68). Most studies scored >75% of the questions positively. Almost all studies scored low on the justification of the sample size and about non-responders. One study [Citation30] scored low on more than half the questions.
Study characteristics
Three studies from the same research group [Citation8,Citation19,Citation20] were combined because they investigated the same study population; therefore, eight studied populations from six different countries were included (). The merging of the three studies was only done for the presentation of the study characteristics and the MSCs outcomes. As the focus of these studies was different, they were analyzed and presented separately. A total of 1712 individuals with ULA participated (AA: n = 1512, RD: n = 200, mean age 53.0 (ranging from 38.0–74.8), 1442 males, 270 females, 1172 prosthesis users). Three studies also included a control group with a total of 811 participants [Citation6,Citation7,Citation19]. One study included all three populations (AA, RD, and controls) [Citation6]. One study [Citation33] had a follow-up one year after baseline measures.
MSCs outcomes
Definitions of MSCs varied (). Four studies showed that MSCs occurred in over half of the participants with ULA [Citation6,Citation8,Citation19,Citation20,Citation30]. One study [Citation6] compared the point and year prevalence to a control group, indicating a prevalence almost twice as high in the population with ULA. Most participants reported multiple pain locations. Aside from the presence, frequency, and locations of MSCs, almost all studies [Citation6–8,Citation18–20,Citation31–33] also described MSCs or pain-related outcomes, e.g., duration, severity, and interference (Supplementary Appendix 4).
Part 3: associated factors
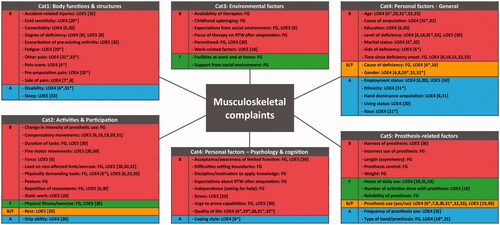
ICF categories Activities and Participation were merged. The category Personal factors was divided into General and Psychology & cognition. The category Body functions and structures and the category Environmental were left unchanged. Factors related to a prosthesis could be categorized as environmental, because it can be seen as an external object. However, users can also see a prosthesis as part of their own body, depending on their level of embodiment, which could make it part of the category body functions and structures. Whenever an individual uses the prosthesis for actions or tasks, it could also be seen as a performance qualifier for activities and participation. To prevent different interpretations, a new category “Prosthesis-related factors” was created. These five main categories contained in total 67 associated factors (); 28 factors were mentioned by the FG, 32 and 23 factors were analyzed or mentioned (expert opinions, references, self-reports) in the studies included in the scoping review with LOE 4 and LOE 5, respectively.
Figure 2. Final overview of factors associated with musculoskeletal complaints in persons with upper limb absence. Divided into barriers (B, red), facilitators (F, green), both barriers and facilitators (B/F, orange), and association undefined (A, blue). Factors were categorized as a barrier if the focus group or the studies mentioned, hypothesized, or analyzed that the factor increases MSCs. Factors that reduced MSCs were categorized as facilitators. If there were contradicting results between the focus group or studies, the factor was classified as both. The remaining associated factors consist of factors of which the association was undefined in the focus group and all studies. References with significant results are displayed with a *. Cat: category; B: barrier; F: facilitator; B/F: barrier and facilitator; A: association undefined; FG: focus group qualified as evidence level 5; LOE4: scoping review results qualified as evidence level 4; LOE5: scoping review results qualified as evidence level 5; RTW: return to work.
Category 1: body functions and structures
The FG participants did not mention any factors from this category. Participants who were sensitive to cold were about 4.4 times more likely to have chronic pain compared to individuals without cold sensitivity (LOE 4) [Citation20]. Participants with higher fatigue scores were more likely to report chronic pain (LOE 4) [Citation20]. A low pain score was a predictor for higher disability (LOE 4) [Citation6]. Pain prior to the amputation was correlated to back and neck pain (LOE 4) [Citation32], the correlation with RLP and non-amputated limb pain was not statistically significant (LOE 4) [Citation32]. Neck pain was reported to be more bothersome contralateral to the amputation in one study (LOE 4) [Citation7], but not in another (LOE 4) [Citation8]. Sleep quantity and quality were suggested to have a relationship with pain (LOE 5) [Citation33]. Comorbidity and degree of deficiency, i.e., how many limbs were affected, did not show a significant result (LOE 4) [Citation6,Citation8,Citation20]. Disability was higher for individuals with ULA and MSCs compared to controls with MSCs (LOE 4) [Citation6]. Disability was also higher for people experiencing severe back pain and people with moderate and severe neck pain [Citation33]. Correlations with other types of pain were also found in two studies (LOE 4) [Citation31,Citation33].
Category 2: activities and participation
Performing physically demanding tasks played a role in the development and perseverance of MSCs according to the participants of the FG, as well as compensatory movements with the non-affected limb (LOE 5). “I am missing my left hand and I, in order to compensate, perform everything with the right side. And that makes me really chronically overloading my right shoulder. But also my head, neck; just the whole area. Sometimes I have, how do you call that stupid, stupid thing, … a tennis elbow.” This was supported by one study [Citation6], which observed significantly higher upper extremity work demands for people with ULA, who had MSCs compared to people with ULA, who did not have MSCs, or those without ULA (LOE 4). Participants from another study [Citation20] reported that heavy physical work increased their pain (LOE 5). Additionally, two case reports [Citation30] showed that physical work could lead to complaints (LOE 5). The possible effect of compensatory movements was mentioned in five studies [Citation6,Citation18–20,Citation33], all expert opinions (LOE 5). Furthermore, participants of the FG mentioned that changes in the intensity of prosthetic use, i.e., receiving a prosthesis after a period of repair, altered their behavior (LOE 5). As soon as the prosthesis was available again, participants performed too many activities in a short while, which resulted in complaints. Task-related factors, such as repetition of movements, the duration of tasks, force, and posture, were also mentioned by the FG (LOE 5) and several studies [Citation6,Citation30] as factors associated with the development of complaints (LOE 5). Several participants reported that static work and fine motor movements increased pain (LOE 5) [Citation20]. Focus group participants mentioned that their physical fitness could be helpful in preventing complaints (LOE 5), which was also observed in the literature [Citation20] which reported physiotherapy, physical activity, and exercise as factors for reducing pain (LOE 5). The influence of rest was mentioned to be a facilitator as well as a barrier (LOE 5) [Citation20]. Grip ability was not significantly associated with chronic pain (LOE 4) [Citation20].
Category 3: environmental factors
Factors that affected MSCs negatively, mentioned by the FG, were the expectations of family members and employers (LOE 5). These expectations could be vocalized expectations, but also entailed the participants’ ideas and perceptions about the expectations. This perception was mentioned to be affected by childhood upbringing (LOE 5). Participants of the FG also mentioned the physical activities that come with parenthood, such as lifting children, as a possible barrier for the development of MSCs (LOE 5), which was not observed in the literature (LOE 4) [Citation20]. In contrast, the environment could also have a positive effect on complaints. The support and help of employers and family were mentioned to influence MSCs in a positive way (LOE 5). “… I do office work. Right away ICT looked at my situation like, what can be useful for you. A regular mouse is not useful. …. It was addressed immediately. Very easy.” Aside from facilities at work, adjustments in a general and home environment, such as multiple bags for groceries in order to lift less weight in one movement, were also mentioned (LOE 5). Two other barriers addressed in the FG were problems regarding rehabilitation (LOE 5). Participants felt that the focus of the therapy was mainly on returning to work and being socially relevant, instead of a personalized plan on what might work for the client (LOE 5). Moreover, the limited knowledge about prosthetic options and available therapies was also discussed as a problem (LOE 5). Dependent on the place of residence some participants had limited access to health care options and were less knowledgeable about the possibilities.
Category 4: personal factors: general
Differences between AA and RD were addressed in the FG (LOE 5). The participants mentioned that after an amputation complaints would occur sooner due to problems with accepting and coping with a decreased function after amputation. Persons with AA experienced more disability when having MSCs, compared to RD (LOE 4) [Citation6]. In contrast, persons with RD did not have problems more often or problems that were more severe than those with an AA (LOE 4) [Citation18]. The level of deficiency was not related to MSCs (LOE 4 [Citation6,Citation18,Citation31,Citation33], LOE 5 [Citation30]). Bilateral amputees were associated with any neck pain (LOE 4) [Citation33]. One participant of the FG assumed an association between longer time since amputation and MSCs (LOE 5); however, this was not observed in five studies (LOE 4) [Citation6,Citation18,Citation31–33]. Higher age was a predictor in two studies [Citation6,Citation33], but not in others (LOE 4) [Citation20,Citation31,Citation32]. While three studies found no significant association between sex and pain [Citation6,Citation8,Citation33], another reported more RLP and neck pain among men [Citation32], whereas a fifth study reported that women were 5.5 times more likely to report chronic pain (LOE 4) [Citation20]. Side of deficiency, i.e., absence of the right limb, and being divorced or widowed were predictors for MSCs (LOE 4) [Citation6]. Individuals with an amputation due to cancer had lower odds of having any neck pain, and due to combat injuries had lower neck pain intensity (LOE 4) [Citation33]. However, the cause of amputation was not significant in another study (LOE 4) [Citation32]. Ethnicity, e.g., people identifying as Hispanic, was significant for any neck pain, but not for back pain or intensity (LOE 4) [Citation33]. Black or unknown race was significantly associated with greater back and neck pain intensity (LOE 4) [Citation33]. Education [Citation6,Citation20], employment status [Citation6,Citation20], amputation of the dominant hand [Citation6,Citation33], and living status [Citation20] were unrelated to MSCs (LOE 4).
Category 4: personal factors: psychology and cognition
The most frequently mentioned factor by the FG concerned problems with setting boundaries (LOE 5). Participants felt that they had to give at least “150%” in all their activities, in order to live up to the expectations of themselves or the environment, resulting in overuse complaints (LOE 5). They also wanted to be independent and not ask other people for help (LOE 5). “…, with what I do have, wanted to overcompensate. More in a way to prove: I can do everything. What you can do, I can do too. And then some extra.” Difficulty to accept that they suffered a loss of function and, especially in those with AAs, their wish to return to work in the same manner as before the amputation, contributed considerably to the development of MSCs (LOE 5). One participant explained that it took a while before he realized that he did not have to perform equally to a two-handed person. “At a certain moment, an occupational physician told me, he really told me: ‘You only have one hand, which means that you don’t have to be able to do as much.’. That really felt like a bowling ball that dropped.” Furthermore, participants mentioned that they sometimes wanted to feel like a normal person who is not completely focused on complaints or disabilities (LOE 5). These feelings lowered participants’ level of discipline and motivation to apply the knowledge given in the therapy sessions (LOE 5). Quality of life was investigated in five studies and showed overall that mental and physical health were lower in people with MSCs or MSC-related disability, and that pain intensity was higher (LOE 4) [Citation6,Citation19,Citation20,Citation32,Citation33]. Stress was reported to increase pain (LOE 5) [Citation20]. Coping style was only significantly different between individuals with ULA and controls on the support seeking subscale (LOE 4) [Citation6].
Category 5: prosthesis-related factors
The participants mentioned the heavy weight of the prosthesis as a contributing factor for the development of MSCs (LOE 5). In order to prevent the prosthesis from pulling on the remaining structures of the upper limb and thus to prevent the development and persistence of MSCs, the prosthetic hand was placed in the pocket of a jacket or on a purse that was hanging from the shoulder. Another factor was the length of the prosthesis, affecting the symmetry of the posture (LOE 5). “This elbow is a bit too long, and sometimes I sit a bit skewed. Well, then after a day, you will have complaints if you wear it [the prosthesis] all day long.” The amount of force and the control needed to perform activities could also lead to complaints according to FG participants (LOE 5). Participants mentioned that it was important to be able to rely on the prosthesis, i.e., on prosthesis fit and the grasp function (LOE 5). Trusting that the prosthesis holds during the execution of tasks, allowed the participants to assume an upright posture and an evenly balanced load on both arms. Furthermore, the participants mentioned that the type of prosthesis and hand could influence MSCs (LOE 5). The type of prosthesis was only significantly associated with the presence of carpal tunnel syndrome (CTS) (LOE 4) [Citation18,Citation33]. Prosthesis use showed no relation with MSCs or pain [Citation6–8,Citation20,Citation31,Citation32], except for any back pain (LOE 4) [Citation33]. But persons who did not wear a prosthesis were more likely to experience MSCs-related disability (LOE 4) [Citation6]. Prosthetic use was not protective for the remaining arm (LOE 5) [Citation30]. The number of hours the prosthesis was worn [Citation18,Citation31,Citation33], the frequency of prosthesis use [Citation33], nor the number of activities performed with the prosthesis [Citation18] were significantly associated with pain nor with the presence of CTS (LOE 4). The complaints of one participant were exacerbated by the harness of the prosthesis (LOE 5) [Citation30].
Discussion
An overview of 67 facilitators and barriers associated with MSCs in individuals with ULA, categorized based on ICF, was created. The participants of the FG predominantly mentioned contextual factors; either personal or environmental. Contrasting, the studies from the scoping review dominantly addressed factors related to functioning, e.g., body functions and structures, and activities and participation. The results of this study, combining two methodologies, showed that biopsychosocial factors from different domains are associated with the presence of MSCs in individuals with ULA.
Category 1: body functions and structures
Individuals with AA reported more neck and back pain if they experienced pain prior to the amputation [Citation32]. Pain prior to the amputation could indicate pre-existing neuroplastic changes [Citation32,Citation34,Citation35]. Neuroplasticity could alter the functional status of nociceptive neurons and central sensitization could occur [Citation36–38]. Pain due to central sensitization can be elicited by normally subthreshold innocuous stimuli (allodynia), can increase and prolong the response to noxious stimuli (hyperalgesia), and can spread to areas larger than the initial pain area (secondary hyperalgesia) [Citation36–38]. The latter was also observed in two studies [Citation31,Citation33] and suggests that different pain mechanisms can (simultaneously) be present. As a nociplastic pain mechanism warrants a different treatment approach compared to a nociceptive pain mechanism, this should be a focus of future studies [Citation36–38].
Category 2: activities and participation
Compensatory movements were frequently mentioned; however, studies only provided expert opinions and suggestions for further research [Citation6,Citation18–20,Citation33]. To execute daily activities, individuals with ULA compensate for the loss of function by using alternative, adjusted, and awkward postures. Especially prosthesis users, often with a stiff wrist or limited function in the elbow, execute activities by moving other joints in order to adequately position the limb. But also when no prosthesis is used tasks are performed differently due to one-handedness. These suboptimal anatomical postures increase strains on different body structures, which could contribute to MSCs [Citation7]. Furthermore, the load is transferred to remaining body structures and repetition and force are increased [Citation3]. As a result, the covering muscles are prone to micro-traumata and inflammation [Citation13]. Ultimately, with insufficient recovery, tissue failure may emerge [Citation13]. These biomechanical factors are substantiated with factors associated with MSCs in the two-handed population [Citation13,Citation39–41]. However, future research should determine in more detail the influence of these factors on the development and persistence of MSCs, including their interaction with other biological and psychosocial factors.
Category 3: environmental factors
Facilities and support of their environment were deemed important by the participants of the FG. In a rehabilitation process, it could therefore be helpful to include significant others and employers in decisions regarding the patient’s work and personal life. It could also be helpful to facilitate peer support to discuss problems and solutions. This could also be suitable to address psychosocial factors (see category 4). Furthermore, future research and practice should also look into possibilities to broaden the patients’ knowledge about prosthetic options and available therapies, to make these accessible to all individuals with ULA.
Category 4: personal factors: general and psychology & cognition
The participants of the FG highlighted the importance of psychosocial factors regarding MSCs. They mentioned that treatment often considered more biological than psychological aspects. Furthermore, they had difficulties setting boundaries and accepting their disability and loss of function. Psychological factors were clearly underrepresented in the studies in this review, which is in contrast to the MSCs studies in the two-handed patients. This may be insightful for clinicians because they should not only look at the biomechanical aspects of functioning but also at the effects of psychosocial aspects on functioning and the development and persistence of MSCs.
Category 5: Prosthesis-related factors
Prosthesis use does not seem to have an effect on MSCs [Citation6–8,Citation20,Citation31–33]. An explanation for this could be that in these studies prosthesis use was defined dichotomously, even though prosthesis type, wearing time, and use may vary considerably [Citation18,Citation31–33,Citation42,Citation43]. Another explanation could be that prosthesis use influences specific regions of the trunk and upper limb, by means of prosthesis weight and alterations of bodily movements. The study by Hanley et al. [Citation32] did observe a positive trend between prosthesis use and RLP, and the study by Resnik et al. [Citation33] found a lower back pain prevalence for prosthesis users, but not for the other body regions. It seemed that the prevalence of MSCs in different body regions varied between wearers and non-wearers [Citation7]. For now, it is still unclear whether prosthesis use prevents or exacerbates MSCs and the evidence is inconclusive.
Strengths and limitations
To our knowledge, this study is the first to consider participant opinions in investigating factors associated with MSCs and to provide an overview of all those factors. This study confirms that target populations should be more involved in the process of creating such an overview. Another strength was that the participants of the FG were diverse with regard to age, cause of ULA, level of limb loss, prosthesis use, and presence of MSCs. However, one must consider that different samples, e.g., another nationality or cultural background, could differ in their opinions and introduce additional factors. Furthermore, other single-handed individuals, e.g., people with brachial plexus injury, may propose other factors associated with MSCs. This may limit the generalizability of the results. Another strength of this study was the combination of qualitative and quantitative data. This showed similarities and differences between the two types of data and could identify gaps and help synthesize research priorities.
For the FG, the participants filled in a questionnaire about socio-demographic data and details about MSCs. As there is no standardized measure for MSCs, we used the same questions as previously used [Citation6]. Additional measures for impairment or disability were not included, because it was not the main focus of this study. Most of the participants wore a myoelectric prosthesis and none of the participants of the FG wore a body-powered prosthesis. This could represent a higher socioeconomic status of the included participants, which might threaten generalizability to persons with a lower socioeconomic status. However, because the studies of the scoping study included body-powered prosthesis users, we argue that the created overview could be used for body-powered prosthesis users as well.
The quotes of the FG could not be assigned to specific participants, and we could therefore not distinguish between individuals with a congenital difference and individuals with an amputation. For future research, we would advise to video-tape the FG session to prevent this issue. The quotes were first translated by a single translator (AAP) as literally as possible and then checked by the co-authors. However, this could still have resulted in biased responses or missed nuances.
MSCs is an umbrella term and definitions varied greatly between studies. The MSCs were self-reported which makes the outcome susceptible to interpretation and bias. We excluded studies that solely presented stump pain and phantom limb pain. Not all studies clearly defined whether stump pain was explicitly excluded, especially when asking about RLP. As the outcomes were subjective and participants could have difficulty distinguishing stump and phantom limb pain from remaining pain problems, we may have overestimated the effects. However, we are confident that the overview we created could aid clinicians in approaching their patients’ problems regarding MSCs from a broader perspective.
In scoping reviews, it is not the standard to perform an assessment of methodological limitations or risk of bias tools. Furthermore, scoping reviews often include multiple types of studies, which makes it challenging to assess the included articles consistently. Only cross-sectional studies were identified, making it more easily comparable after the assessment of methodological limitations. Some points on the quality assessment stood out. All studies scored low on the question about the justification of the sample size. This could perhaps be explained by the design of the studies and an overall small population of individuals with ULA. Almost all articles also scored low on the question about non-respondents. Due to privacy regulations, demographic data of the non-respondents often is not allowed to be obtained, so the reason for not participating remains unknown. One study [Citation30] scored low on several points, this is probably due to the fact that this is an older article, and standards regarding reporting and methodological assessments might have been different.
If we had performed the study in a reverse order (starting with the scoping review, followed by the FG), the factors resulting from the scoping review could have been validated with the participants of the FG. It is however possible that factors not presented in the literature, e.g., psychosocial factors, would have remained underexposed in this way. Allowing the participants to speak freely about their experiences without constraining them towards certain topics, may have resulted in more factors and probably has broadened the overview.
Conclusions
The FG and scoping review provided an extensive overview of 67 factors that are associated with the development and persistence of MSCs in people with ULA. The large number of psychosocial factors mentioned by the FG addresses the need for awareness of clinicians in that domain. It also reinforces the need for patients, clinicians, and researchers to address this topic from a biopsychosocial perspective. Consequently, future research should also integrate pain mechanisms other than nociceptive and how it interacts with biomechanical factors. This overview can help in the better understanding of MSCs and aid clinicians in their plan to prevent or to treat MSCs in patients with ULA.
Appendices_revised.docx
Download MS Word (97.2 KB)Acknowledgements
The authors would like to thank the focus group members for their valuable time and contributions. The authors would also like to thank S. van Twillert for moderating the focus group. Furthermore, the authors would like to thank M.A. de Bruin for her contribution as second assessor, helping with the coding and reviewing process.
Disclosure statement
S.G. Postema, M.F. Reneman, and C.K. van der Sluis were authors of one of the included studies in the scoping review. To prevent competing interests, that study was assessed by the other reviewers. The authors report no conflict of interest.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- van Tulder M, Malmivaara A, Koes B. Repetitive strain injury. Lancet. 2007;369(9575):1815–1822.
- Yassi A. Repetitive strain injuries. Lancet. 1997;349(9056):943–947.
- Huisstede BM, Miedema HS, Verhagen AP, et al. Multidisciplinary consensus on the terminology and classification of complaints of the arm, neck and/or shoulder. Occup Environ Med. 2007;64(5):313–319.
- Volkgezondheidsenzorg.info: Ranglijst aandoeningen op basis van ziektelast (in DALY's) [Internet]; 2021 [cited 2021 Jul 23]. Available from: https://www.volksgezondheidenzorg.info/ranglijst/ranglijst-aandoeningen-op-basis-van-ziektelast-dalys
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1789–1858.
- Postema SG, Bongers RM, Brouwers MA, et al. Musculoskeletal complaints in transverse upper limb reduction deficiency and amputation in The Netherlands: prevalence, predictors, and effect on health. Arch Phys Med Rehabil. 2016;97(7):1137–1145.
- Østlie K, Franklin RJ, Skjeldal OH, et al. Musculoskeletal pain and overuse syndromes in adult acquired major upper-limb amputees. Arch Phys Med Rehabil. 2011;92(12):1967–1973.e1.
- Johansen H, Østlie K, Andersen LØ, et al. Adults with congenital limb deficiency in Norway: demographic and clinical features, pain and the use of health care and welfare services. A cross-sectional study. Disabil Rehabil. 2015;37(22):2076–2082.
- Marshall M, Helmes E, Deathe AB. A comparison of psychosocial functioning and personality in amputee and chronic pain populations. Clin J Pain. 1992;8(4):351–357.
- World Health Organization. Towards a common language for functioning, disability and health. ICF – The International Classification for Functioning, Disability and Health. Geneva: CHE; 2002.
- Ariëns GA, Bongers PM, Hoogendoorn WE, et al. High quantitative job demands and low coworker support as risk factors for neck pain: results of a prospective cohort study. Spine. 2001;26(17):1896–1901.
- Feveile H, Jensen C, Burr H. Risk factors for neck-shoulder and wrist-hand symptoms in a 5-year follow-up study of 3,990 employees in Denmark. Int Arch Occup Environ Health. 2002;75(4):243–251.
- Huang GD, Feuerstein M, Kop WJ, et al. Individual and combined impacts of biomechanical and work organization factors in work-related musculoskeletal symptoms. Am J Ind Med. 2003;43(5):495–506.
- van Rijn RM, Huisstede BM, Koes BW, et al. Associations between work-related factors and specific disorders of the shoulder—a systematic review of the literature. Scand J Work Environ Health. 2010;36(3):189–201.
- Kraatz S, Lang J, Kraus T, et al. The incremental effect of psychosocial workplace factors on the development of neck and shoulder disorders: a systematic review of longitudinal studies. Int Arch Occup Environ Health. 2013;86(4):375–395.
- Picavet HSJ, Schouten JSAG. Musculoskeletal pain in The Netherlands: prevalences, consequences and risk groups, the DMC3-study. Pain. 2003;102(1):167–178.
- van den Heuvel SG, van der Beek AJ, Blatter BM, et al. Psychosocial work characteristics in relation to neck and upper limb symptoms. Pain. 2005;114(1–2):47–53.
- Burger H, Vidmar G. A survey of overuse problems in patients with acquired or congenital upper limb deficiency. Prosthet Orthot Int. 2016;40(4):497–502.
- Johansen H, Østlie K, Andersen LØ, et al. Health-related quality of life in adults with congenital unilateral upper limb deficiency in Norway. A cross-sectional study. Disabil Rehabil. 2016;38(23):2305–2314.
- Johansen H, Bathen T, Andersen LØ, et al. Chronic pain and fatigue in adults with congenital unilateral upper limb deficiency in Norway. A cross-sectional study. PLOS One. 2018;13(1):e0190567.
- Creswell JW. A concise introduction to mixed methods research. Thousands Oaks (CA): SAGE Publications, Inc.; 2015.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357.
- Peters AA, Postema SG, Reneman MF, et al. Associated factors for musculoskeletal complaints in subjects with upper limb absence – focus group results and a scoping review; [Internet]. OSF; 2020 [cited 2021 Jul 23]. Available from: osf.io/qcy2m
- Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473.
- Peters MDJ, Godfrey C, McInerney P, et al. Chapter 11: scoping reviews (2020 version). In: Aromataris E, Munn Z, editors. [Internet]; [cited 2021 Jul 23]; JBI; 2020. Available from: https://synthesismanual.jbi.global
- Downes MJ, Brennan ML, Williams HC, et al. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open. 2016;6(12):e011458.
- Barnett-Page E, Thomas J. Methods for the synthesis of qualitative research: a critical review. BMC Med Res Methodol. 2009;9:59.
- Pope C, Ziebland S, Mays N. Qualitative research in health care. Analysing qualitative data. BMJ. 2000;320(7227):114–116.
- OCEBM Levels of Evidence Working Group. The Oxford levels of evidence 2 [Internet]; 2011 [cited 2021 Jul 23]. Available from: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence
- Jones LE, Davidson JH. Save that arm: a study of problems in the remaining arm of unilateral upper limb amputees. Prosthet Orthot Int. 1999;23(1):55–58.
- Desmond DM, Maclachlan M. Prevalence and characteristics of phantom limb pain and residual limb pain in the long term after upper limb amputation. Int J Rehabil Res. 2010;33(3):279–282.
- Hanley MA, Ehde DM, Jensen M, et al. Chronic pain associated with upper-limb loss. Am J Phys Med Rehabil. 2009;88(9):742–751.
- Resnik L, Borgia M, Clark MA. The prevalence and impact of back and neck pain in veterans with upper limb amputation. Am J Phys Med Rehabil. 2021;100(11):1042–1053.
- Katz J, Melzack R. Pain 'memories' in phantom limbs: review and clinical observations. Pain. 1990;43(3):319–336.
- Flor H. The modification of cortical reorganization and chronic pain by sensory feedback. Appl Psychophysiol Biofeedback. 2002;27(3):215–227.
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926.
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl.):S2–S15.
- Woolf CJ. What is this thing called pain? J Clin Invest. 2010;120(11):3742–3744.
- Celik S, Celik K, Dirimese E, et al. Determination of pain in musculoskeletal system reported by office workers and the pain risk factors. Int J Occup Med Environ Health. 2017;31(1):91–111.
- Gómez-Galán M, Pérez-Alonso J, Callejón-Ferre Á, et al. Musculoskeletal disorders: OWAS review. Ind Health. 2017;55(4):314–337.
- Bozkurt S, Demirsoy N, Günendi Z. Risk factors associated with work-related musculoskeletal disorders in dentistry. Clin Invest Med. 2016;39(6):27527.
- Datta D, Selvarajah K, Davey N. Functional outcome of patients with proximal upper limb deficiency-acquired and congenital. Clin Rehabil. 2004;18(2):172–177.
- Dudkiewicz I, Gabrielov R, Seiv-Ner I, et al. Evaluation of prosthetic usage in upper limb amputees. Disabil Rehabil. 2004;26(1):60–63.

