Abstract
Purpose
The purpose of this review was to compare all intervention modalities aimed at increasing skeletal muscle mass (SMM) in the paralysed limbs of persons with chronic (>1-year post-injury), motor complete spinal cord injury (SCI).
Materials and methods
A systematic review of EMBASE, MEDLINE, Scopus, and SPORTDiscus databases was conducted from inception until December 2021. Published intervention studies aimed to increase SMM (measured by magnetic resonance imaging, computed tomography, ultrasound, muscle biopsy, or lean soft tissue mass by dual X-ray absorptiometry) in the paralysed limbs of adults (>18 years) with SCI were included.
Results
Fifty articles were included that, overall, demonstrated a high risk of bias. Studies were categorised into six groups: neuromuscular electrical stimulation (NMES) with and without external resistance, functional electrical stimulation cycling, walking- and standing-based interventions, pharmacological treatments, and studies that compared or combined intervention modalities. Resistance training (RT) using NMES on the quadriceps produced the largest and most consistent increases in SMM of all intervention modalities.
Conclusions
Current evidence suggests that clinical practise aiming to increase SMM in the paralysed limbs of persons with motor complete SCI should perform NMES-RT. However, more high-quality randomised control trials are needed to determine how training variables, such as exercise volume and intensity, can be optimised for increasing SMM.
Persons with spinal cord injury (SCI) experience severe reductions in skeletal muscle mass (SMM) post-injury, which may exacerbate their risk of obesity and metabolic disease.
Out of all exercise and non-exercise-based interventions, this systematic review shows that neuromuscular electrical stimulation-based resistance training demonstrates the most robust and consistent evidence for increasing skeletal muscle mass in the paralysed limbs of adults with motor complete spinal cord injury.
The findings from this review can be used to inform evidence-based practise for exercise practitioners, as well as direct future research focused on increasing muscle mass in this population.
Implications for rehabilitation
Introduction
Spinal cord injury (SCI) impairs neurological communication below the level of injury resulting in varying degrees of sensory, motor, and autonomic impairments depending on the location and severity of the lesion. Skeletal muscle mass (SMM) is lost rapidly due to immobilisation and reductions in mechanical loading, with up to 45% of paralysed muscle cross-sectional area being lost within the first six weeks post-injury [Citation1,Citation2]. A lack of voluntary neural muscle activation also induces atrophy at an accelerated rate compared to persons without SCI (non-SCI) [Citation3].
Beyond its primary locomotive function, skeletal muscle performs many vital metabolic functions and contributes to resting metabolic rate (RMR) [Citation4–6]. A loss of SMM therefore predisposes to an increase in adiposity and associated long-term conditions including type 2 diabetes and cardiovascular disease [Citation7–9]. For these reasons, strategies to maintain, and subsequently enhance SMM post SCI, are of high importance.
In individuals prone to muscle loss, such as those with sarcopenia or multiple sclerosis, exercise and non-exercise (e.g., dietary, pharmacological) approaches are commonly used to prevent and reverse atrophy. Regular exercise that involves loading of the muscle (i.e., resistance training [RT]) is universally accepted as a fundamental method for maintaining and increasing muscle mass in healthy individuals. However, traditional RT is not feasible for persons with motor complete SCI. Neuromuscular electrical stimulation (NMES) is used in persons with SCI to induce involuntary contractions in the skeletal muscle. Using NMES, simple movements, such as knee extensions [Citation10], and functional movements, such as cycling, rowing, and standing, known as functional electrical stimulation (FES) [Citation11–13], can be performed. Thus, electrical stimulation can facilitate structured RT- and aerobic-based exercise, which can elicit numerous cardio-metabolic benefits, including restoring SMM [Citation14,Citation15]. However, the optimal strategy for inducing muscle hypertrophy has not been systematically evaluated.
Non-exercise adjunctive interventions are also well-established for restoring skeletal muscle in atrophy-prone non-SCI individuals [Citation16,Citation17]. For example, older individuals with sarcopenia are recommended to increase total daily protein intake from 0.8 to 1–1.5 g/kg/day in conjunction with vitamin D supplementation and regular RT (20–30 min, thrice weekly) to slow and prevent atrophy [Citation18]. There is also substantial evidence to show that pharmacological agents such as testosterone [Citation19,Citation20], nandrolone [Citation21,Citation22], and growth hormone [Citation23,Citation24] are effective in attenuating the effects of sarcopenia. However, little is known about the efficacy of dietary and pharmacological interventions in persons with motor complete SCI.
In order to identify all strategies for increasing SMM in persons with SCI, and contribute to developing best-practise guidelines, we conducted a systematic review to identify, appraise, and compare all current intervention modalities to increase SMM in persons with chronic, motor complete SCI.
Methods
Searches
A systematic review was conducted using Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines by two independent examiners (JMF and SPH) [Citation25]. Four databases (EMBASE, MEDLINE, Scopus, and SPORTDiscus) were used to search for the title, abstract and keywords of relevant articles from inception to 31 December 2021. Terms relating to SCI (e.g., “spinal cord injury,” “paraplegia”, “tetraplegia”) and skeletal muscle (e.g., “fat-free mass,” “muscle mass”) were used in the literature search (a full list of search terms can be found in Supplementary File 1: Table e-1). The following search limitations were used in conjunction with the search terms where possible: articles originally written in English, human participants and participants ≥18 years of age. After removing duplicate records, studies were selected by reviewing the title and abstract, followed by the whole text. Reference lists of the selected articles and related review papers were also assessed to find additional relevant studies. Differences in selected articles between reviewers were discussed to produce the final selection of studies.
Study inclusion and exclusion criteria
Published randomised control trials (RCTs), uncontrolled trials, longitudinal studies, case series and case reports investigating interventions that aimed to increase SMM were included. Abstracts, observational studies, editorials, commentaries, reviews, book chapters, and animal studies were excluded. Articles were included if all participants had a traumatic or non-traumatic motor complete SCI were over 18 years of age and were a minimum of one-year post-injury [Citation26]. The American Spinal Injury Association impairment scale (AIS), a universal classification tool for spinal cord injuries, was used to define motor complete (AIS grade “A” or “B”) and motor incomplete injuries (AIS grade “C” or “D”) in the present review. Included studies had at least one outcome measure of changes in SMM, muscle fibre size or cross-sectional area, including magnetic resonance imaging (MRI), computed tomography (CT), ultrasound, and skeletal muscle biopsy. Studies that measured fat- and bone-free lean soft tissue mass (LSTM) using dual X-ray absorptiometry (DXA) were also included, given the strong association between MRI- and DXA-derived values [Citation27]. Studies exclusively using skinfold measurements, bioelectrical impedance, or circumferences to analyse body composition were excluded based on their reduced validity for people with SCI [Citation28,Citation29].
Data extraction
Once all the studies that fulfilled the eligibility criteria were identified, they were categorised into one of the following groups based on the intervention investigated: neuromuscular electrical stimulation-RT-based interventions (NMES-RT), NMES interventions without resistance, functional electrical stimulation (FES) cycling-based interventions, standing- and walking-based interventions, pharmacological interventions, and mixed modality interventions. Data relating to the study design, cohort size, participant age, duration and level of injury, intervention duration and frequency, changes in, and method of measuring, SMM/LSTM were extracted. Where relevant data were not reported, the study authors were contacted asking for data and if no response had been received after two weeks, the study was excluded.
Quality assessment
Two independent reviewers (JMF and SPH) assessed the risk of bias using methods described previously [Citation30]. Briefly, methodological quality was determined initially by study design, and secondly using cut-off scores from the Physiotherapy Evidence Database (PEDro) Score [Citation31] and the Downs and Black Tool [Citation32], which assess RCTs and non-randomised studies, respectively. Each study was categorised into a four-level rating system, with Level 1 studies demonstrating the lowest risk of bias and Level 4 studies the highest. For example, an RCT with a PEDro score ≥6 is Level 1, and a Uncontrolled intervention study with a Downs and Black score <21 is Level 4 (Supplementary File 1: Table e-2). In the event of any discrepancies in scoring, reviewers discussed the article until a consensus was reached.
Results
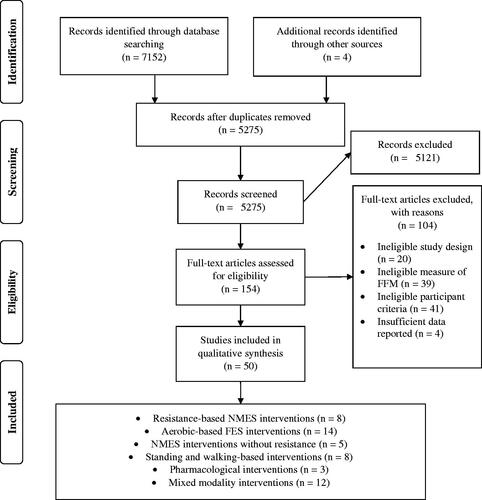
The database searches yielded 7152 articles. Following deduplication and screening for eligibility, 50 studies remained and were included in the review, as illustrated in . There were no discrepancies regarding the inclusion decisions between the two independent reviewers.
Study design & quality assessment
The selected studies demonstrated a range of study designs, including uncontrolled trials (30% 15/50), RCTs (20%, 10/50), longitudinal studies (8%, 4/50), case series (4%, 2/50), within-subject controlled trials (4%, 2/50) a crossover study (2%, 1/50), and most frequently, case reports (32%, 16/50). Consequently, the quality of evidence illustrated an overall high risk of bias, with 35 studies classified as Level 4 (70%), 3 studies as Level 3 (6%), 4 studies as Level 2 (8%), and 8 studies as Level 1 (16%). However, one Level 1 study split the results across two papers [Citation15,Citation33], and another Level 1 study published their results over four separate papers [Citation34–37], meaning only four unique RCTs were identified. Interventions lasted from 2 to 113 weeks and were conducted 1 to 7 times per week.
Participants
There were a total of 303 unique participants, of which at least 258 (86%) were male (two studies did not report their subject’s sex) [Citation38,Citation39]. Time since injury ranged from 1 to 35 years, and level of injury ranged from C4-T12, with one study recruiting participants with lumbar SCI (level not specified) [Citation40]. Participants’ ages ranged from 18 to 68 years.
Interventions
There was a high degree of heterogeneity in study designs, making further analysis (i.e., meta-analysis) untenable.
NMES-RT interventions
Eight studies (16%) investigated the effects of transcutaneous NMES-RT of the quadriceps on changes in SMM and LSTM using dynamic (n = 7) [Citation10,Citation15,Citation33,Citation41–44] or isometric (n = 1) [Citation45] knee extensions (). Five studies used MRI to measure changes in quadriceps SMM, demonstrating a significantincrease of 18–43% over the intervention period [Citation15,Citation41–44]. Only one study reported no significant change in quadriceps muscle size, despite a numerical 10% increase in vastus lateralis cross-sectional area [Citation45]. Surrounding muscles, including the hamstring, hip adductor, and hip flexor muscle groups, also showed positive changes in size ranging from 4 to 43% [Citation15,Citation33,Citation41,Citation43].
Table 1. Neuromuscular electrical stimulation-based interventions (n = 13).
NMES interventions without resistance
Five additional articles (10%) conducted NMES interventions without external resistance (). Surface electrodes were used to stimulate the quadriceps (n = 2) [Citation46,Citation47] and tibialis anterior (n = 1) [Citation48], and percutaneous electrodes were used to stimulate the glute muscles (n = 1) [Citation49]. One study also investigated increasing blood pressure via epidural electrical stimulation on changes in LSTM [Citation50]. Although CT scans from two case reports revealed large increases in quadriceps (45–61%) [Citation46], and gluteal (44–50%) [Citation49] cross-sectional areas, MRI scans established no statistically significant changes in quadriceps SMM (1–6%) [Citation47]. A statistically significant increase in LSTM of the legs (9%) was only reported in one study [Citation50].
FES cycling interventions
Fourteen studies (28%) utilised FES cycling as the main intervention, which featured asynchronous stimulation of the quadriceps, hamstrings, and gluteal muscle groups using surface electrodes for 30 min [Citation11,Citation14,Citation51–54], 60 min [Citation55–59], or for an increasing duration during the intervention [Citation60–62] (). Training sessions were conducted between 20 and 50 revolutions per minute (RPM) and with a stimulation frequency of 30–60 Hz. DXA was used to measure whole-body and leg LSTM in nine [Citation52–54,Citation57–62] and five [Citation52,Citation54,Citation59,Citation61,Citation62] studies, respectively, with positive changes varying from 2–10% and 4–7%. Studies in this category also illustrated increments of thigh SMM of 12–20% via MRI [Citation51,Citation55,Citation56] and 23–129% in vastus lateralis cross-sectional area [Citation11,Citation14].
Table 2. Functional electrical stimulation cycling-based interventions (n = 14).
Standing- and walking-based interventions
As shown in , standing- and walking-based interventions were used in eight of the selected studies (16%), four of which utilised body-weight supported treadmill training (BWSTT) [Citation12,Citation63–66]. BWSTT was supplemented by quadriceps NMES in one study [Citation12] and epidural electrical stimulation in the second phase of two studies [Citation64,Citation66]. Other intervention modalities included the use of a robotic exoskeleton (n = 2) [Citation67,Citation68] and passive standing on a vibrating platform (n = 1) [Citation69]. Changes in whole-body LSTM were varied, ranging from a 1–17% increase [Citation65–68].
Table 3. Standing and walking-based interventions (n = 8).
Pharmacological interventions
Three studies (6%) examined the independent effects of pharmacological interventions (). Despite the low sample sizes, MRI scans illustrated a positive influence of transdermal testosterone patches on thigh and quadriceps SMM (9–13% and 11–14% increase, respectively), as well as whole-body LSTM (7%) [Citation70,Citation71]. A significant increase in whole body, but not arm, LSTM were also seen following orally administered oxandrolone treatment (2% and 5%, respectively) [Citation72].
Table 4. Pharmacological interventions (n = 3).
Mixed modality interventions
Twelve studies (24%) incorporated two or more intervention modalities in their study design (). These included studies that compared the efficacy of different interventions (n = 6), studies that utilised interventions with two or more phases (n = 4), and studies that examined the synergistic effects of two treatments or interventions simultaneously (n = 2). Four studies incorporated an initial muscle conditioning (NMES) phase before FES cycling [Citation73,Citation74], FES rowing [Citation75], or NMES-RT [Citation40]. These studies documented significant increases in SMM of the thigh, though different measures were used. Another two studies used muscle biopsies to examine the impact of FES cycling on changes in SMM (1) in combination with oral salbutamol supplementation [Citation38] and (2) in comparison to isometric NMES-RT [Citation39]. Vastus lateralis cross-sectional area increased by 18–63% and 119% in these FES cycling and NMES-RT interventions, respectively. An electrical stimulation-based protocol was also combined with whole-body vibration [Citation76], yielding a significant improvement in calf muscle area (8–22%). Gorgey et al. reported (across four separate studies) that testosterone replacement therapy (TRT) in adjunction to NMES-RT stimulated greater increments in leg SMM and whole-body LSTM compared to TRT alone [Citation34–37]. Finally, no difference in leg LSTM was detected following an 8-week high protein diet. Further information on the study designs used in each category can be seen in Supplementary File 1: Tables e3-8.
Table 5. Mixed modality interventions (n = 12).
Outcome measurements
DXA was the most frequently used method of measuring changes in body composition (22/50 studies), followed by MRI (15/50 studies), muscle biopsy (10/50 studies), CT (7/50 studies) and ultrasound (2/50 studies). While these outcomes all represent a measure of skeletal muscle, they are measurements that yield distinctly different estimates, and thus we determined that they could not readily be compared [Citation77]. Six studies used multiple forms of measurement [Citation15,Citation44,Citation52,Citation67,Citation71,Citation78]. Most studies measured compositional changes of the legs (43/50 studies), predominantly the quadriceps (21/50 studies). Nineteen studies (38%) examined differences in whole-body LSTM, and 10 studies (20%) measured other body parts, including the trunk and upper limbs.
Discussion
The purpose of this systematic review was to evaluate all published methods of increasing SMM (and LSTM) in the paralysed limbs of persons with motor complete SCI. Overall, heterogeneity in study design and outcome measures, and low methodological quality, made it difficult to compare and identify optimal protocols for increasing SMM. Despite this, the present review highlights consistent evidence that electrical stimulation-based exercise, NMES-RT in particular, results in an increase in SMM in this population and thus is worthy of further review.
In the resistance-based NMES category, six studies illustrated that the Dudley Protocol [Citation79], 3–4 sets of 10 electrically stimulated knee extensions (30–35 Hz) performed twice a week with progressive resistance, elicited a significant increase in SMM over 8–16 weeks [Citation15,Citation33,Citation41–44]. These increments were seen predominantly in the quadriceps muscle group; however, two RCTs and one case report stated that, due to indirect stimulation and/or a localised adaptive response, the surrounding muscles of the upper leg also increased in size [Citation15,Citation33,Citation41]. Despite the mixed quality of evidence, we observed consistent findings that this particular NMES-RT protocol effectively increases SMM in persons with motor complete SCI. In the non-SCI literature, a substantial amount of research has been conducted which explores how training volume and variables (e.g., number of weekly sessions, number of sets and reps, training load) can be manipulated to optimise muscle hypertrophy [Citation80,Citation81]. Subsequently, additional research is warranted to explore the dose– and intensity–response relationship of NMES-RT volume on SMM in this population.
We also note that longer term NMES-RT may result in ongoing muscle hypertrophy. For example, a 39% increase in quadriceps muscle mass after 16 weeks [Citation44], 35% after 12 weeks [Citation15], and 18% after 8 weeks of using the Dudley Protocol (albeit only 3 sets of 10 repetitions were performed in this 8-week study) [Citation43]. Additionally, a review of this study design, including previously unpublished data, demonstrated that quadriceps SMM continued to grow from ∼44 to 78% from three to six months, relative to baseline [Citation82]. Longitudinal studies are, therefore, needed to confirm the ongoing effects of NMES-RT on muscle hypertrophy in this population.
The Dudley Protocol has also been investigated in conjunction with TRT, with MRI results showing an increase in quadriceps SMM by ∼31% after 16 weeks [Citation35]. However, in this RCT, no changes in SMM were reported for the TRT alone control group indicating that muscular contraction is required for hypertrophic effects, at least in persons with SCI. Interestingly, larger increments in quadriceps muscle mass can be seen (35–43%) in other studies that have used the Dudley Protocol for just 12 weeks, suggesting that TRT does not accentuate the hypertrophic response when administered alongside NMES-RT [Citation15,Citation41,Citation42]. On the contrary, case reports from the same research group recorded a 9-14% increase in quadriceps SMM after 16 weeks of TRT without NMES (using the same dosage) [Citation70,Citation71]. Thus, additional studies are required to understand the efficacy of TRT, with or without NMES, on SMM growth in the SCI population.
Two studies in the present review utilised a virtually supervised, home-based NMES-RT program, which elicited a significant increase in SMM of the quadriceps [Citation43,Citation44] and surrounding muscles [Citation43]. Home-based interventions have been recognised as an effective means of increasing intervention compliance in persons with SCI due to the attenuation of barriers such as transportation difficulties, and travel time and cost [Citation43,Citation83]. Furthermore, the minimal, lightweight equipment necessary for NMES allows participants to complete the training in any location (several participants completed the NMES protocol at work), and easily and autonomously set up the device. Thus, in addition to, training frequency, intensity, and stimulation parameters, location should also be considered as an important training variable when planning a NMES-RT intervention. As mentioned in a recent commentary, more home- or community-based interventions in persons with SCI may also help establish “real-world” effectiveness [Citation84].
Studies employing FES cycling protocols generally report mixed efficacy in increasing SMM and LSTM. Furthermore, the low overall methodological quality and heterogeneity in study design make it difficult to determine the best protocol parameters. Crameri et al. illustrated that NMES-RT evokes a more potent stimulus for muscle hypertrophy than FES cycling (119 vs. 18% increase in vastus lateralis muscle fibre cross-sectional area, respectively) [Citation39]. Notably, thrice weekly FES cycling for 26 weeks induced roughly half of the quadriceps growth compared to twice weekly NMES-RT for 12 weeks (10–20 vs. 35–43%, respectively) [Citation15,Citation41,Citation42,Citation55,Citation56]. This suggests that future research and rehabilitation programs designed to induce increases in SMM may be better directed toward optimising NMES-RT protocols for muscle hypertrophy instead of FES cycling. However, it is worth noting that results from Johnston et al. suggest a lower FES cycling cadence (20 RPM) may be preferable to a higher cadence (50 RPM) (20 vs. 11% increase in SMM, respectively) [Citation56]. These findings may have been due to the increased muscle time-under-tension, and the ability for the muscle to withstand greater levels of torque without fatigue compared to a higher RPM. This thesis is supported by a study of people with motor complete and incomplete injuries (AIS A-C) that documented a greater increase in thigh girth and torque after six weeks of low- (10 RPM) versus high-cadence (50 RPM) FES cycling [Citation85]. Thus, although FES cycling did not generally appear to generate the same level of muscle growth as NMES-RT, our findings imply that this exercise modality should not be dismissed as a redundant method of increasing muscle mass if sufficient stress on the muscle is induced.
The effectiveness of standing- and walking-based interventions on skeletal muscle is equivocal. Though two case reports found modest increments in lower limb LSTM [Citation63,Citation65], two non-controlled studies reported non-significant changes in leg LSTM and calf SMM after 6 and 40 weeks, respectively [Citation67,Citation69]. Furthermore, another case report documented a decrease in LSTM from months 6 to 12 of standing- and stepping-based activities [Citation64], suggesting that the benefits of this intervention modality do not extend to increasing muscle mass. Interestingly, the only study demonstrating a significant increase in quadriceps SMM supplemented gait training (30–50% body weight supported) with NMES [Citation12]. This finding further supports the conclusion that loaded NMES effectively induces muscle hypertrophy in a motor complete SCI population.
Ours is the first review to summarise and compare all studies aiming to increase SMM in a motor complete SCI population. The current findings can be used as a foundation developing exercise- and health-related guidelines and future rehabilitation-based research and inform evidence-based practice for rehabilitation professionals. The primary limitation of this review was the low number (studies and subjects), the heterogeneity in study designs and the overall low methodological quality of the included studies. The high proportion of low-quality research likely reflects the difficulties in conducting high-quality research (RCTs) in people with SCI. Factors such as difficulties with transportation, out of pocket expenses, and potential side effects or a decline in functionality have been identified as substantial barriers to participation in clinical research trials in this population [Citation86]. By enhancing facilitators and minimising the barriers to participation, such as reducing the number of visits to the research facility, using telemonitoring software and reimbursing travel costs, more persons with SCI may be willing to participate in RCTs [Citation86]. Furthermore, multi-centre approaches may be an additional strategy in the design of high-quality intervention studies. This meant that further analysis on the influence of variables such as age and time since injury was not possible. Another methodological limitation was that in only 4/50 studies were participants’ diets standardised (45% carbohydrate, 30% fat, 25% protein and caloric intake based on RMR) [Citation15,Citation33,Citation34,Citation71]. There is a well-established synergistic effect of increased dietary protein and exercise, RT in particular, on the growth and maintenance of muscle mass in non-SCI and atrophy-prone populations [Citation87,Citation88]. Consequently, the impact of diet on the magnitude of change in SMM is unclear for most studies in this review. It should also be noted that although strong correlations have been reported between cross-sectional point-estimates of MRI-derived values of muscle volume and DXA-derived values of lean mass [Citation27], the correlation between changes over time is less convincing [Citation27,Citation89]. Thus, studies using measures of changes in LSTM as a proxy for SMM should be interpreted with caution. Finally, although an increase in muscle size is important for maintaining metabolic health in persons with SCI, muscle quality and function also play a role. Future research should endeavour to address the efficacy of different interventions on these outcome measures.
Conclusions
In summary, the findings of the current review are limited by the low methodological quality and heterogeneity in study design. Nonetheless, NMES-RT demonstrated the most robust and consistent evidence for increasing SMM in adults with motor complete SCI of all interventions identified. Future studies should explore how training variables, such as volume, frequency, and stimulation parameters, can be manipulated to optimise hypertrophy. The ability to conduct home-based, virtually supervised NMES-RT may also be a more practical, low-cost, and feasible option over other intervention modalities, positively influencing adherence and providing more insight into the interventions’ “real-world” effectiveness.
Supplementary_file_1.docx
Download MS Word (138.2 KB)Acknowledgements
We acknowledge the funding from the Loughborough University, Peter Harrison Foundation, the Matt Hampson Foundation, and KC Suri. The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research (NIHR) or the Department of Health and Social Care. SMP thanks the Canada Research Chairs Program for support.
Disclosure statement
SMP is a named inventor on Canadian patent 3052324 and US patent 16/182891 (pending) both issued to Exerkine but reports no financial gains. SMP is an unpaid advisor on the scientific board of Enhanced Recovery. The results of the present study do not constitute endorsement by American College of Sports Medicine. We also declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Singh R, Rohilla R, Saini G, et al. Longitudinal study of body composition in spinal cord injury patients. IJOO. 2014;48(2):168–177.
- Castro MJ, Apple DF, Hillegass EA, et al. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol. 1999;80(4):373–378.
- Spungen AM, Adkins RH, Stewart CA, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol. 2003;95(6):2398–2407.
- Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84(3):475–482.
- Müller MJ, Wang Z, Heymsfield SB, et al. Advances in the understanding of specific metabolic rates of major organs and tissues in humans. Curr Opin Clin Nutr Metab Care. 2013;16(5):501–508.
- Johnstone AM, Murison SD, Duncan JS, et al. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am J Clin Nutr. 2005;82(5):941–948.
- Cragg JJ, Noonan VK, Krassioukov A, et al. Cardiovascular disease and spinal cord injury: results from a national population health survey. Neurology. 2013;81(8):723–728.
- Cragg JJ, Noonan VK, Dvorak M, et al. Spinal cord injury and type 2 diabetes: results from a population health survey. Neurology. 2013;81(21):1864–1868.
- Gorgey AS, Dolbow DR, Dolbow JD, et al. Effects of spinal cord injury on body composition and metabolic profile - part I. J Spinal Cord Med. 2014;37(6):693–702.
- Gorgey AS, Caudill C, Khalil RE. Effects of once weekly NMES training on knee extensors fatigue and body composition in a person with spinal cord injury. J Spinal Cord Med. 2016;39(1):99–102.
- Chilibeck PD, Jeon J, Weiss C, et al. Histochemical changes in muscle of individuals with spinal cord injury following functional electrical stimulated exercise training. Spinal Cord. 1999;37(4):264–268.
- de Abreu DCC, Cliquet A, Rondina JM, et al. Muscle hypertrophy in quadriplegics with combined electrical stimulation and body weight support training. Int J Rehabil Res. 2008;31(2):171–175.
- Kim DI, Park DS, Lee BS, et al. A six-week motor-driven functional electronic stimulation rowing program improves muscle strength and body composition in people with spinal cord injury: a pilot study. Spinal Cord. 2014;52(8):621–624.
- Crameri RM, Weston A, Climstein M, et al. Effects of electrical stimulation-induced leg training on skeletal muscle adaptability in spinal cord injury. Scand J Med Sci Sports. 2002;12(5):316–322.
- Gorgey AS, Mather KJ, Cupp HR, et al. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc. 2012;44(1):165–174.
- Malafarina V, Uriz-Otano F, Iniesta R, et al. Effectiveness of nutritional supplementation on muscle mass in treatment of sarcopenia in old age: a systematic review. J Am Med Dir Assoc. 2013;14(1):10–17.
- Neto WK, Gama EF, Rocha LY, et al. Effects of testosterone on lean mass gain in elderly men: systematic review with meta-analysis of controlled and randomized studies. Age. 2015;37(1):9742.
- Morley J, Argiles J, Evans W, et al. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010;11(6):391–396.
- Wittert G, Chapman I, Haren M, et al. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci. 2003;58(7):618–625.
- Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90(2):678–688.
- Macdonald J, Marcora S, Jibani M, et al. Nandrolone decanoate as anabolic therapy in chronic kidney disease: a randomized phase II dose-finding study. Nephron Clin Pract. 2007;106(3):c125–c135.
- Frisoli A, Chaves P, Pinheiro M, et al. The effect of nandrolone decanoate on bone mineral density, muscle mass, and hemoglobin levels in elderly women with osteoporosis: a double-blind, randomized, placebo-controlled clinical trial. J Gerontol A Biol Sci Med Sci. 2005;60(5):648–653.
- Rudman D, Feller A, Nagraj H, et al. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990;323(1):1–6.
- Blackman M, Sorkin J, Münzer T, et al. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288(18):2282–2292.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and Meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009; 339:b2700.
- Rodriguez DJ, Clevenger FW, Osler TM, et al. Obligatory negative nitrogen balance following spinal cord injury. JPEN J Parenter Enteral Nutr. 1991;15(3):319–322.
- Tavoian D, Ampomah K, Amano S, et al. Changes in DXA-derived lean mass and MRI-derived cross-sectional area of the thigh are modestly associated. Sci Rep. 2019;9(1):1–9.
- Goosey-Tolfrey V, Keil M, Brooke-Wavell K, et al. A comparison of methods for the estimation of body composition in highly trained wheelchair games players. Int J Sports Med. 2016;37(10):799–806.
- Mojtahedi MC, Valentine RJ, Evans EM. Body composition assessment in athletes with spinal cord injury: comparison of field methods with dual-energy X-ray absorptiometry. Spinal Cord. 2009;47(9):698–704.
- Van Der Scheer JW, Ginis KAM, Ditor DS, et al. Effects of exercise on fitness and health of adults with spinal cord injury: a systematic review. Neurology. 2017;89(7):736–745.
- Moseley AM, Herbert RD, Sherrington C, et al. Evidence for physiotherapy practice: a survey of the physiotherapy evidence database (PEDro). Aust J Physiother. 2002;48(1):43–49.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- Gorgey AS, Dolbow DR, Cifu DX, et al. Neuromuscular electrical stimulation attenuates thigh skeletal muscles atrophy but not trunk muscles after spinal cord injury. J Electromyogr Kinesiol. 2013;23(4):977–984.
- Gorgey AS, Khalil RE, Gill R, et al. Low-dose testosterone and evoked resistance exercise after spinal cord injury on cardio-metabolic risk factors: an open-label randomized clinical trial. J Neurotrauma. 2019;36(18):2631–2645.
- Holman ME, Gorgey AS. Testosterone and resistance training improve muscle quality in spinal cord injury. Med Sci Sports Exerc. 2019;51(8):1591–1598.
- Gorgey AS, Abilmona SM, Sima A, et al. A secondary analysis of testosterone and electrically evoked resistance training versus testosterone only (TEREX-SCI) on untrained muscles after spinal cord injury: a pilot randomized clinical trial. Spinal Cord. 2020;58(3):298–308.
- Gorgey AS, Graham ZA, Chen Q, et al. Sixteen weeks of testosterone with or without evoked resistance training on protein expression, fiber hypertrophy and mitochondrial health after spinal cord injury. J Appl Physiol. 2020;128(6):1487–1496.
- Murphy RJL, Hartkopp A, Gardiner PF, et al. Salbutamol effect in spinal cord injured individuals undergoing functional electrical stimulation training. Arch Phys Med Rehabil. 1999;80(10):1264–1267.
- Crameri RM, Cooper P, Sinclair PJ, et al. Effect of load during electrical stimulation training in spinal cord injury. Muscle Nerve. 2004;29(1):104–111.
- Kern H, Rossini K, Carraro U, et al. Muscle biopsies show that FES of denervated muscles reverses human muscle degeneration from permanent spinal motoneuron lesion. J Rehabil Res Dev. 2005;42(3 Suppl 1):43–53.
- Gorgey AS, Shepherd C. Skeletal muscle hypertrophy and decreased intramuscular fat after unilateral resistance training in spinal cord injury: case report. J Spinal Cord Med. 2010;33(1):90–95.
- Mahoney ET, Bickel CS, Elder C, et al. Changes in skeletal muscle size and glucose tolerance with electrically stimulated resistance training in subjects with chronic spinal cord injury. Arch Phys Med Rehabil. 2005;86(7):1502–1504.
- Gorgey AS, Lester RM, Wade RC, et al. A feasibility pilot using telehealth videoconference monitoring of home-based NMES resistance training in persons with spinal cord injury. Spinal Cord Ser Cases. 2017;3(1):17039.
- Ryan TE, Brizendine JT, Backus D, et al. Electrically induced resistance training in individuals with motor complete spinal cord injury. Arch Phys Med Rehabil. 2013;94(11):2166–2173.
- Gerrits HL, Hopman MTE, Offringa C, et al. Variability in fibre properties in paralysed human quadriceps muscles and effects of training. Pflugers Arch. 2003;445(6):734–740.
- Kern H, Salmons S, Mayr W, et al. Recovery of long-term denervated human muscles induced by electrical stimulation. Muscle Nerve. 2005;31(1):98–101.
- Erickson ML, Ryan TE, Backus D, et al. Endurance neuromuscular electrical stimulation training improves skeletal muscle oxidative capacity in individuals with motor-complete spinal cord injury. Muscle Nerve. 2017;55(5):669–675.
- Martin TP, Stein RB, Hoeppner PH, et al. Influence of electrical stimulation on the morphological and metabolic properties of paralyzed muscle. J Appl Physiol. 1992;72(4):1401–1406.
- Bogie KM, Reger SI, Levine SP, et al. Electrical stimulation for pressure sore prevention and wound healing. Assist Technol. 2000;12(1):50–66.
- Legg Ditterline B, Harkema SJ, Willhite A, et al. Epidural stimulation for cardiovascular function increases lower limb lean mass in individuals with chronic motor complete spinal cord injury. Exp Physiol. 2020;105(10):1684–1691.
- Mohr T, Andersen JL, Biering-Sørensen F, et al. Long term adaptation to electrically induced cycle training in severe spinal cord injured individuals. Spinal Cord. 1997;35(1):1–16.
- Hjeltnes N, Aksnes AK, Birkeland KI, et al. Improved body composition after 8 wk of electrically stimulated leg cycling in tetraplegic patients. Am J Physiol Regulat Integr Comp Physiol. 1997;273(3):42–43.
- Sköld C, Lönn L, Harms-Ringdahl K, et al. Effects of functional electrical stimulation training for six months on body composition and spasticity in motor complete tetraplegic spinal cord-injured individuals. J Rehabil Med. 2002;34(1):25–32.
- Dolbow DR, Credeur DP. Effects of resistance-guided high intensity interval functional electrical stimulation cycling on an individual with paraplegia: a case report. J Spinal Cord Med. 2018;41(2):248–252.
- Johnston TE, Marino RJ, Oleson CV, et al. Cycling with functional electrical stimulation before and after a distal femur fracture in a man with paraplegia. Top Spinal Cord Inj Rehabil. 2015;21(4):275–281.
- Johnston TE, Marino RJ, Oleson CV, et al. Musculoskeletal effects of 2 functional electrical stimulation cycling paradigms conducted at different cadences for people with spinal cord injury: a pilot study. Arch Phys Med Rehabil. 2016;97(9):1413–1422.
- Gorgey AS, Graham ZA, Bauman WA, et al. Abundance in proteins expressed after functional electrical stimulation cycling or arm cycling ergometry training in persons with chronic spinal cord injury. J Spinal Cord Med. 2017;40(4):439–448.
- Gorgey AS, Harnish CR, Daniels JA, et al. A report of anticipated benefits of functional electrical stimulation after spinal cord injury. J Spinal Cord Med. 2012;35(2):107–112.
- Farkas GJ, Gorgey AS, Dolbow DR, et al. Energy expenditure, cardiorespiratory fitness, and body composition following arm cycling or functional electrical stimulation exercises in spinal cord injury: a 16-week randomized controlled trial. Top Spinal Cord Inj Rehabil. 2021;27(1):121–134.
- Dolbow DR, Gorgey AS, Cifu DX, et al. Feasibility of home-based functional electrical stimulation cycling: case report. Spinal Cord. 2012;50(2):170–171.
- Dolbow DR, Gorgey AS, Moore JR, et al. Report of practicability of a 6-month home-based functional electrical stimulation cycling program in an individual with tetraplegia. J Spinal Cord Med. 2012;35(3):182–186.
- Dolbow DR, Gorgey AS, Gater DR, et al. Body composition changes after 12 months of FES cycling: case report of a 60-year-old female with paraplegia. Spinal Cord. 2014;52(S1):S3–S4.
- Adams MM, Ditor DS, Tarnopolsky MA, et al. The effect of body weight-supported treadmill training on muscle morphology in an individual with chronic, motor-complete spinal cord injury: a case study. J Spinal Cord Med. 2006;29(2):167–171.
- Beck L, Veith D, Linde M, et al. Impact of long-term epidural electrical stimulation enabled task-specific training on secondary conditions of chronic paraplegia in two humans. J Spinal Cord Med. 2020;1:1–6.
- Forrest GF, Sisto SA, Barbeau H, et al. Neuromotor and musculoskeletal responses to locomotor training for an individual with chronic motor complete AIS-B spinal cord injury. J Spinal Cord Med. 2008;31(5):509–521.
- Terson de Paleville DGL, Harkema SJ, Angeli CA. Epidural stimulation with locomotor training improves body composition in individuals with cervical or upper thoracic motor complete spinal cord injury: a series of case studies. J Spinal Cord Med. 2019;42(1):32–38.
- Karelis AD, Carvalho LP, Castillo MJE, et al. Effect on body composition and bone mineral density of walking with a robotic exoskeleton in adults with chronic spinal cord injury. J Rehabil Med. 2017;49(1):84–87.
- Asselin P, Cirnigliaro CM, Kornfeld S, et al. Effect of exoskeletal-assisted walking on soft tissue body composition in persons with spinal cord injury. Arch Phys Med Rehabil. 2021;102(2):196–202.
- Masani K, Alizadeh-Meghrazi M, Sayenko DG, et al. Muscle activity, cross-sectional area, and density following passive standing and whole body vibration: a case series. J Spinal Cord Med. 2014;37(5):575–581.
- Gorgey AS, Moore PD, Wade RC, et al. Disruption in bone marrow fat may attenuate testosterone action on muscle size after spinal cord injury: a case report. Eur J Phys Rehabil Med. 2017;53(4):625–629.
- Gorgey AS, Lester RM, Ghatas MP, et al. Dietary manipulation and testosterone replacement therapy may explain changes in body composition after spinal cord injury: a retrospective case report. World J Clin Cases. 2019;7(17):2427–2437.
- Halstead LS, Groah SL, Libin A, et al. The effects of an anabolic agent on body composition and pulmonary function in tetraplegia: a pilot study. Spinal Cord. 2010;48(1):55–59.
- Scremin AME, Kurta L, Gentili A, et al. Increasing muscle mass in spinal cord injured persons with a functional electrical stimulation exercise program. Arch Phys Med Rehabil. 1999;80(12):1531–1536.
- Frotzler A, Coupaud S, Perret C, et al. High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone. 2008;43(1):169–176.
- Deley G, Denuziller J, Casillas JM, et al. One year of training with FES has impressive beneficial effects in a 36-year-old woman with spinal cord injury. J Spinal Cord Med. 2017;40(1):107–112.
- Menéndez H, Ferrero C, Martín-Hernández J, et al. Chronic effects of simultaneous electromyostimulation and vibration on leg blood flow in spinal cord injury. Spinal Cord. 2016;54(12):1169–1175.
- Haun CT, Vann CG, Roberts BM, et al. A critical evaluation of the biological construct skeletal muscle hypertrophy: size matters but so does the measurement. Front Physiol. 2019;10(MAR):247.
- Yarar-Fisher C, Polston KFL, Eraslan M, et al. Paralytic and nonparalytic muscle adaptations to exercise training versus high-protein diet in individuals with long-standing spinal cord injury. J Appl Physiol. 2018;125(1):64–72.
- Dudley GA, Castro MJ, Rogers S, et al. A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol. 1999;80(4):394–396.
- Schoenfeld B, Grgic J. Evidence-based guidelines for resistance training volume to maximize muscle hypertrophy. Strength Condition J. 2018;40(4):107–112.
- Krzysztofik M, Wilk M, Wojdała G, et al. Maximizing muscle hypertrophy: a systematic review of advanced resistance training techniques and methods. Int J Environ Res Public Health. 2019;16(24):4897.
- Bickel CS, Yarar-Fisher C, Mahoney ET, et al. Neuromuscular electrical stimulation-induced resistance training after SCI: a review of the Dudley protocol. Top Spinal Cord Inj Rehabil. 2015;21(4):294–302.
- Dolbow DR, Gorgey AS, Ketchum JM, et al. Exercise adherence during home-based functional electrical stimulation cycling by individuals with spinal cord injury. Am J Phys Med Rehabil. 2012;91(11):922–930.
- de Groot S, Cowan RE. Exercise for people with SCI: so important but difficult to achieve. Spinal Cord. 2021;59(1):1–2.
- Fornusek C, Davis GM, Russold MF. Pilot study of the effect of low-cadence functional electrical stimulation cycling after spinal cord injury on thigh girth and strength. Arch Phys Med Rehabil. 2013;94(5):990–993.
- Anderson KD, Cowan RE, Horsewell J. Facilitators and barriers to spinal cord injury clinical trial participation: multi-national perspective of people living with spinal cord injury. J Neurotrauma. 2016;33(5):493–499.
- Liao Y, Peng Z, Chen L, et al. Prospective views for whey protein and/or resistance training against age-related sarcopenia. Aging Dis. 2019;10(1):157–174.
- Morton RW, Murphy KT, McKellar SR, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52(6):376–384.
- Lee S, Kuk JL. Changes in fat and skeletal muscle with exercise training in obese adolescents: comparison of whole-body MRI and dual energy X-ray absorptiometry. Obesity. 2013;21(10):2063–2071.