Abstract
Purpose
To determine the feasibility of using parent-reported outcome measures of the Paediatric Pain Profile (PPP), Sleep Disturbance Scale for Children (SDSC) and Care and Comfort Hypertonicity Questionnaire (CCHQ) as repeated outcome measures of change at weekly intervals for children with dyskinetic cerebral palsy (CP). The secondary aim was to explore the efficacy of individualised movement intervention.
Material and methods
In this pilot feasibility study a single subject research design was utilised. Three children with dyskinetic CP, completed 5 weeks of parent-reported baseline assessments, 8 weekly sessions of intervention and 5 weeks of follow up.
Results
All children completed 18 weeks of the study, with no missing data. There was evidence of parent-reported improvements in their child’s pain and care and comfort between the baseline and intervention phases.
Conclusions
The PPP, SDSC and CCHQ were feasible to assess pain, sleep and comfort before and after an intervention in children with dyskinetic CP. There is preliminary evidence that individualised movement intervention as little as once a week may help improve pain, sleep and improve ease of care and comfort.
The Paediatric Pain Profile is feasible to identify and monitor pain, as frequently as weekly, in children with dyskinetic cerebral palsy (CP).
There is preliminary evidence that movement can decrease pain in children with dyskinetic CP.
Assessments and treatment in this group may be interrupted due to their complex health issues which may be a limitation when collecting repeated measures.
IMPLICATIONS FOR REHABILITATION
Introduction
Dyskinesia describes abnormal postures or movements associated with impaired muscle tone regulation, movement control, and coordination [Citation1]. Children with dyskinetic cerebral palsy (CP) make up to 7–15% of the total population of children with CP, however this may be underreported due to mixed presentations of movement disorder [Citation2–4]. Dystonia and choreoathetosis are simultaneously present in children with dyskinetic movement disorders, however dystonia is more predominant in the majority of children [Citation5]. Dystonia is defined as involuntary sustained or intermittent muscle contractions causing twisting or repetitive movements, abnormal postures or both [Citation6,Citation7]. It has been identified as one of the most common causes of pain in children and youth with CP [Citation8] and found to be a major predictor of emotional and behavioural problems [Citation9]. Gross motor function, activity and participation are negatively impacted by the presence of dystonia, suggesting that dystonia should be addressed as a priority in children with CP [Citation1].
Pharmacotherapy, occupational therapy and physiotherapy are common management options for children with dystonia, however there is limited evidence for any of these interventions [Citation10]. Lin and colleagues [Citation11] observed that two thirds of the families of 279 children, aged 5–18 years referred for tertiary management, perceived no improvement in their child’s dystonia despite complex medical management, with most reporting it stayed the same or worsened over time [Citation11]. A systematic review of pharmacological and neurosurgical interventions suggests there is weak evidence for current medical management options, at best some are “possibly effective” [Citation12]. These statistics highlight the difficulties treating children with dyskinetic CP. There is emerging evidence that intrathecal baclofen (ITB) improves both dyskinesia and has a positive impact towards goal attainment focussed on improving pain and ease of caregiving [Citation13,Citation14].
In a retrospective audit of children with dyskinetic CP, attending the movement disorders clinic at the sole tertiary paediatric hospital for the state of Western Australia – between 1st January 2016 and 31st December 2016, 82% of children described as having dyskinetic CP identified pain as an issue [Citation15]. Strategies identified by carers that reduced their child’s pain consistently included, gentle stretching, repositioning and massage [Citation15]. These strategies may form part of routine occupational therapy and physiotherapy treatment for children with dyskinetic CP. Occupational and physiotherapists assess and treat children using individualised movement interventions aimed at promoting postural symmetry, positioning for function and varied movement opportunities to reduce the impact of dystonia on the developing musculoskeletal system, however there is currently little evidence to guide clinical practice.
This pilot feasibility study explored the use of goal directed, individualised movement intervention (described in Supplementary material 1) for children with dyskinetic CP, delivered weekly for eight weeks. The primary aim was to evaluate the feasibility of the weekly assessments to measure pain, sleep and care and comfort. The secondary aim was to explore the efficacy of individualised movement on pain, care and comfort and quality of life.
Materials and methods
Study design
This pilot feasibility study employed a single subject research design (SSRD) A-B-A withdrawal design (Supplementary material 2). The baseline 5-week pre- intervention period (A) (T1-T5) was followed by an 8-week intervention period (B) (T6-T13), which was followed by a 5-week post intervention withdrawal period (A) (T14-T19). The SSRD methodology allowed us to: collect data prospectively; have a blinded assessor and control period; reduce the bias that can be seen in traditional case studies; and use the participant as his/her own control. This method was preferred to a randomised controlled trial due to the large numbers of participants required and the heterogeneous nature of children with CP. Outcome assessments were collected once a week in all three phases. The study protocol was approved by the Child and Adolescent Health Service Human Research Ethics Committee, PRN 0000000701.
Participants
Three participants were identified and recruited from a rehabilitation clinic of a tertiary paediatric hospital. Children were eligible to participate if they were classified as Gross Motor Function Classification level extended and revised (GMFCS-E&R) [Citation16], level IV or V, were aged between 5 and 18 years and had dyskinetic CP and significant pain on the Paediatric Pain Profile [Citation17] interfering with their cares, and function. Exclusion criteria were; commencement of any new therapy interventions during the 18 weeks of the study, orthopaedic surgery within the previous 6 months and inability of the family to attend the tertiary paediatric hospital for the intervention period. Families of children who met the criteria were invited to participate by the clinical nurse specialist (PW). Written and verbal consent was obtained from a parent/guardian for their child to participate in the study as the children did not have the cognitive ability to provide assent.
The 8 weeks of intervention were conducted in the therapy gym area of a paediatric tertiary hospital and home visits were conducted during the baseline and follow up periods to obtain video assessments and collect the completed patient reported outcome measures.
Equipment
A double therapy plinth, therapy balls, individualised developmentally appropriate toys and play were used during the sessions to engage the children in the movement intervention. Specialised equipment was not required to deliver the intervention. A video camera was used to record the child’s movement to score the Dyskinesia Impairment Scale (DIS) and Barry Albright Dystonia (BAD) scale assessments.
Intervention
Following baseline data collection, the children participated in 8 weeks of once-weekly, individualised movement intervention targeting parent reported goals of improving pain, sleep and care and comfort. For each child, dyskinetic movement and pain were the problems interfering with the parent identified goals.
Movement intervention was the term used to reflect the multidisciplinary approach used by occupational therapists and physiotherapists using movement to treat children with dyskinesia. The principles of symmetry were considered important for this group of children as end range asymmetrical posturing of the neck, trunk and upper and lower limbs can cause pain and impact function such as positioning in a supported seating system to see and interact with others.
The intervention is described in Supplementary material 1 according to the Consensus on Exercise Reporting Template (CERT) [Citation18].
The movement intervention was provided by a senior occupational and physiotherapist with more than 15 years of individual experience working with children with dyskinetic CP in a paediatric tertiary hospital.
Each movement intervention session was an hour in duration, with individualised activities to meet the parent identified goals formulated by using the Canadian Occupational Performance Measure (COPM)[Citation19]. The intervention consisted of movement opportunities for the whole body with the child’s active participation wherever possible.
Each session included:
Passive and active assisted movements of all joints including neck, spine, upper and lower limbs in a variety of positions, including supine lie, side lying, and supported sitting.
Symmetry and alignment of head and trunk combined with gentle movement opportunities for spine and neck in supported sitting.
Modified weight bearing through arms and legs.
Facilitated rolling, moving from lying to sitting and sit to stand.
The targeted aspect to the intervention referred to the individualised movement that was appropriate for each child based on each child’s movement characteristics and/or musculoskeletal impairments. For example, participant 1 had a significant pes excavatum and scoliosis that affected certain movement opportunities in some planes, whereas participant 2 had a dislocated hip and costopelvic impingement that changed the way the therapists had to manually support the movement opportunities, such as supported sitting. In addition, dystonia may have varied from week to week at each session, which also altered the movement intervention.
Parents and caregivers were present throughout and helped to guide and modify the intervention sessions accordingly. Participant’s parents were instructed to continue the child’s usual daily activities during the block of intervention, for example, school and usual therapy routines.
Measures
The outcome measures used in this study are outlined in . All outcome measures were parent reported.
Table 1. Description and psychometric properties of outcome measures used.
Procedure
The assessment time points and select outcome measures for the primary dependent variables which took place at each time point are outlined in Supplementary material 2. The primary outcomes of interest, pain measured with Paediatric Pain Profile (PPP) [Citation17]; sleep measured with the Sleep Disturbance Scale for children (SDSC) [Citation20]; and care and comfort measured with the Care and Comfort Hypertonicity Questionnaire (CCHQ) [Citation21], took place at each time point (each week). The COPM [Citation19] and the Cerebral Palsy Quality of Life (CP QoL) [Citation22] were probe assessments, performed at the start and end of baseline phase (A), middle intervention phase (B), and at follow-up assessment (A). The complete set of outcome measures were assessed at “probe” time points denoted by the larger arrows (T1, T6, T10, T14 and T19)
During the intervention phase, each weekly set of outcome measures were collected before the start of the intervention, reflecting the outcomes for the previous week. The same two therapists (NS and SG) completed all the treatment sessions ensuring intervention fidelity.
At the probe time points, the COPM was used to ensure the intervention was individualized to parent-identified goals. The CP QoL was used to measure perceived improvement in quality of life as a result of reduced pain. The BAD Scale [Citation23] and the DIS [Citation24] were used to objectively describe the type and level of movement disorder for each child. Video footage for the BAD Scale and DIS were randomised using a computer-generated tool. The randomised footage was scored by a trained assessor, who was blind to each participant’s time point in the study.
Statistical analysis
To assess the feasibility of repeated measures, each outcome for each participant was assessed for missing data. To examine the effects of the intervention for each participant, the seven outcomes collected weekly (PPP, CCHQ personal care, CCHQ comfort CCHQ positioning subsections, SDSC overall score, SDSC excessive somnolence, and SDSC maintaining sleep subsections) were plotted over the three phases. Visual analysis was primarily performed and aided by quasi-statistical methods. Changes from phase A (baseline) to phase B (intervention) were the focus. Visual analysis was performed independently by four reviewers using visual inspection guidelines [Citation25] and discordant responses were reviewed as a team for agreement. Quasi-statistical methods included change in means, the 2-standard deviation (2SD) band method, non-overlapping data points, non-overlapping of all pairs (NAP) [Citation26,Citation27]. Effect size was determined using standard mean difference (SMD) [Citation28,Citation29]. For each outcome the impact of the intervention was categorised as positive, unclear or nil. An outcome was positive if supported by visual analysis. Outcomes were marked as unclear if they were not supported by visual analysis but received support from at least three statistical methods including (1) a change in means (2) 2SD band method, (3) at least 50% non-overlapping data points, (4) high NAP (>0.7) or 5) a large effect size (SMD >0.8). Outcomes were listed as nil if there was no positive visual analysis finding or consistent statistical support.
Results
describes the characteristics of the study participants and their identified goals.
Table 2. Participant characteristics.
Feasibility of assessments
For participant 1 we were unable to collect continuous weekly data due to an unplanned hospital admission for a medical illness. Despite this, we were still able to collect all data points so there was no missing data. Seven of the nine parent identified goals of intervention fell within the ICF categories of activity and participation. The other two fell into the ICF body structure and function categories (refer to ) [Citation30].
and show stability of the patient reported outcome measures. A stable baseline was achieved in two of the three participants for the PPP (Participants 2 and 3), two of the three participants for the CCHQ personal care (Participants 1 and 3), positioning (Participants 1 and 2) and comfort sections (Participants 1 and 3). There was a stable baseline for the total SDSC in two out of three participants (Participants 1 and 2), all three participants for maintaining sleep.
Figure 1. Participant 1 results.
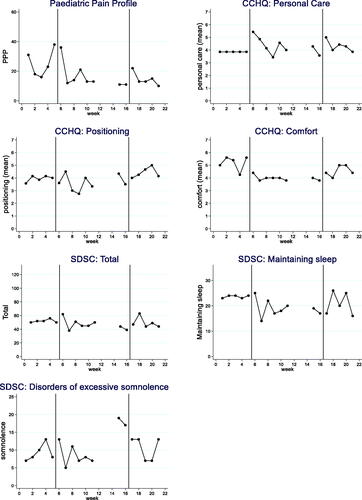
Figure 2. Participant 2 results.
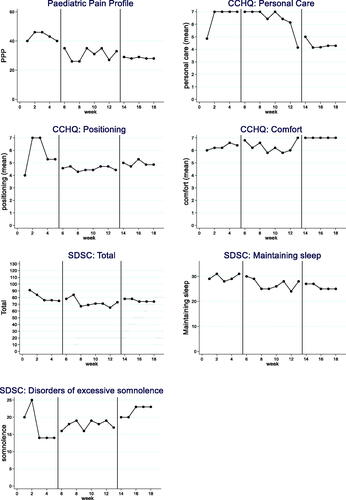
Figure 3. Participant 3 results.
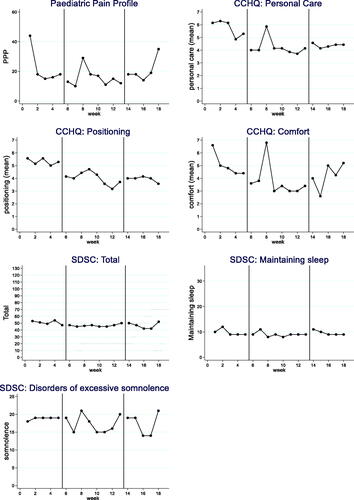
Table 3. Results of weekly outcome measures.
Feasibility of the intervention
The intervention for participant 1 was disrupted for three weeks following treatment session 6 due to a respiratory related hospital admission. The final two treatment sessions were completed once the child was well enough to participate resulting in a longer phase B to complete the 8 weeks of intervention.
Each participant’s 7 weekly outcomes are displayed in : the PPP, CCHQ personal care, comfort and positioning subsections and the SDSC overall score and the excessive somnolence, maintaining sleep subsections. Results for the three participants are summarised in . There was evidence of significant improvements in pain between baseline and the intervention phases and some significant improvements in care and comfort subsections.
Probe assessments results are displayed in Supplementary material 3. The BAD was mostly consistent with the most variability with Participant 1 in the intervention period in the weeks prior to their respiratory related hospital admission. The DIS and CP QoL varied over time. The COPM performance and satisfaction increased in scores for all participants at follow up (week 15) although not to a clinically meaningful level.
Participant 1
Visual analysis change and evidence of baseline to treatment improvements (phase A to B) was noted in three outcome measures, the PPP, the CCHQ comfort subsection and the SDSC maintaining sleep subsection.
The PPP did not display a stable baseline but despite this, visual and statistical analysis indicated an improvement. The PPP recorded a reduction in the mean (SD) score from 25.2 (9.2) at baseline to 16.4 (8.6) during the intervention period with changes from phase A to B of large effect size (SMD) of 0.96; 6/8 non-overlapping time points and a NAP of 0.85. Improvement was maintained in the follow up period. There was a large effect size for the CCHQ comfort and SDSC maintain sleep (SMD 2.10 and 8.4 respectively), with a high number of non-overlapping time points (both 7/8).
The outcome was unclear for CCHQ positioning and SDSC total with no clear visual analysis change, but quantitative findings included significant results from the 2SD band method, at least 50% of non-overlapping data points and large effect sizes (SMD 1.32 and 2.14 respectively). There was no impact on CCHQ personal care and SDSC somnolence.
Participant 2
Visual analysis change and evidence of baseline to treatment improvements (phase A to B) was noted in five outcome measures. These included the PPP, the CCHQ personal care, CCHQ positioning, CCHQ comfort and SDSC somnolence.
The PPP recorded a reduction in the mean (SD) score from 22.2 (12.3) at baseline to 15.6 (6.1) during the intervention period with changes from phase A to B showing a large effect size (SMD) of 0.96; 4/8 non-overlapping time points and a NAP of 0.76. CCHQ personal care and CCHQ comfort did not demonstrate a stable baseline, however visual analysis and all statistical analyses consistently suggested an improvement with large effect sizes (SMD 2.38 and 1.42 respectively), high non overlapping data points (both 7/8) and high NAP (0.95 and 0.88 respectively). CCHQ positioning demonstrated a clear change, with 8/8 non overlapping time points, a large effect size (SMD 5.13) and a high NAP of 1.00. The outcome was unclear for SDSC total, and no impact for SDSC maintain sleep for participant 2.
Participant 3
Visual analysis change and evidence of baseline to treatment improvements (phase A to B) was noted in four outcome measures, the PPP, SDSC total and SDSC maintaining sleep subsection.
SDSC total did not display a stable baseline, but visual and statistical analysis indicated an improvement with a large effect size (SMD 1.17), high NAP (0.84) and 6/8 non overlapping data points. Visual analysis for the PPP displayed a clear visual improvement, with all statistical analysis supporting this trend with no overlapping data points (8/8), a large effect size (SMD 4.00) and a high NAP of 1.00.
The outcome was unclear for CCHQ positioning, and no impact for CCHQ personal care, CCHQ comfort and SDSC somnolence. For CCHQ personal care there was an improvement from phase B (Intervention) to C (follow up).
Discussion
We aimed to assess the feasibility of adherence to both assessments and attendance to a weekly movement intervention for children with dystonia. Our results support the feasibility of our research design and methods. All movement intervention was delivered despite the break in the treatment phase for participant 1. The potential for sessions to be interrupted in this population by other health issues is a limitation when collecting repeated measures.
Our results supported that the PPP, SDSC and CCHQ are feasible for use in this population and can be used to establish baseline levels of pain, sleep and comfort before initiating an intervention. The achievement of baseline stability of the SDSC and CCHQ may have been affected by lack of sensitivity to change in some sections of these outcome measures. For example, items relating to ability to walk or get in and out of a car, which is not applicable and do not change for a child classified as GMFCS level V. It is worth noting that in clinical practice weekly assessment is not required.
We were interested in parent’s perception of the usefulness of the PPP, SDSC and CCHQ for improving awareness of their child’s pain, sleep and comfort levels and acceptability of weekly assessment. We had no missing data points across the full ABA study period, suggesting the acceptability of weekly assessments.
All participants had secondary consequences of CP, including significant kyphoscoliosis, hip dysplasia and upper and lower limb muscle contracture. They were not able to initiate any independent mobility and some therapy approaches would be contraindicated due to the risk of causing pain or distress. There is no evidence to date that movement decreases pain in children with CP. Many children with dyskinetic cerebral palsy have a significant motor impairment and are described as GMFCS level IV and V with no ability for independent mobility [Citation31]. All three children in this study had pain from muscle stiffness and dystonia, as reflected by the PPP and the goals identified by parents. Our results supported that targeted and individualised movement and mobilisation of joints helped improve pain, sleep and ease of care and comfort. This suggests that a larger trial with more participants is worthwhile and feasible.
Many families may not be able to commit to intensive therapy blocks due to competing demands including multiple medical appointments, school and other family commitments. Our research protocol was informed by consumer input from the inception of the study. Consumers highlighted that it would be good to know if less intensive therapy could deliver benefits to the child. The 8-week duration was chosen to reflect the current reported durations of interventions [Citation32]. We showed preliminary evidence that gentle movement opportunities as little as once a week provided positive outcomes for the child, with carers reporting minimal burden.
Strengths of this current study include the use of a multiple SSRD based on recent guidelines [Citation33] and use of a valid and reliable pain assessment tool (PPP) for children who are unable to communicate verbally or with augmentative alternative communication.
Several limitations of this study are notable. Baseline stability was not achieved in all participants for all assessment measures, therefore changes in the treatment phase are difficult to interpret. One participant had a break in the treatment phase which could have impacted the results. However, we were still able to resume intervention and collect all data points which meant we had no missing data. There was also no random allocation of the participants to the treatment which should be considered in a larger trial.
Any future studies with larger numbers of enrolled participants should consider the addition of rigorous qualitative methodology to assess parent perceptions of the worth of the interventions and outcomes.
Conclusion
This study provides evidence that the parent-reported assessments were feasible to collect on a weekly basis and preliminary evidence that movement intervention reduced pain and improved care and comfort on these assessments. Consideration should be given to the potential for interruption due to medical issues in this population.
Supplementary_material_1_2_3.pdf
Download PDF (392.3 KB)Acknowledgements
Natalie Dewson for completing blinded assessment. Dr Jane Valentine and Dr Jon Silberstein for assistance with funding applications.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Monbaliu E, Himmelmann K, Lin J-P, et al. Clinical presentation and management of dyskinetic cerebral palsy. Lancet Neurol. 2017;16(9):741–749.
- Australian Cerebral Palsy Register. Report of the australian cerebral palsy register: birth years 1995-2012. Sydney: Australian Cerebral Palsy Register; 2019.
- Rice J, Skuza P, Baker F, et al. Identification and measurement of dystonia in cerebral palsy. Dev Med Child Neurol. 2017;59(12):1249–1255.
- Himmelmann K, Uvebrant P. The panorama of cerebral palsy in Sweden part XII shows that patterns changed in the birth years 2007–2010. Acta Paediatr. 2018;107(3):462–468.
- Sanger TD, Delgado MR, Gaebler-Spira D, et al. Classification and definition of disorders causing hypertonia in childhood. Pediatrics. 2003;111(1):e89–e97.
- Monbaliu E, De Cock P, Ortibus E, et al. Clinical patterns of dystonia and choreoathetosis in participants with dyskinetic cerebral palsy. Dev Med Child Neurol. 2016;58(2):138–144.
- Sanger TD, Chen D, Fehlings DL, et al. Definition and classification of hyperkinetic movements in childhood. Mov Disord. 2010;25(11):1538–1549.
- Penner M, Xie WY, Binepal N, et al. Characteristics of pain in children and youth with cerebral palsy. Pediatrics. 2013;132(2):e407–e13.
- Ramstad K, Jahnsen R, Skjeldal OH, et al. Characteristics of recurrent musculoskeletal pain in children with cerebral palsy aged 8 to 18 years. Dev Med Child Neurol. 2011;53(11):1013–1018.
- Novak I, Morgan C, Fahey M, et al. State of the evidence traffic lights 2019: systematic review of interventions for preventing and treating children with cerebral palsy. Curr Neurol Neurosci Rep. 2020;20(2):1–21.
- Lin J-P, Lumsden DE, Gimeno H, et al. The impact and prognosis for dystonia in childhood including dystonic cerebral palsy: a clinical and demographic tertiary cohort study. J Neurol Neurosurg Psychiatry. 2014;85(11):1239–1244.
- Fehlings D, Brown L, Harvey A, et al. Pharmacological and neurosurgical interventions for managing dystonia in cerebral palsy: a systematic review. Dev Med Child Neurol. 2018;60(4):356–366.
- Bonouvrié LA, Becher JG, Vles JS, et al. The effect of intrathecal baclofen in dyskinetic cerebral palsy: the IDYS trial. Ann Neurol. 2019;86(1):79–90.
- Stewart K, Copeland L, Lewis J. The impact of intrathecal baclofen therapy on health-related quality of life for children with marked hypertonia. Dev Neurorehabil. 2020;23(8):542–547.
- Smith N, Garbellini S, Gibson N, et al. Children with dyskinetic cerebral palsy experience high levels of pain on a good day. Dev Med Child Neurol. 2018;60(27):30.
- Palisano RJ, Rosenbaum P, Bartlett D, et al. Content validity of the expanded and revised gross motor function classification system. Dev Med Child Neurol. 2008;50(10):744–750.
- Hunt A, Goldman A, Seers K, et al. Clinical validation of the paediatric pain profile. Dev Med Child Neurol. 2007;46(1):9–18.
- Slade SC, Dionne CE, Underwood M, et al. Consensus on exercise reporting template (CERT): explanation and elaboration statement. Br J Sports Med. 2016;50(23):1428–1437.
- Law M, Baptiste S, McColl M, et al. The Canadian occupational performance measure: an outcome measure for occupational therapy. Can J Occup Ther. 1990;57(2):82–87.
- Bruni O, Ottaviano S, Guidetti V, et al. The sleep disturbance scale for children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res. 1996;5(4):251–261.
- Nemer R, Blasco PA, Russman BS, et al. Validation of a care and comfort hypertonicity questionnaire. Dev Med Child Neurol. 2006;48(3):181–187.
- Waters E, Davis E, Mackinnon A, et al. Psychometric properties of the quality of life questionnaire for children with CP. Dev Med Child Neurol. 2007;49(1):49–55.
- Barry MJ, VanSwearingen JM, Albright AL. Reliability and responsiveness of the Barry-Albright Dystonia Scale. Dev Med Child Neurol. 1999;41(6):404–411.
- Monbaliu E, Ortibus E, De Cat J, et al. The dyskinesia impairment scale: a new instrument to measure dystonia and choreoathetosis in dyskinetic cerebral palsy. Dev Med Child Neurol. 2012;54(3):278–283.
- Kazdin AE. Single-case experimental designs. Evaluating interventions in research and clinical practice. Behav Res Ther. 2019;117:3–17.
- Lobo MA, Moeyaert M, Cunha AB, et al. Single-case design, analysis, and quality assessment for intervention research. J Neurol Phys Ther. 2017;41(3):187–197.
- Parker RI, Vannest KJ, Davis JL. Effect size in single-case research: a review of nine nonoverlap techniques. Behav Modif. 2011;35(4):303–322.
- Gierut JA, Morrisette ML, Dickinson SL. Effect size for single-subject design in phonological treatment. J Speech Lang Hear Res. 2015;58(5):1464–1481.
- Tate RL, Perdices M. Single-case experimental designs for clinical research and neurorehabilitation settings: Planning, conduct, analysis and reporting. Boca Raton (FL): Routledge; 2019.
- World Health Organization. A practical manual for using the international classification of functioning, disability and health (ICF). Geneva: WHO 2013.
- Préel M, Rackauskaite G, Larsen ML, et al. Children with dyskinetic cerebral palsy are severely affected as compared to bilateral spastic cerebral palsy. Acta Paediatr. 2019;108(10):1850–1856.
- Hsu C-W, Kang Y-N, Tseng S-H. Effects of therapeutic exercise intensity on cerebral palsy outcomes: a systematic review with meta-regression of randomized. Front Neurol. 2019;10:657.
- Logan LR, Hickman RR, Harris SR, et al. Single-subject research design: recommendations for levels of evidence and quality rating. Dev Med Child Neurol. 2008;50(2):99–103.
