Abstract
Purpose
(1) To investigate the differences in the course of participation up to one year after stroke between distinct movement behavior patterns identified directly after discharge to the home setting, and (2) to investigate the longitudinal association between the development of movement behavior patterns over time and participation after stroke.
Materials and methods
200 individuals with a first-ever stroke were assessed directly after discharge to the home setting, at six months and at one year. The Participation domain of the Stroke Impact Scale 3.0 was used to measure participation. Movement behavior was objectified using accelerometry for 14 days. Participants were categorized into three distinct movement behavior patterns: sedentary exercisers, sedentary movers and sedentary prolongers. Generalized estimating equations (GEE) were performed.
Results
People who were classified as sedentary prolongers directly after discharge was associated with a worse course of participation up to one year after stroke. The development of sedentary prolongers over time was also associated with worse participation compared to sedentary exercisers.
Conclusions
The course of participation after stroke differs across distinct movement behavior patterns after discharge to the home setting. Highly sedentary and inactive people with stroke are at risk for restrictions in participation over time.
The course of participation in people with a first-ever stroke up to one year after discharge to the home setting differed based on three distinct movement behavior patterns, i.e., sedentary exercisers, sedentary movers and sedentary prolongers.
Early identification of highly sedentary and inactive people with stroke after discharge to the home setting is important, as sedentary prolongers are at risk for restrictions in participation over time.
Supporting people with stroke to adapt and maintain a healthy movement behavior after discharge to the home setting could prevent potential long-term restrictions in participation.
Implications for rehabilitation
Introduction
Stroke is the second leading cause of death and the third leading cause of disability worldwide [Citation1]. Stroke prevalence has increased over the last decades, most likely because of longer survival and reduced mortality of people experiencing stroke [Citation2] due to improved stroke care and risk factor management [Citation3,Citation4]. This results in more people living with the long-term consequences of stroke, such as physical, emotional, and cognitive problems, that contribute to restrictions in participation and quality of life [Citation5–7]. Participation defined as “the person’s involvement in a life situation” [Citation8] is among the most impacted domains of health-related quality of life after stroke [Citation9]. Participation is considered an important outcome of stroke rehabilitation as it provides clinicians with valuable person-centered information on the impact of stroke on daily life [Citation10,Citation11]. Improvements in participation mainly occur in the first six months after stroke and recovery are often incomplete, remaining lower compared to the general population [Citation12,Citation13]. Consequently, many people experience considerable and ongoing restrictions in participation after stroke [Citation14], including the domains of outdoor mobility, work and physical exercise [Citation15].
Physical activity has been shown to be one of the key components in stroke rehabilitation, as interventions that enhance physical activity were associated with improvement in participation after stroke [Citation16,Citation17]. Three small cross-sectional studies (n = 19–31) in community-living chronic stroke patients (ranging from six months to ten years after stroke onset) reported moderate correlations between the participation domain of the Stroke Impact Scale (SIS) and the Six-Meter Walk Test [Citation18], steps per day using pedometry [Citation19] and physical activity using accelerometry [Citation20]. However, stroke patients are not only less physically active in all phases after stroke, but also spend more time sedentary than healthy individuals [Citation21], especially in prolonged periods of time [Citation22–24].
Movement behavior is defined as the composition of time spent sedentary and time spent in light, moderate or vigorous physical activity [Citation25]. Research has shown that time spent in moderate to vigorous physical activity (MVPA), light physical activity (LPA) and sedentary behavior all interact to impact physical functioning in people after stroke [Citation24]. These are partly independent behaviors, and it is suggested that research should focus on movement behavior patterns instead of separate aspects of movement behavior [Citation24]. Currently, physical activity is the most studied aspect of movement behavior, and longitudinal studies investigating the relationship between physical activity and participation are lacking [Citation5]. To our knowledge, the association between participation and other types of movement behavior, such as sedentary behavior, has rarely been studied [Citation26].
Recently, three distinct movement behavior patterns were identified based on accelerometry data in people with a first-ever stroke after discharge to the home setting [Citation24]. The so-called ‘sedentary exercisers’ were the most active group as they spent sufficient time in MVPA (1.3 h/day). Although they also spent the majority of their waking hours sedentary (61%), the periods of uninterrupted sedentary time were relatively short. The ‘sedentary movers’ showed similar sedentary behavior compared to the sedentary exercisers but spent less time in MVPA (0.4 h/day). The ‘sedentary prolongers’ spent similar time in MVPA compared to the sedentary movers, but also spent more time sedentary (78%) and the periods of uninterrupted sedentary time were longer [Citation24]. These movement behavior patterns turned out to be predictive of the course of physical functioning up to two years after stroke, as sedentary prolongers showed worse physical functioning over time compared to the sedentary movers and sedentary exercisers [Citation25]. Furthermore, more individuals develop a more unfavorable movement behavior pattern over time [Citation27]. Namely, after an initial improvement in the first six months after discharge to the home setting, movement behavior often deteriorated and the proportion of sedentary prolongers (the most unfavorable movement behavior pattern) increased [Citation27].
Whether these distinct movement behavior patterns are also predictive of the course of participation after stroke is currently unknown. Gaining a better understanding of the relationship between movement behavior and participation may yield possibilities for the development of tailored interventions targeting movement behavioral change. Therefore, this study aims (1) to explore the differences in the course of participation up to one year after stroke between distinct movement behavior patterns identified directly after discharge to the home setting, and (2) to investigate the longitudinal association between the development of movement behavior patterns over time and participation after stroke.
Materials and methods
Participants and study design
This prospective cohort study is part of the RISE study [Citation24]. Participants were recruited from four stroke units in the Netherlands. Participants were deemed eligible to participate when: presenting with a clinically confirmed first-ever stroke, expected to return home (with or without inpatient rehabilitation before returning home), activities of daily living independent (Barthel index >18) before stroke [Citation28], >18 years old, able to maintain a conversation (score >4 on the Utrecht Communication assessment) [Citation29], and at least able to walk with supervision (score ≥3 in the Functional Ambulation Categories) when they returned home [Citation30]. People with subarachnoid hemorrhage were excluded. Written informed consent was obtained at the stroke unit. The study was approved by the Medical Ethics Research Committee of the University Medical Center Utrecht (study number 14/76).
Demographic, stroke, and care characteristics were obtained from medical health records. Within three weeks after discharge from inpatient care, six months after discharge from inpatient care and one year after discharge from inpatient care, participants were visited at home by trained researchers to obtain measurements. Before the participant was visited at home, a postal questionnaire was sent to get information on emotional symptoms. During the visits at home, data on physical functioning and a self-report participation questionnaire were obtained. After each visit, participants wore an accelerometer to objectify movement behavior for 14 days.
Dependent variable
Participation was measured using the Participation domain of the Stroke Impact Scale 3.0 (SIS Participation) [Citation31,Citation32]. The SIS is a self-administered questionnaire that estimates how stroke affects health and quality of life. It comprises 59 questions divided into eight domains, including strength, hand function, mobility, activities of daily living (ADL), emotion, communication, memory and thinking, and participation. The SIS Participation has eight questions that ask the participant to range his or her limitations in the past four weeks in (1) work, volunteer or other activities; (2) social activities; (3) quiet recreation; (4) active recreation; (5) role as a family member or friend; (6) participation in spiritual or religious activities; (7) ability to control life as he or she wished; and (8) ability to help others. Each question is rated on a five-point scale to indicate how often the participant has experienced restrictions in certain activities. The possible scores range from 1 to 5, where 1 is always, and 5 is never. The SIS 3.0 is valid and sensitive to changes in stroke-related recovery [Citation31,Citation32], and evaluation of the use of separate domain scores have also shown excellent validity [Citation33].
For a particular participant, if ≥50% of the questions had missing responses, the SIS Participation was assigned as missing. Otherwise, scores for the SIS Participation were computed using the following equation: SIS Participation = (mean-1/5-1) × 100, where the mean is the mean of the non-missing item scores within the domain. Using this algorithm, the SIS Participation has a range from 0 to 100 [Citation32].
Independent variables
Movement behavior
Movement behavior was measured by an accelerometer (Activ8, a three-axial accelerometer) worn on the thigh for two consecutive weeks during waking hours. The Activ8 is validated in community-living ambulatory people with stroke [Citation34]. Ten different movement behaviors were calculated from data supplied by the accelerometer, including mean time spent sedentary, the mean time spent in uninterrupted periods of sitting and lying down (bouts), measured in periods of ≥5 min per day, ≥30 min a day or ≥60 min per day. Also, time spent in LPA and time spent in MVPA were measured.
In total, three distinct movement behavior patterns were identified: sedentary exercisers, sedentary movers and sedentary prolongers [Citation24]. Sedentary exercisers were characterized by interrupted sedentary and active movement patterns, sedentary movers were characterized by interrupted sedentary and inactive movement patterns, and sedentary prolongers were characterized by a prolonged and highly sedentary and inactive movement pattern. The sedentary exercisers group was sedentary for 9.0 h per day and had a mean MVPA time of 1.4 h a day, while the sedentary prolongers were sedentary for on average 10.7 h per day and spent a mean time of 0.4 h per day on MVPA () [Citation25].
Table 1. Baseline characteristics.
Demographic characteristics
Demographic characteristics included age, sex, educational level, comorbidities and living situation. Educational level was asked using the Dutch classification system and dichotomized into low (score 1–5, up to completed secondary education) and high (score 6–7, completed secondary professional education, university or higher) [Citation35]. Comorbidity was assessed using the Cumulative Illness Rating Scale (Range 0–52, a higher score indicates more comorbidities) [Citation36]. The living situation was divided into living with a partner and living without a partner.
Stroke characteristics
Stroke characteristics obtained from medical records included type, location, severity of stroke symptoms, and discharge destination. The severity of symptoms was measured within four days after stroke with the National Institutes of Health Stroke Scale (NIHSS, range 0–42) and was divided into (1) no stroke symptoms (0 points), (2) minor stroke symptoms (1–4 points); and (3) moderate to severe stroke symptoms (≥5 points) [Citation37]. Discharge destination after hospitalization was categorized into home or inpatient rehabilitation.
Physical functioning
Physical functioning at discharge from inpatient care was measured with the physical domain of the Stroke Impact Scale 3.0 (SIS Physical) and the Five Meter Walk Test (5MWT). The SIS Physical consists of ten questions regarding ADL, eight regarding mobility, and five regarding hand function [Citation32]. Scores range from 0 to 100, and lower scores indicate lower levels of physical functioning. Walking speed was measured with the 5MWT [Citation38]. Participants were asked to perform this test three times. The mean test time was calculated. A higher score on the 5MWT reflects a lower walking speed.
Psychological and cognitive factors
Cognitive functioning after stroke was assessed with the Montreal Cognitive Assessment (MoCA). Scores range from 0 to 30 (<26 indicates impaired cognitive function), and higher scores indicate better cognitive functioning [Citation39]. The Hospital Anxiety and Depression Scale (HADS) was used to assess the presence of symptoms of anxiety or depression. The HADS consists of 14 items, divided into seven items about anxiety (HADS-A) and seven items about depression (HADS-D). Both the HADS-A and HADS-D scores range from 0 to 21; scores ≥8 indicate the presence of symptoms of anxiety and depression respectively [Citation40]. Self-efficacy was evaluated with the Self-Efficacy for Symptom Management Scale (SEsx) which consists of 13 items. Scores range from 13 to 130, and scores < 115 indicate low/moderate self-efficacy [Citation41].
Statistical analysis
All analyses were conducted with IBM SPSS statistics version 26 (IBM Corp., Armonk, NY). To describe the patients’ characteristics and independent variables, descriptive statistics were used. Missing data were considered missing at random because data were more often missing for female participants. For that reason, multiple imputations using Multivariate Imputation by Chained Equation was used [Citation42]. Multiple imputations was performed by fitting models to predict missing outcomes based on all other observed variables. Five imputed data sets were created and combined with a pooled set using Rubin’s rules [Citation43].
Generalized estimating equations (GEE) were performed to explore the relationship between movement behavior patterns and participation over time. An exchangeable correlation structure was used to correct for within-subject correlations [Citation44]. SIS Participation (measured at discharge, at six months and at one year) was entered as a dependent variable and time as a categorical within-subject variable.
By adding age, sex, stroke severity (NIHSS), discharge destination, cognitive functioning (MoCA), anxiety symptoms (HADS-A), depressive symptoms (HADS-D) and self-efficacy (SEsx) to the model, potential confounding variables were identified. A variable was considered to be a confounder if the coefficient of the movement behavior patterns changed more than 10% after adding the variable to the model. If not, the variable was left out of the analyses. Results are expressed as regression coefficients (β) with 95% confidence intervals (CI). A positive score implies an improvement in SIS Participation scores compared to the reference category with β units, containing both within-subject as between-subject effects. P-values of < 0.05 were considered statistically significant. To answer both research questions, two GEE models were performed.
In the first model, the course of participation over time for each movement behavior pattern at baseline was explored. Therefore, movement behavior patterns at baseline (measured directly after discharge to the home setting) were entered as an independent factor. As a non-linear recovery pattern of participation over time was expected [Citation5], time was added to the model as a categorical variable, modelling each time interval separately. Interaction terms between time and movement behavior patterns at discharge were added to the model to explore the course of participation over time for each movement behavior pattern at discharge.
In the second model, the longitudinal association between the development of movement behavior patterns over time and participation was explored. Therefore, the development of movement behavior patterns over time (measured at discharge, six months and one year) was entered as an independent factor, and differences between the movement behavior patterns were calculated.
Results
In total, 262 people from the stroke unit agreed to participate in the study. A total of 200 people were included in the study at discharge from inpatient care, of whom 184 (92%) participated after six months and 175 (88%) after one year (flowchart and reasons for refusal are presented in ) [Citation25].
Figure 1. RISE study flowchart [Citation24].
![Figure 1. RISE study flowchart [Citation24].](/cms/asset/03b197e5-0aa3-45fc-a507-743a8a965115/idre_a_2109071_f0001_b.jpg)
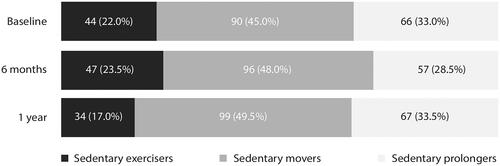
The participants’ characteristics at baseline are presented in [Citation25]. The mean age at stroke onset was 67.8 years, 64.0% were male and 91.5% had an ischemic stroke. The majority of the participants experienced no or minor stroke symptoms (68.5%) and were discharged home after hospitalization (73.5%). A total of 44 people (22%) were classified as sedentary exercisers, 90 people (45%) were classified as sedentary movers, and 66 people (33%) were classified as sedentary prolongers directly after discharge to the home setting (). Sedentary exercisers were younger, had fewer comorbidities, had better physical functioning and had higher walking speed compared to the sedentary movers and sedentary prolongers. Sedentary exercisers had higher levels of self-efficacy compared to sedentary prolongers. The composition of the movement behavior patterns remained relatively stable over time ().
Figure 2. Distribution movement behavior pattern groups at baseline, 6 months and 1 year (n = 200).
The course of participation over time across the movement behavior patterns
All movement behavior patterns showed significant improvement in the first six months after discharge to the home setting (), adjusted for the confounding effects of age, sex, stroke severity (NIHSS), and anxiety symptoms (HADS-A) and discharge destination. Between six months and one year, a non-significant decline in participation was observed in all movement behavior groups, most distinctive in the sedentary prolongers (). Overall, only the sedentary movers showed significant improvement over time in the first year after discharge to the home setting.
Figure 3. The modelled course of participation during the first year after discharge to the home setting in people with a first-ever stroke per movement behavior pattern at baseline. Abbreviations: SIS Participation: Stroke Impact Scale 3.0 Participation domain. Outcomes are adjusted for the confounding effects of stroke severity (NIHSS), age, sex, symptoms of anxiety (HADS-A) and discharge destination after hospitalization. Note: higher SIS Participation scores indicate better participation outcomes. The error bars represent the standard error (SE). *p < 0.05, comparing mean SIS Participation scores between consecutive time points.
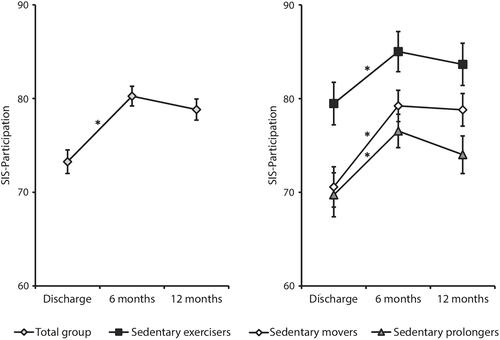
Table 2. Changes in SIS Participation during the first year after discharge across different movement behavior patterns identified directly after discharge to the home setting.
Sedentary exercisers had better participation outcomes compared to the sedentary movers and sedentary prolongers at discharge (p = 0.006 and p = 0.003, respectively) and at six months (p = 0.036 and p = 0.003, respectively), but at one year, the differences between sedentary exercisers and sedentary movers were no longer statistically significant. Sedentary prolongers had the most unfavorable course of participation up to one year after stroke (), as they had the worst participation scores at all time points.
The longitudinal association between movement behavior patterns and participation
Sedentary prolongers were associated with worse participation compared to sedentary exercisers, adjusted for the confounding effects of age, sex, stroke severity (NIHSS), anxiety symptoms (HADS-A) and discharge destination (). No association was found between sedentary exercisers and sedentary movers, and between sedentary exercisers and sedentary movers.
Table 3. The longitudinal association between the course of SIS Participation and the development of movement behavior patterns over time.
Discussion
In this prospective cohort study, we showed that the course of participation over time differed based on distinct movement behavior patterns identified directly after discharge to the home setting. Sedentary prolongers, being inactive and highly sedentary, experienced the most restrictions in participation over time. Furthermore, a longitudinal association between the participation and the development of movement behavior patterns over time was observed after stroke, showing worse participation in sedentary prolongers compared to sedentary exercisers. Our results show that supporting people with stroke to adapt and maintain a healthy movement behavior after discharge to the home setting could prevent potential long-term restrictions in participation. As participation is considered the cornerstone of successful rehabilitation after stroke [Citation11], these new insights into the longitudinal association between movement behavior and participation provide potential targets and strategies for rehabilitation interventions to improve long-term outcomes in people with stroke.
The course of participation over time across the movement behavior patterns
Our results showed that participation improved in the first six months after stroke and stabilized from six months onwards, which is in accordance with previous literature on the course of participation over time [Citation5]. Similar recovery patterns of participation were observed across the different movement behavior patterns, but baseline and long-term levels of participation differed. At discharge to the home setting, both sedentary prolongers and sedentary movers were already experiencing worse participation outcomes compared to sedentary exercisers. However, the sedentary movers were able to catch up with the sedentary exercisers one year after discharge, whereas the sedentary prolongers were never able to close this gap and still experienced considerably worse participation one year after discharge compared to the sedentary exercisers. A comparison between the recovery patterns of physical functioning (using the SIS Physical) across movement behavior patterns yielded somewhat similar results: after an initial improvement in physical functioning in the first six months, sedentary prolongers were at risk for decline in physical functioning from six months onwards [Citation25].
Although no minimal clinically important difference has been defined for SIS Participation in current literature, the difference between sedentary exercisers and sedentary prolongers at one year (10 points) seems clinically meaningful [Citation45]. Among all SIS domains, SIS Participation has been identified as the SIS domain showing the most clinically meaningful changes between three months and one year after stroke in a similar Swedish stroke cohort, as many people experienced either improvement or deterioration in participation within this time period [Citation46]. More improvement in SIS Participation over time was observed in our study, resulting in slightly higher SIS Participation scores one year after stroke in our study compared to the Swedish cohort (78.8 versus 70.3) [Citation46].
The longitudinal association between movement behavior patterns and participation
To the best of our knowledge, this is the first study exploring the longitudinal association between the development of movement behavior patterns over time and participation after stroke. Similar to our study, a Canadian study exploring the longitudinal effect of a home-based sedentary behavior change intervention in 34 people with stroke after discharge from inpatient rehabilitation, found improvement in SIS Participation in the first 16 weeks after discharge to the home setting [Citation47]. Also, a Chinese prospective cohort study in first-ever ischemic stroke patients reported the longitudinal relation between quality of life (measured with the 12-item Short-Form Health Survey) and exercise frequency and exercise time up to two years after stroke onset [Citation48]. They concluded that irregular exercisers had an unfavorable course of quality of life over time compared to regular exercisers after stroke. Although exercise frequency and exercise time were only measured using self-report questionnaires and no other types of movement behavior (such as sedentary behavior or LPA) were taken into account, these results are in line with the unfavorable course of participation in sedentary prolongers as stated in our study.
Furthermore, our results revealed worse participation in sedentary prolongers versus sedentary exercisers over time. A recent study found that people’s movement behavior patterns often deteriorated over time (as for example 9.0% of the sedentary prolongers at one year were actually classified as sedentary exercisers at baseline) [Citation27]. Therefore, early identification of sedentary prolongers and prolonged follow-up to prevent a relapsing sedentary and inactive lifestyle is essential. On the other hand, no differences in the course of participation between the development of other movement behavior patterns over time were found in our study. Premorbid movement behavior patterns, the growing contribution of other factors associated with long-term participation and movement behavior over time, such as personal and environmental factors, and the stabilization of participation outcome after six months, maybe possible explanations [Citation49,Citation50].
Future research
As this study identified movement behavior as an important determinant of participation after stroke, we also recommend future studies consider movement behavior as a whole (combining LPA, MVPA and sedentary behavior), instead of focusing on just one aspect of movement behavior. Currently, exercise interventions in stroke rehabilitation are mainly focused on raising MVPA levels, while attention to other essential components of movement behavior such as sedentary behavior and LPA is often lacking [Citation51]. Preliminary results of interventions incorporating sedentary behavior and LPA already yielded promising results in older adults and people with stroke [Citation47,Citation52]. Interventions including tailored counseling toward behavioral change were found to be effective to pursue sustainable long-term change in movement behavior [Citation53]. Therefore, these interventions may also have the potential to promote the course of participation after a stroke.
Strengths & limitations
The longitudinal design, the large sample size and the stratification based on the individual’s movement behavior patterns are among the main strengths of this study. Furthermore, extensive measurements on the individual’s movement behavior took place using accelerometry for 14 days, enabling accurate insights into the habitual movement behavior of the participants over time. Last but not least, our results were highly robust due to the statistical technique used for the longitudinal data analysis (GEE), which took into account that the repeated observations within one subject are not independent and allowed adjustment for confounding effects in the analyses.
Although participants were directly recruited from the stroke units to enable an accurate representation of the general stroke population, the study population largely consisted of relatively mild stroke patients with mostly ischemic strokes. This could negatively affect the generalizability of the results to more severely affected stroke patients and those with hemorrhagic strokes. Furthermore, causality between movement behavior and participation could not be proven with our study design, as an association does not imply causation. Therefore, whether or not participation could be modified by improving movement behavior is not entirely certain.
Conclusions
In conclusion, this study shows that the course of participation in people with a first-ever stroke up to one year after discharge to the home setting differed based on three distinct movement behavior patterns, i.e., sedentary exercisers, sedentary movers and sedentary prolongers. Early identification of highly sedentary and inactive people with stroke after discharge to the home setting is important, as sedentary prolongers are associated with an unfavorable course of participation compared to sedentary exercisers over time. To unravel the potential of movement behavior as a modifiable factor to improve participation after stroke, more research about the effectiveness of tailored interventions targeting movement behavioral change on long-term participation is needed.
Ethics approval and consent to participate
All participants gave written informed consent. The study was approved by the METC of UMC Utrecht 14/076.
Acknowledgments
We would like to thank all participants for their contribution to the RISE-study. Furthermore, we would like to thank the staff of Catharina Hospital (Eindhoven), Jeroen Bosch Ziekenhuis (‘s Hertogenbosch), Maxima Medisch Centrum (Veldhoven) and Sint-Jans Gasthuis (Weert), and we would like to thank Thirsa Koebrugge and Joeri Polman, who helped with the data collection.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1736–1788.
- Avan A, Digaleh H, Di Napoli M, et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: an ecological analysis from the global burden of disease study 2017. BMC Med. 2019;17(1):191.
- Lakshminarayan K, Berger AK, Fuller CC, et al. Trends in 10-year survival of patients with stroke hospitalized between 1980 and 2000: the Minnesota stroke survey. Stroke. 2014;45(9):2575–2581.
- Waziry R, Heshmatollah A, Bos D, et al. Time trends in survival following first hemorrhagic or ischemic stroke between 1991 and 2015 in the rotterdam study. Stroke. 2020;51(3):824–829.
- Ezekiel L, Collett J, Mayo NE, et al. Factors associated with participation in life situations for adults with stroke: a systematic review. Arch Phys Med Rehabil. 2019;100(5):945–955.
- Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38(2):208–211.
- Broussy S, Saillour-Glenisson F, García-Lorenzo B, et al. Sequelae and quality of life in patients living at home 1 year after a stroke managed in stroke units. Front Neurol. 2019;10:907.
- World Health Organization. The international classification of functioning, disability and Health – ICF. Geneva: World Health Organization; 2001.
- Thilarajah S, Mentiplay BF, Bower KJ, et al. Factors associated with post-stroke physical activity: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2018;99(9):1876–1889.
- Engel-Yeger B, Tse T, Josman N, et al. Scoping review: the trajectory of recovery of participation outcomes following stroke. Behav Neurol. 2018;2018:5472018.
- Woodman P, Riazi A, Pereira C, et al. Social participation post stroke: a meta-ethnographic review of the experiences and views of community-dwelling stroke survivors. Disabil Rehabil. 2014;36(24):2031–2043.
- van Mierlo ML, van Heugten CM, Post MWM, et al. Quality of life during the first two years post stroke: the Restore4Stroke cohort study. Cerebrovasc Dis. 2016;41(1–2):19–26.
- Desrosiers J, Demers L, Robichaud L, et al. Short-term changes in and predictors of participation of older adults after stroke following acute care or rehabilitation. Neurorehabil Neural Repair. 2008;22(3):288–297.
- de Graaf JA, van Mierlo ML, Post MWM, et al. Long-term restrictions in participation in stroke survivors under and over 70 years of age. Disabil Rehabil. 2018;40(6):637–645.
- van der Zee C, Visser-Meily JMA, Lindeman E, et al. Participation in the chronic phase of stroke. Top Stroke Rehabil. 2013;20(1):52–61.
- Kim M, Cho K, Lee W. Community walking training program improves walking function and social participation in chronic stroke patients. Tohoku J Exp Med. 2014;234(4):281–286.
- Obembe AO, Eng JJ. Rehabilitation interventions for improving social participation after stroke. Neurorehabil Neural Repair. 2016;30(4):384–392.
- Danielsson A, Willén C, Sunnerhagen KS. Is walking endurance associated with activity and participation late after stroke? Disabil Rehabil. 2011;33(21–22):2053–2057.
- Baert I, Feys H, Daly D, et al. Are patients 1 year post-stroke active enough to improve their physical health? Disabil Rehabil. 2012;34(7):574–580.
- Fulk GD, He Y, Boyne P, et al. Predicting home and community walking activity post-stroke. Stroke. 2017;48(2):406–411.
- Butler EN, Evenson KR. Prevalence of physical activity and sedentary behavior among stroke survivors in the United States. Top Stroke Rehabil. 2014;21(3):246–255.
- English C, Healy GN, Coates A, et al. Sitting and activity time in people with stroke. Phys Ther. 2016;96(2):193–201.
- Moore SA, Hallsworth K, Plötz T, et al. Physical activity, sedentary behaviour and metabolic control following stroke: a cross-sectional and longitudinal study. PLOS One. 2013;8(1):e55263.
- Wondergem R, Veenhof C, Wouters E, et al. Movement behavior patterns in people with first-ever stroke. Stroke. 2019;50(12):3553–3560.
- Wondergem R, Pisters MF, Wouters EJM, et al. The course of physical functioning in the first two years after stroke depends on peoples’ individual movement behavior patterns. Int J Stroke. 2022;17(1):83–92.
- English C, Manns PJ, Tucak C, et al. Physical activity and sedentary behaviors in people with stroke living in the community: a systematic review. Phys Ther. 2014;94(2):185–196.
- van der Laag P, Wondergem R, Pisters M. Movement behavior patterns composition remains stable, but individuals’ membership changes over time in people with a first-ever stroke. Eur Rev Aging Phys Act. 2022;19(1):11.
- Collin C, Wade DT, Davies S, et al. The barthel ADL index: a reliability study, international disability studies. Int Disabil Stud. 1988;10(2):61–63.
- Pijfers L, de Vries LA, Messing-Peterson H, editors. The Utrecht communication assessment [het utrechts communicatie onderzoek]. Utrecht: Stichting Afasie Nederland; 1985.
- Holden MK, Gill KM, Magliozzi MR. Gait assessment for neurologically impaired patients: standards for outcome assessment. Phys Ther. 1986;66(10):1530–1539.
- Vellone E, Savini S, Fida R, et al. R. Psychometric evaluation of the stroke impact scale 3.0. J Cardiovasc Nurs. 2015;30(3):229–241.
- Duncan PW, Bode RK, Lai M, et al. Rasch analysis of a new stroke-specific outcome scale: the stroke impact scale. Arch Phys Med Rehabil. 2003;84(7):950–963.
- Lin K, Fu T, Wu C, et al. Minimal detectable change and clinically important difference of the stroke impact scale in stroke patients. Neurorehabil Neural Repair. 2010;24(5):486–492.
- Fanchamps MHJ, Horemans HLD, Ribbers GM, et al. The accuracy of the detection of body postures and movements using a physical activity monitor in people after a stroke. Sensors. 2018;18(7):2167.
- Verhage F. Intelligence and age: research in a dutch sample. In: Intelligentie en leeftijd: Onderzoek bij nederlanders van twaalf tot zevenenzeventig jaar. Assen: Van Gorcum; 1964.
- de Groot V, Beckerman H, Lankhorst GJ, et al. How to measure comorbidity: a critical review of available methods. J Clin Epidemiol. 2003;56(3):221–229.
- Meyer BC, Hemmen TM, Jackson CM, et al. Modified national institutes of health stroke scale for use in stroke clinical trials: prospective reliability and validity. Stroke. 2002;33(5):1261–1266.
- van Bloemendaal M, van de Water Alexander TM, van de Port Ingrid GL, et al. Walking tests for stroke survivors: a systematic review of their measurement properties. Disabil Rehabil. 2012;34(26):2207–2221.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699.
- Spinhoven PH, Ormel J, Sloekers PPA, et al. A validation study of the hospital anxiety and depression scale (HADS) in different groups of dutch subjects. Psychol Med. 1997;27(2):363–370.
- Cicerone KD, Azulay J. Perceived self-efficacy and life satisfaction after traumatic brain injury. J Head Trauma Rehabil. 2007;22(5):257–266.
- Lee JA, Gill J. Missing value imputation for physical activity data measured by accelerometer. Stat Methods Med Res. 2018;27(2):490–506.
- Royston P, Carlin JB, White IR. Multiple imputation of missing values: new features for mim. Stata J. 2009;9(2):252–264.
- Twisk J. Applied longitudinal data analysis for epidemiology: a practical guide. second edition. Cambridge: University Press; 2011.
- Duncan PW, Wallace D, Lai SM, et al. The stroke impact scale version 2.0. evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30(10):2131–2140.
- Guidetti S, Ytterberg C, Ekstam L, et al. Changes in the impact of stroke between 3 and 12 months post-stroke, assessed with the stroke impact scale. J Rehabil Med. 2014;46(10):963–968.
- Ezeugwu VE, Manns PJ. The feasibility and longitudinal effects of a home-based sedentary behavior change intervention after stroke. Arch Phys Med Rehabil. 2018;99(12):2540–2547.
- Hou L, Du X, Chen L, et al. Exercise and quality of life after first-ever ischaemic stroke: a two-year follow-up study. Int J Neurosci. 2018;128(6):540–548.
- Robinson CA, Shumway-Cook A, Ciol MA, et al. Participation in community walking following stroke: Subjective versus objective measures and the impact of personal factors. Phys Ther. 2011;91(12):1865–1876.
- Foley EL, Nicholas ML, Baum CM, et al. Influence of environmental factors on social participation post-stroke. Behav Neurol. 2019;2019:2606039.
- Moore SA, Hrisos N, Flynn D, et al. How should long-term free-living physical activity be targeted after stroke? A systematic review and narrative synthesis. Int J Behav Nutr Phys Act. 2018;15(1):100.
- Rosenberg DE, Gell NM, Jones SMW, et al. The feasibility of reducing sitting time in overweight and obese older adults. Health Educ Behav. 2015;42(5):669–676.
- Morris JH, Macgillivray S, McFarlane S. Interventions to promote long-term participation in physical activity after stroke: a systematic review of the literature. Arch Phys Med Rehabil. 2014;95(5):956–967.
