Abstract
Purpose
To evaluate physical fitness and its association with fatigue in patients with low grade glioma (LGG).
Methods
Cross-sectional study. Muscle strength was measured with a digital dynamometer, cardiorespiratory fitness (peak oxygen uptake (VO2peak), maximal workload (MWL)) by cardiopulmonary-exercise-testing, and fatigue by using the Multidimensional Fatigue Inventory.
Results
Thirty patients were included, mean age of 44.1 (SD11.2) years, and 67% were men, 31.2 (SD18) months post-diagnosis. Muscle strength (p < 0.01), and cardiorespiratory fitness (VO2peak, MWL) (p < 0.01) were significantly decreased compared to predicted values based on age and gender. Thirty percent of the patients experienced severe physical fatigue, and severe mental fatigue was reported in 57% of the patients. Cardiorespiratory fitness showed weak to moderate (r − 0.46 to r − 0.52) but significant (p < 0.01) correlations with physical fatigue, not with mental and general fatigue. Muscle strength was not associated with fatigue. A lower VO2peak was independently associated with a higher level of physical fatigue, adjusted for Karnofsky Performance Status (R2 0.40).
Conclusions
Physical fitness (muscle strength, cardiorespiratory fitness) is reduced in patients with LLG, and a lower level of cardiorespiratory fitness (VO2peak) is independently associated with a higher level of experienced physical fatigue. Trials to explore the benefit of exercise programs to improve cardiorespiratory fitness and, consequently, fatigue are warranted.
Physical fitness (muscle strength and cardiorespiratory fitness) is reduced in patients with low-grade glioma.
Patients with low-grade glioma should be screened for fatigue with the multidimensional fatigue inventory, to differentiate between mental and physical fatigue.
Patients with low-grade glioma with severe physical fatigue should be screened for reduced physical fitness, especially cardiorespiratory fitness by objective cardiopulmonary-exercise-testing.
Rehabilitation exercise programs to improve cardiorespiratory fitness and, consequently, (physical) fatigue could be warranted in patients with low-grade glioma.
Implications for rehabilitation
Introduction
Gliomas encompass 30% of all tumors in the brain and central nervous system and 80% of all malignant brain tumors [Citation1]. Nowadays, these tumors are classified according to their genetic abnormalities; most of the histologically lower grade diffuse glioma’s (LGG, in particular, astrocytoma and oligodendroglioma) are characterized by the presence of a mutation in the gene encoding for isocitrate dehydrogenase (IDH). Patients with an IDH mutated grade 2 or 3 gliomas have a median survival time between eight and fifteen years, emphasizing the importance of quality of life after treatment [Citation2–4]. Fatigue is considered to be one of the most prevalent and disturbing long-term effects of cancer, significantly affecting the ability to perform activities of daily living and reducing a patient’s quality of life [Citation5,Citation6]. Cancer-related fatigue is defined as a “persistent, subjective sense of tiredness related to cancer and cancer treatment that interferes with usual daily functioning,” and is described as a multidimensional phenomenon where physical, cognitive and emotional issues interfere [Citation7,Citation8]. Unfortunately, little is known about the specific determinants of cancer-related fatigue, especially in patients with glioma, which makes treatment difficult [Citation9–11].
The pathophysiology of cancer-related fatigue can be viewed from a mental and physical perspective [Citation12]. The mental perspective of fatigue focuses on the increased cognitive effort required by patients with brain injury to meet the demands of everyday life [Citation13,Citation14] and, according to previous findings, provides a plausible partial explanation for (mental) fatigue in glioma patients [Citation15]. The physical perspective focuses on health-related components of physical fitness, including cardiorespiratory fitness and muscle strength [Citation16,Citation17]. Poorer fitness means that carrying out ordinary daily tasks becomes more physically demanding, which can lead to fatigue [Citation18]. A decrease in cardiorespiratory fitness and muscle strength is reported in patients with other types of cancer [Citation18,Citation19], and relations between decreased physical fitness and fatigue were shown [Citation20,Citation21]. Moreover, studies in patients with all sorts of cancer have shown that exercise therapy during and after cancer is beneficial in improving physical fitness [Citation22,Citation23] and reducing fatigue [Citation24,Citation25]. Exercise therapy may therefore be an effective intervention to reduce fatigue. However, levels of fatigue in patients with a brain tumor are 40–50% higher than in patients suffering from other types of cancer [Citation26,Citation27]. Therefore, it is important to evaluate the relationship between physical fitness levels and fatigue in LGG patients. So far, the physical fitness of LGG patients has only been reported in 2 studies. Both studies reported reduced fitness compared to the norm. However, one of these studies only included patients who had recently (<10 days) undergone surgical treatment whereas the other study selected patients with demonstrated low to moderate physical fitness [Citation28,Citation29]. Therefore, the reported physical fitness of these patients may not be representative of the total group of patients with LGG in the chronic phase. To the best of our knowledge, there are no studies on LGG patients that have examined the relationship between physical fitness and fatigue.
Therefore, the aim of this study was to evaluate physical fitness in terms of muscle strength and cardiorespiratory fitness and to study its relationship with fatigue in patients with low-grade glioma. Knowledge about this can contribute to the development of appropriate rehabilitation programs aimed at reducing fatigue, expanding the ability to perform activities of daily living and improving the patient’s quality of life.
Methods
Design and procedure
This study has an observational cross-sectional single-centre design. The study is part of the Assessment of Fatigue in Glioma Patients [AFIG] study. Patients were invited for a study visit to Erasmus MC, University Medical Center Rotterdam in the Netherlands, where a blood sample was taken and physical fitness tests were performed. Standardized questionnaires and cognitive tests were completed during a study visit at the patient’s home. Cognitive outcomes were analysed in a separate study [Citation15]. The study was approved by the Medical Ethics Committee of Erasmus MC (METC-2015-577). All patients provided written informed consent before the start of the study.
Participants
Consecutive patients were recruited between May 2016 and September 2018 from the neuro-oncology and neurosurgery department of Erasmus MC. Patients in the stable chronic phase were included if they met the following inclusion criteria [Citation1]: histologically proven grade II glioma or grade III IDH mutant glioma [Citation2], time post histological diagnosis >6 months and ≤5 years [Citation3], in clinical follow-up at Erasmus MC and [Citation4] age at diagnosis ≥18 years.
Patients were excluded if they [Citation1] underwent active treatment or were treated with surgery, radiotherapy and/or chemotherapy in the previous three months [Citation2]; were diagnosed with any additional progressive neurological disease and/or psychiatric disorder according to the DSM-IV; or [Citation3] did not have sufficient command of the Dutch language to understand instructions during the psychometric investigation and exercise testing. Patients in the active treatment phase (surgery, radiotherapy, chemotherapy) were included at a later time (≥3 months after treatment) because the temporary side effects of treatment may interfere with the outcomes of the physical fitness tests and other outcomes of the study.
Outcome measures
Physical fitness
Tests were performed in a fixed order, starting with the muscle strength test, and ending with the cardiopulmonary exercise test. Muscle strength was determined from the hand grip strength of the dominant hand with a custom-made digital dynamometer (Hartech, Wormerveer, The Netherlands) at the most common grip width [Citation30]. During measurement, patients sat on a chair without armrests with zero shoulder rotation, with the elbow flexed at 90 degrees, and with the forearm and wrist in the neutral position. Hand grip strength was expressed in kg, and the mean of 3 maximum voluntary contractions was analysed. Individual predicted values were calculated using norm values specified for gender, age and preferred hand [Citation31].
Cardiorespiratory fitness was assessed by cardiopulmonary exercise testing (CPET), using a cycle ergometer (Excalibur Sport, Lode, Groningen, the Netherlands). Gas exchange was analysed by indirect calorimetry (Oxycon Pro; ViaSys Healthcare, Houten, the Netherlands). After instructions and calibration, patients warmed up for 4 min without resistance, after which a ramp protocol started to reach their maximum physical effort within 10–14 min. Resistance increased every 10 s, and varied by gender (women: 12 W/min, men: 16 W/min). Verbal encouragement was provided to continue as long as possible until maximum exhaustion. The test stopped when the participants were not able to maintain the target pedal rate (70–90 rpm). The respiratory exchange ratio (RER) was used to determine whether participants had reached maximal physical exertion (RER > 1.0) [Citation32]. Directly after stopping the test, patients were asked to rate their subjective feeling of exertion using the revised category-ratio Borg-scale. The scale ranges from 0 (no exertion at all) to 10 (maximal exertion) [Citation33].
Peak oxygen uptake (VO2peak) was defined as the highest mean value during a 15 s window of exercise and was expressed in mL/kg/min. Maximal workload (MWL) was defined as the maximal wattage sustained for 10 s and expressed in Watt/kg. Measured values for VO2peak and MWL were compared to individual predicted values calculated from published equations specified for gender and age (VO2peak) [Citation34] and gender, age, weight and height (MWL) [Citation35]. The ventilatory anaerobic threshold was estimated by two assessors using the ventilator equivalent method, defined as the moment at which the ventilatory equivalent for CO2 (VE/VCO2) and end-tidal CO2 partial pressure (PETCO2) remained stable, and the VE/VO2 and the end-tidal O2 partial pressure (PETO2) increased disproportionally [Citation36]. Additionally, the metabolic equivalent of task (MET) at the anaerobic threshold was calculated [Citation37]. The MET concept represents a simple, practical, and easily understood the procedure for expressing the energy cost of all kinds of daily functioning and physical activities, as a multiple of the resting metabolic rate. An anaerobic threshold ≥5 MET is required to perform basic daily self-care (ADL) and instrumental activities of daily living (IADL) [Citation38].
Fatigue
Fatigue was measured using the Multi-dimensional Fatigue Index (MFI-20), which is a self-report questionnaire [Citation39]. The MFI is a 20-item questionnaire designed to evaluate five domains of fatigue: general fatigue, physical fatigue, reduced motivation, reduced activity, and mental fatigue [Citation39,Citation40]. Each domain contains 4 items, with scores on a 1–5 point Likert scale. Each subdomain score ranges from 4 to 20, with higher scores indicating more fatigue. Normative values were used to classify patients into subgroups of severe and non-severe fatigue adjusted for age and sex [Citation41]. Mild fatigue was defined as a score >1 and ≤2 standard deviations (SD) above the norm value and severe fatigue > 2 SD above the norm [Citation42].
Other outcomes
Clinical characteristics (e.g., tumor type, tumor treatment and Karnofsky Performance Status) were retrieved from patient records and sociodemographic characteristics were collected by a questionnaire developed for the purpose of the study. Depression was measured with the Center for Epidemiological Studies Depression Scale (CES-D) [Citation43]. The CES-D uses a 20-item scale, with a total score ranging from 0 to 60. A higher score indicates more symptoms of depression. A score ≥ 16 indicates the presence of depression [Citation43,Citation44]. Data on anthropometry (body mass index) and body composition (fat mass) were collected. A general screening laboratory (blood)test for known and easily treatable causes of fatigue such as anemia, thyroid dysfunction and vitamin deficiency was performed. The screening consisted of determining Hemoglobin, Haematocrit, Mean Corpuscular Volume, Ferritin, Vitamin B12, Vitamin B11, 25-OH-Vitamin D, thyroid stimulating hormone, leucocytes and differentiation.
Statistics/data-analysis
IBM SPSS software, version 23, was used for the statistical analyses. Descriptive statistics and frequency distributions were generated for the sociodemographic and clinical characteristics of the study population. Paired t-tests were used to compare the physical outcomes to predicted values. Associations between physical outcomes and fatigue were expressed in Pearson correlations. A correlation coefficient between 0.3 and 0.5 is considered a weak correlation, between 0.5 and 0.7 is moderate, and ≥0.7 is a strong correlation [Citation45]. A multivariable linear regression analysis was performed to evaluate the independent association between physical fitness, as expressed by VO2peak, and physical fatigue, the dependent variable. The relationship was adjusted for potential confounders, which included age, sex, tumor type, Karnofsky Performance Status (KPS), presence of epilepsy, and symptoms of depression. First univariable analyses were performed and, if significant, variables were subsequently added to the multivariable model. Variables were kept in the multivariable model if they significantly added to the explained variance of the model. For all tests, the level of statistical significance was set at a p value < 0.05.
Results
Study population
Ninety-seven patients diagnosed with LGG were invited to participate in the study by a letter from their oncologist. Fifty-one patients returned the reply form, of which 20 refused participation [poor health condition (n = 6), duration of the assessments (n = 7), or unknown reason (n = 7)]. Thirty-one patients provided written informed consent and were included in the study. The health status of one patient deteriorated due to a new, unrelated neurological condition (Multiple Sclerosis) and was excluded. In total, 30 patients underwent all physical tests.
The mean age of the study population was 44.1 (SD 11.2) years, 67% were male, and the mean time post-diagnosis was 31.2 (SD 18) months. Twenty-nine patients had a grade II tumor, one patient had a grade III IDH mutant tumor. The most common histopathological diagnoses were diffuse astrocytoma II IDH mutant (43%) and oligodendroglioma II IDH mutant/1p19q codeleted (40%). Most patients had undergone surgical resection (97%). In 40% of the patients, surgical resection was the only treatment. Half of the patients (50%) had received a combination of surgery, radiotherapy, and chemotherapy. None of the patients used steroid medication. The outcomes of the blood sample screening of the patients showed no obvious reasons for fatigue complaints or reduced physical fitness. The baseline characteristics of the patients are listed in .
Table 1. Socio-demographic, clinical, and treatment characteristics (N = 30).
Physical fitness
Muscle strength tests were successfully performed on all 30 patients. During the cardiopulmonary exercise test, one patient did not reach the maximal physical exertion criterion (RER > 1.0), and therefore the results of this test were excluded from the analyses. The mean rate of exertion on the BORG scale was 8.7 (range 7–10). Both muscle strength and cardiorespiratory fitness were significantly lower than the predicted values. The average MET value at the anaerobic threshold was 6.5 (SD 2.2). Eight patients (28%) had a MET < 5. All physical outcomes are shown in .
Table 2. Outcomes of physical fitness.
Fatigue
Fatigue outcomes are shown in and the distribution of severity of fatigue in . Severe mental fatigue was present in 17 patients (57%) and 9 patients (30%) experienced severe physical fatigue. Of the 17 patients who experienced severe mental fatigue, 8 (47%) also experienced severe physical fatigue. Twenty-one patients (70%) had severe fatigue in at least one domain of fatigue.
Figure 1. Proportions of no, mild, and severe fatigue for the physical, mental and general fatigue domains of the Multi-dimensional Fatigue Index.
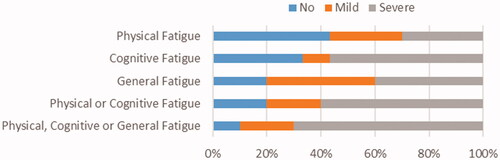
Table 3. Fatigue as measured with the Multi-dimensional Fatigue Index (N = 30).
In Pearson correlations between physical fitness outcomes and physical, mental and general fatigue are presented. No significant association was found between muscle strength and fatigue. Cardiorespiratory fitness outcomes showed significant weak to moderate inverse correlations with physical fatigue. A lower cardiorespiratory fitness correlated with a higher level of physical fatigue.
Table 4. Pearson correlations between physical fitness outcomes and physical, mental and general fatigue.
Clinical characteristics and physical fatigue
shows the results of the multivariable regression analysis. In univariable analyses, peak oxygen uptake (p = 0.02) and Karnofsky Performance Status (p = 0.03) were significantly associated with physical fatigue, whereas depression (p = 0.92), epilepsy (p = 0.29), tumor type (p=.92), sex (p = 0.87) and age (p = 0.68) were not. In the final multivariable model, VO2peak was significantly associated with physical fatigue, adjusted for Karnofsky's Performance Status.
Table 5. Multivariable linear regression model of Peak Oxygen Uptake (VO2peak) in relation to physical fatigue, adjusted for Karnofsky Performance Status.
Discussion
This study shows that patients with LGG in the chronic phase after treatment have impaired physical fitness. Muscle strength and cardiorespiratory fitness were significantly reduced when compared to predicted values. Thirty percent of the study population experienced severe physical fatigue, severe mental fatigue was present in 57% of the patients with LGG. Cardiorespiratory fitness outcomes showed weak to moderate (r between −0.46 and −0.52) but significant correlations with physical fatigue, but not with mental and general fatigue. A more impaired cardiorespiratory fitness correlated with higher levels of physical fatigue. Multivariable regression analysis showed that a lower VO2peak was independently associated with a higher level of physical fatigue, adjusted for KPS. With respect to cardiorespiratory fitness, VO2peak and maximal workload were significantly lower than the predicted reference values based on gender and age, showing a reduction of, respectively, 24% and 20%. We found that reduced cardiorespiratory fitness was related to increased physical fatigue. This is in agreement with previous studies on patients with different types of cancer [Citation20–22,Citation46]. Twenty-eight percent of the patients in our study had a MET score <5 at their anaerobic threshold. This means that just carrying out daily routines (ADL/IADL) is already a great effort for these patients and that they may experience metabolic acidosis when carrying out such tasks. Previous research reported that most patients with primary brain cancer are open to information about exercise and estimated that these patients are able to participate in an exercise program during and after cancer treatment [Citation26,Citation47]. A study about a home-based exercise intervention in patients with stable glioma who had low to moderate fitness levels reported a trend of improvement in VO2peak and reduction in perceived physical fatigue in the exercise group [Citation48]. Exercise interventions in several other populations of patients with cancer also show positive effects on cardiorespiratory fitness and decreased levels of fatigue [Citation21]. Therefore, improving cardiorespiratory fitness, aimed at increasing VO2peak and anaerobic threshold, should be considered a treatment goal in severely physically fatigued patients with LGG, with the aim of reducing the effort required to perform daily activities.
The reduction in muscle strength relative to predicted values (9%) was significant but probably not to an extent that it affects everyday activities. The decrease in hand grip strength confirms the results of another study in patients with high-grade glioma [Citation44]. We found no relation between hand grip strength and fatigue. Studies in other patient groups with a different type of cancer also failed to demonstrate correlations between hand grip strength and fatigue [Citation49–51]. A relationship has been found between decreased quadriceps muscle strength and increased general fatigue in patients with low-grade glioma who recently underwent surgical tumor resection (<1 month) [Citation28]. In addition, reduced functional capacity (e.g., timed sit-to-stand test) was found to be related to increased cancer-related fatigue [Citation49]. In contrast to hand grip strength, lower limb strength is more decisive for endurance and energy consumption, and may therefore be more strongly related to fatigue [Citation52].
Although this study focused on the physical domain of fatigue, a large proportion of our patients also experienced severe mental fatigue. Mental fatigue may be associated with cognitive and psychosocial disorders, from which patients with LGG may suffer [Citation53]. Acquired brain injury patients with cognitive disorders, need to deliver more cognitive effort in order to handle the demands of everyday life (ADL/IADL), which is likely to lead to mental fatigue [Citation14]. An important psychosocial factor is a mood. Depression has been associated with cancer-related fatigue but the nature and directionality of the depression-fatigue relationship are incompletely understood [Citation46,Citation54]. In our study population, 37% had symptoms of depression, but we found no association between depression and physical fatigue.
A limitation of this study is in particular the cross-sectional study design from which no causal relationships can be established. Furthermore, the study included a relatively small sample from a single academic medical center. Together with the low response rate, this may have resulted in selection bias and therefore the results should be interpreted with caution.
In conclusion, this study indicates that physical fitness (muscle strength and cardiorespiratory fitness) is reduced in patients with LGG in the chronic phase after treatment and that lower cardiorespiratory fitness is associated with a higher level of experienced physical fatigue. Physically fatigued patients with LGG may benefit from a personalized rehabilitation program to improve cardiorespiratory fitness.
Acknowledgments
The authors thank Emiel Sneekes for his contribution to the data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Grier JT, Batchelor T. Low-grade gliomas in adults. Oncologist. 2006;11(6):681–693.
- van den Bent MJ. Practice changing mature results of RTOG study 9802: another positive PCV trial makes adjuvant chemotherapy part of standard of care in low-grade glioma. Neuro Oncol. 2014;16(12):1570–1574.
- Shields LB, Choucair AK. Management of low-grade gliomas: a review of patient-perceived quality of life and neurocognitive outcome. World Neurosurg. 2014;82(1–2):e299-309–e309.
- Soffietti R, Baumert BG, Bello L, et al. Guidelines on management of low-grade gliomas: report of an EFNS-EANO task force. Eur J Neurol. 2010;17(9):1124–1133.
- Gustafsson M, Edvardsson T, Ahlstrom G. The relationship between function, quality of life and coping in patients with low-grade gliomas. Support Care Cancer. 2006;14(12):1205–1212.
- Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4–10.
- Mock V, Atkinson A, Barsevick A, et al. NCCN practice guidelines for cancer-related fatigue. Oncology. 2000;14(11A):151–161.
- Mock V, Atkinson A, Barsevick AM, et al. Cancer-related fatigue. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2007;5(10):1054–1078.
- Lee EQ, Muzikansky A, Drappatz J, et al. A randomized, placebo-controlled pilot trial of armodafinil for fatigue in patients with gliomas undergoing radiotherapy. Neuro Oncol. 2016;18(6):849–854.
- Prue G, Rankin J, Allen J, et al. Cancer-related fatigue: a critical appraisal. Eur J Cancer. 2006;42(7):846–863.
- Struik K, Klein M, Heimans JJ, et al. Fatigue in low-grade glioma. J Neurooncol. 2009;92(1):73–78.
- Al-Majid S, Gray DP. A biobehavioral model for the study of exercise interventions in cancer-related fatigue. Biol Res Nurs. 2009;10(4):381–391.
- van Zomeren AH, van den Burg W. Residual complaints of patients two years after severe head injury. J Neurol Neurosurg Psychiatry. 1985;48(1):21–28.
- Zomeren AH, vanBrouwer WH, Deelman BG. Attentional deficits: the riddles of selectivity, speed and alertness. In: N. Brooks, editor. Closed Head Injury: Psychological, Social and Family Consequences. New York (NY): Oxford University Press; 1984. p. 398–415.
- van Coevorden-van Loon EMP, Heijenbrok-Kal MH, Horemans HLD, et al. The relationship between mental fatigue, cognitive functioning, and employment status in patients with low-grade glioma: a cross-sectional single-center study. Disabil Rehabil. 2021[Oct 25];[1–7]. doi:10.1080/09638288.2021.1991013
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131.
- McArdle WK, Katch VL. Exercise physiology. Energy, nutrition and performance. 5th ed. Philadelphia: Williams & Wilkins; 2001.
- Lucía A, Earnest C, Pérez M. Cancer–related fatigue: can exercise physiology assist oncologists? Lancet Oncol. 2003;4(10):616–625.
- Chinapaw MJ, Buffart LM, van Mechelen W, et al. Alpe d‘HuZes cancer rehabilitation (A-CaRe) research: four randomized controlled exercise trials and economic evaluations in cancer patients and survivors. Int J Behav Med. 2012;19(2):143–156.
- Kilgour RD, Vigano A, Trutschnigg B, et al. Cancer-related fatigue: the impact of skeletal muscle mass and strength in patients with advanced cancer. J Cachexia Sarcopenia Muscle. 2010;1(2):177–185.
- Repka CP, Peterson BM, Brown JM, et al. Cancer type does not affect exercise-mediated improvements in cardiorespiratory function and fatigue. Integr Cancer Ther. 2014;13(6):473–481.
- Jones LW, Liang Y, Pituskin EN, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist. 2011;16(1):112–120.
- Speck RM, Courneya KS, Masse LC, et al. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100.
- Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012;11:CD006145.
- Kampshoff CS, Chinapaw MJ, Brug J, et al. Randomized controlled trial of the effects of high intensity and low-to-moderate intensity exercise on physical fitness and fatigue in cancer survivors: results of the resistance and endurance Exercise After ChemoTherapy (REACT) study. BMC Med. 2015;13:275.
- Cormie P, Nowak AK, Chambers SK, et al. The potential role of exercise in neuro-oncology. Front Oncol. 2015;5:85.
- Asher A, Fu JB, Bailey C, et al. Fatigue among patients with brain tumors. CNS Oncol. 2016;5(2):91–100.
- Jones LW, Friedman AH, West MJ, et al. Quantitative assessment of cardiorespiratory fitness, skeletal muscle function, and body composition in adults with primary malignant glioma. Cancer. 2010;116(3):695–704.
- Gehring K, Kloek CJ, Aaronson NK, et al. Feasibility of a home-based exercise intervention with remote guidance for patients with stable grade II and III gliomas: a pilot randomized controlled trial. Clin Rehabil. 2018;32(3):352–366.
- Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–429.
- Werle S, Goldhahn J, Drerup S, et al. Age- and gender-specific normative data of grip and pinch strength in a healthy adult swiss population. J Hand Surg Eur Vol. 2009;34(1):76–84.
- Prinsen H, Hopman MT, Zwarts MJ, et al. Maximal exercise performance in patients with postcancer fatigue. Support Care Cancer. 2013;21(2):439–447.
- Borg E, Kaijser L. A comparison between three rating scales for perceived exertion and two different work tests. Scand J Med Sci Sports. 2006;16(1):57–69.
- van der Steeg GE, Takken T. Reference values for maximum oxygen uptake relative to body mass in dutch/flemish subjects aged 6-65 years: the lowlands fitness registry. Eur J Appl Physiol. 2021;121(4):1189–1196.
- Van de Poppe DJ, Hulzebos E, Takken T. Low-Land fitness registry study g. Reference values for maximum work rate in apparently healthy dutch/flemish adults: data from the LowLands fitness registry. Acta Cardiol. 2019;74(3):223–230.
- Reinhard U, Muller PH, Schmulling RM. Determination of anaerobic threshold by the ventilation equivalent in normal individuals. Respiration. 1979;38(1):36–42.
- Jette M, Sidney K, Blumchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13(8):555–565.
- Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–S504.
- Smets EM, Garssen B, Bonke B, et al. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325.
- Kuhnt S, Ernst J, Singer S, et al. Fatigue in cancer survivors–prevalence and correlates. Onkologie. 2009;32(6):312–317.
- Schwarz R, Krauss O, Hinz A. Fatigue in the general population. Onkologie. 2003;26(2):140–144.
- Hendricks MPH, MBAW RP, Kessels C. Uniformiteit in de kwalitatieve beschrijving van scores op prestatietaken. Tijdschrift Voor Neuropsychologie. 2020;15(3):166–176.
- Radloff. The CES-D scale a self-reported depression scale for research in the general population. 1977.
- Bouma JR, Sanderman R, van Sonderen E. Het meten van symptomen van een depressie met de CED-D: Een Handleiding; 1995.
- Akoglu H. User’s guide to correlation coefficients. Turk J Emerg Med. 2018;18(3):91–93.
- Brownstein CG, Twomey R, Temesi J, et al. Physiological and psychosocial correlates of cancer-related fatigue. J Cancer Surviv. 2021 [Oct 5]. doi:10.1007/s11764-021-01115-6
- Jones LW, Guill B, Keir ST, et al. Exercise interest and preferences among patients diagnosed with primary brain cancer. Support Care Cancer. 2007;15(1):47–55.
- Gehring K, Stuiver MM, Visser E, et al. A pilot randomized controlled trial of exercise to improve cognitive performance in patients with stable glioma: a proof of concept. Neuro Oncol. 2020;22(1):103–115.
- Brown DJ, McMillan DC, Milroy R. The correlation between fatigue, physical function, the systemic inflammatory response, and psychological distress in patients with advanced lung cancer. Cancer. 2005;103(2):377–382.
- Stone P, Hardy J, Broadley K, et al. Fatigue in advanced cancer: a prospective controlled cross-sectional study. Br J Cancer. 1999;79(9–10):1479–1486.
- Schvartsman G, Park M, Liu DD, et al. Could objective tests Be used to measure fatigue in patients With advanced cancer? J Pain Symptom Manage. 2017;54(2):237–244.
- White C, Dixon K, Samuel D, et al. Handgrip and quadriceps muscle endurance testing in young adults. Springerplus. 2013;2:451.
- van Loon EM, Heijenbrok-Kal MH, van Loon WS, et al. Assessment methods and prevalence of cognitive dysfunction in patients with low-grade glioma: a systematic review. J Rehabil Med. 2015;47(6):481–488.
- Brown LF, Kroenke K. Cancer-related fatigue and its associations with depression and anxiety: a systematic review. Psychosomatics. 2009;50(5):440–447.
