Abstract
Purpose
Conservative management of lumbar radiculopathy (LR) is the first treatment option. To date, systematic reviews and clinical practice guidelines have not considered the most appropriate timing of management. This study aimed to establish consensus on effective conservative treatment modalities across different stages (i.e., acute, sub-acute, or chronic) of LR.
Materials and methods
Through an iterative multistage Delphi process, experts rated agreement with proposed treatment modalities across stages of LR and could suggest additional treatment modalities. The agreement was measured using a 5-point Likert scale. Descriptive statistics were used to measure agreement (median, interquartile ranges, and percentage of agreement). Consensus criteria were defined a priori for each round.
Results
Fourteen panelists produced a consensus list of effective treatment modalities across stages of LR. Acute stage management should focus on providing patients with information about the condition including pain education, individualized physical activity, and directional preference exercises, supported with NSAIDs. In the sub-acute stage, strength training and neurodynamic mobilization could be added and transforaminal/epidural injections considered. In the chronic stage, spinal manipulative therapy, specific exercise, and function-specific physical training should be combined with individualized vocational, ergonomic and postural advice.
Conclusions
Experts agree effectiveness of interventions differs through the evolution of LR.
To date clinical guideline for conservative management of lumbar radiculopathy do not consider the evolution of the condition.
Acute stage management of lumbar radiculopathy should focus on providing information about the condition and support individualized physical activity with pain medication.
Sub-acute management should add neurodynamic mobilization to strength training, while transforaminal and/or epidural injections could be considered.
Chronic stage management should consider spinal manipulative therapy and focus on restoring personalized functional capacity.
IMPLICATIONS FOR REHABILITATION
Introduction
Lumbar radiculopathy (LR), is a condition characterized by motor, reflex, and/or sensory changes, such as radicular pain, paresthesia, or numbness in the lower limb which may be provoked by spinal posture(s) and/or movement(s) [Citation1]. While radiculopathy and radicular pain commonly occur together, radiculopathy can occur in the absence of pain and radicular pain can occur in the absence of radiculopathy [Citation2,Citation3]. A prolapsed disc is a frequent cause of LR, but other causes include spinal or lateral recess stenosis, tumors, and radiculitis [Citation4]. Exact data on the incidence and prevalence of LR vary [Citation5,Citation6]. The incidence of LR in the Netherlands is estimated at 5 per 1000 persons a year [Citation7] and generally, a lifetime prevalence of around 40–90% has been reported [Citation6,Citation8]. The annual prevalence of LR in the general population is estimated at 2.2% [Citation9]. There is limited research on the economic burden of LR. A Dutch study estimated societal LR costs of about 13% of overall low back pain-related costs, equivalent to a current annual impact on the United Kingdom (UK) economy of over £500 million in healthcare costs and £3.8 billion in indirect costs related to LR [Citation10,Citation11].
In general, the clinical course of acute LR is favorable and most pain and related disability resolves within two weeks, however, a substantial proportion of patients (up to 30%) continue to have pain for a year or longer [Citation12,Citation13]. Unless emergency surgery is necessary (e.g., in the case of severe, progressive loss of function or cauda equine symptoms), conservative management is the preferred first treatment option, since the risk-benefit ratio for surgery is less favorable [Citation5,Citation14–19]. However, there is a lack of evidence for the effectiveness of conservative interventions [Citation14,Citation20,Citation21].
Systematic reviews, traditionally include outcomes from randomized controlled trials (RCTs) and sometimes controlled clinical trials (CCTs). RCTs in general do not examine management strategies during different stages of the studied condition (i.e., acute, sub-acute, chronic). Instead, they often manage all participants identically, regardless of the stage of the investigated condition [Citation22]. Rehabilitation programs, however, are based on the clinical reasoning that some treatment modalities might potentially be more effective in the early acute stage of the disorder, while others might be more effective during the subacute or chronic phases [Citation23,Citation24]. In the absence of data from randomized trials, consensus on how clinicians should approach different stages of LR can be gathered from a group of experts, using a Delphi approach.
Current evidence on the effectiveness of conservative management of patients with LR as well as cervical radiculopathy (CR) reports a lack of consensus on the optimal timing and dosage of treatment modalities [Citation21,Citation25–27]. A recent study on the timing of conservative treatment interventions reported a consensus on the effectiveness of different interventions in different stages of recovery of CR, using a Delphi study methodology [Citation28].
A Delphi technique is an approach that could be used to determine expert opinion on the most suitable timing of different interventions for the conservative management of LR representing the first step for a posterior validation study. The Delphi technique is described as “a method used to obtain the most reliable consensus of opinion of a group of experts by a series of intensive questionnaires interspersed with controlled feedback” [Citation29,Citation30]. Delphi studies are often used to combine clinical expertise and achieve consensus on what preferred management options should or could be included in the management of conditions including patients with LR at varying stages [Citation30,Citation31].
Objective
To establish consensus on effective conservative treatment modalities for patients in different stages (acute, sub-acute, and chronic) of LR, using the Delphi method approach.
Methods and analysis
Steering committee
A steering committee was formed, consisting of the five authors of this study: the lead investigator (ET) and four senior academics (MTdG, JAC, AG, and DF), all with experience in the Delphi technique, qualitative and quantitative research methods, and more than 10 years of clinical experience within musculoskeletal medicine and/or physiotherapy. The responsibility of the committee was to recruit experts and to design, circulate and analyze the questionnaires. The steering committee made collective decisions regarding methodology, data analysis, and quality assurance. The steering committee also composed an initial list of proposed treatment modalities collated from systematic reviews and (inter)national guidelines [Citation13,Citation20,Citation21,Citation25–27,Citation32–34].
Design
Similar to recent studies [Citation35–38] an electronic version of the Delphi method [Citation30,Citation31,Citation39,Citation40], was used and modified for the purpose of this study. An electronic platform was used to construct and distribute three rounds of surveys to an international expert panel in an iterative process of an e-Delphi technique [Citation39,Citation41]. This design allowed the recruitment of a homogenous group of international experts without geographical constraints and avoided dominance of opinion from minority members, offering anonymity, encouraging freedom of expression, and removing peer or authoritative pressure [Citation31]. The study is reported in line with the Conducting and Reporting Delphi Studies (CREDES) recommendations (Supplemental File 1) to ensure appropriate methodological rigor [Citation30].
Ethical approval was granted from the University of Birmingham ethics committee (ERN_21-0786). Formal consent and declaration of conflict of interests were required before participation in the study. Quasi-anonymity, which refers to blinding of participation between panel members but not to the researchers, was guaranteed. All participants were assigned a unique identification code to aid the feedback process and to protect the confidentiality of responses.
Definition of stages of LR terminology
Panelists were provided with a definition of LR being: “a clinical condition characterized by motor, reflex and/or sensory changes, such as radicular pain, paresthesia or numbness in the lower limb which may be provoked by spinal posture(s) and/or movement(s) [Citation1].
For this study, we chose to align the different clinical stages of LR with established pain terminology, e.g., “acute,” “sub-acute,” and “chronic” as proposed by the International Association for the Study of Pain (IASP) [Citation42,Citation43]. “Acute” pain being pain present for up to 6 weeks [Citation43]. “Subacute” pain is a subset of acute pain: being pain present for at least 6 weeks but <3 months [Citation44]. “Chronic” pain is defined as pain that persists or recurs for more than 3 months [Citation42,Citation43].
Participants
In line with the CREDES recommendations, experts were sought globally from a variety of different professional backgrounds (medicine, allied health care, academia) [Citation30]. The steering committee agreed on pre-defined eligibility criteria, informed by similar studies [Citation35,Citation37,Citation38] which defined experts. To serve as panelists, experts had to meet ≥1 criterion listed below:
Being the first author of ≥1 peer-reviewed publication on clinically relevant LR or lumbar spinal entrapment neuropathies within the past 10 years
Have ≥10 years of experience working in a pain/musculoskeletal outpatient of either primary and/or secondary care service with patients with LR or lumbar spinal entrapment neuropathies
Potential panelists also needed to have sufficient English and computer literacy skills, judged by either the language of authored publications and/or being the corresponding author of that publication.
Past research has suggested that 20 panelists are appropriate in a Delphi study to enable consensus [Citation31,Citation45,Citation46]. An upper limit for panelist numbers was not defined.
Recruitment
Electronic libraries (PubMed, Embase, CINAHL, Google Scholar) were searched by the recruiter (ET) for individuals meeting the eligibility criteria. Potential panelists were then contacted via e-mail and informed that they had been identified by the steering committee as an expert within the field, and simultaneously received an outline of the Delphi procedure and a provision of the study objective. The recruiter collated demographic data (e.g., country of residence, profession, and current occupation) for potential panelists. The recruitment period duration was limited to 6 weeks. The recruiter (ET) requested contact panelists to recommend peers who also satisfied the eligibility criteria, creating a snowballing strategy. Members of the steering committee were not allowed to complete the Delphi questionnaire but could recommend potential panelists from their professional network to the recruiter (ET). Following receipt of a signed consent form, conflict of interest form as well as a participant information form, participation was confirmed electronically.
Delphi procedure
Before the start of the study, a pre-notification period of 6 weeks was allocated to recruit participants. Questions were sent to the panelists “en bloc” and comments were returned in a non-blinded fashion to the lead investigator (ET), who incorporated the comments. Panelists received an email containing a link to the platform hosted on LimeSurvey® (www.limesurvey.com). All the participants’ characteristics, such as age, country of origin, current country of residence/work, highest qualification, current occupation, professional background, and amount of time working with patients who have LR or nerve root-related leg pain were collected.
Panelists were invited to provide their level of agreement for each proposed treatment modality for each stage of LR. Additionally, an open question was provided in each section to explore any additional treatment modalities which might have been overlooked by the steering committee. All the additional treatment modalities suggested by the participants were added to round two, when the questionnaire was returned to each participant, allowing them to compare their response from round 1 with the overall panel’s response. If treatment modalities did not reach pre-defined agreement criteria, they were excluded from the next round. The third round of this process was carried out to reach a consensus [Citation47]. The treatment modalities generated following round 3 were collated to create the final list of treatment modalities for each stage of LR. In line with similar studies, panelists were allowed four weeks to complete each round and three weeks per round were allocated for data analysis [Citation31,Citation35,Citation37,Citation38]. Non-responders were sent two reminders per round at equally distributed intervals. details the procedure and timeline for the study. Round 1 of the questionnaire (Supplemental File 2) was sent out in September 2021.
Figure 1. Procedure and timelines for participants in Delphi study.
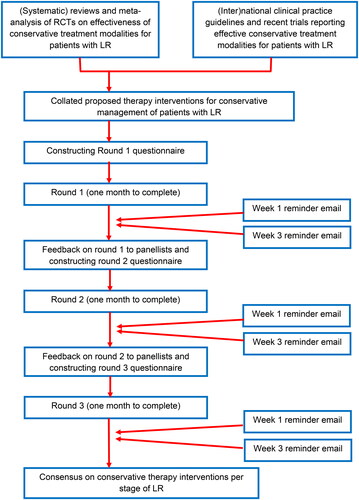
A five-point Likert scale (1 = strongly disagree, 2 = disagree, 3 = do not agree or disagree, 4 = agree, 5 = strongly agree) evaluated the level of agreement throughout [Citation48]. The consensus was assessed by analyzing descriptive statistics against pre-defined criteria for consensus.
A pilot study was conducted with eight students at the University of Birmingham with musculoskeletal expertise (PhD/MRes/MSc) who were invited to complete the round 1 survey over a 1-week period and asked to provide feedback to help improve the usability of the survey. Due to their feedback, clarification and/or elaboration with respect to some of the treatment categories and interventions was made and consistency of UK/US spelling was checked.
Data collection and analysis
Content analysis was used to analyze data from the free text boxes; treatment modalities were identified by two authors (ET, MTdG) which helped to inform the construction of the next round of survey. Results of the descriptive statistics and content analysis were discussed with the steering committee before constructing the next round of survey. The five-point ordinal Likert scale is an ordinal scale [Citation48–50]. Qualitative data was extracted deductively (to identify treatment modalities) and inductively (to identify additional treatment modalities). Descriptive statistics including (where applicable) means or median, IQR, quartile, and percentage of agreement [Citation48] were used to assess consensus in each round according to the following established criteria [Citation35,Citation36,Citation51]:
Round 1: criteria of consensus
Median value of participants’ Likert scale data ≥3
Percentage of agreement ≥50%
Round 2: criteria of consensus
Median value of participants’ Likert scale data ≥3.5
IQR value of participants’ Likert scale data ≤2
Percentage of agreement ≥60%
Round 3: criteria of consensus
Median value of participants’ Likert scale data ≥4
IQR value of participants’ Likert scale data ≤1
Percentage of agreement ≥70%
Two or more criteria needed to be met for consensus to be reached. Descriptive statistics and quantitative data were analyzed using IBM SPSS V.28.
Results
A total of 47 potential experts were identified and contacted, of which 1 responded with a reason to decline and 32 did not respond to three repeated and individualized emails, which left a total of 15 panelists who met the inclusion criteria and agreed to participate (). None of the potential experts suggested peers that had not already been identified in the literature search. One of the participants did not complete Round 1 even after repeated individualized reminders, leaving a total of 14 panelists for Round 1. All panelists were still clinically actively involved in patients with CR. There were no differences in panelist characteristics (with respect to profession and country of residence) between the experts who participated and those that declined or did not respond to the invitation. All 14 panelists completed all three survey rounds and none needed additional time to complete a round. The deletion, addition, and modification of treatments based on previous rounds is listed in Supplemental Table 2 (Supplemental File 3).
Table 1. Panelist characteristics.
Table 2. Proposed effective interventions per stage.
Round 1
Feedback from panelists on Round 1 focused mainly on the importance of providing an individualized management program based on individual assessment and sound clinical reasoning. Mentioning of this was added to the comments of relevant questions in Round 2. The panelists were unanimous in their agreement on the items “Providing information” and “Pain education” across all stages. Only in the acute stage, the item “Behavioral therapy” (as part of the intervention group heading “Counseling, Advice and Behavioral Therapy”) had a Likert scale median of 3 and an agreement of 61.5%, necessitating the need to include this for further assessment in Round 2 of the survey.
Agreement of deletion was reached with regards to thirteen proposed physical therapy interventions and two proposed medications in the acute stage, eleven proposed physical therapy interventions and two proposed medications in the sub-acute stage, and eleven proposed physical therapy interventions and four proposed medications in the chronic stage. With regards to the “traction” treatment modalities (as part of the intervention group heading “Physical Therapy”), “manual traction” in the “acute stage” was the only intervention carried forward to Round 2. In the “Miscellaneous” section only “acupuncture” as a treatment modality in the sub-acute and chronic stages was carried forward to Round 2.
Several additional treatment interventions were suggested and these were added to Round 2 of the survey, e.g., “bio stimulation laser,” “percutaneous electrical nerve stimulation (PENS),” “Interferential Current Therapy (IFC),” “transforaminal and/or epidural corticosteroids,” “vocational, ergonomic and postural advice,” and “therapeutic exercise with supervised tele-physiotherapy” (see Supplemental Table 2; Supplemental File 3).
Round 2
Additional feedback from panelists on Round 2 consisted of only three individual comments on interventions in the acute stage.
With regards to “Counselling” in the acute stage, the item “Behavioral therapy” now had a Likert scale median of 3.5 and an agreement of 78.6%, which led to being able to change this group of questions to one statement: “At the end of Round 1, both “Providing Information” as well as “Pain Education” were unanimously (100%) considered useful in the Acute stage. “Behavioral Therapy” was also considered useful (62%) in the Acute stage. In the Sub-acute stage “Providing Information” (92.3%). Pain Education” (100%) and “Behavioral Therapy” (92.3%) were all considered highly useful. In the Chronic stage, “Providing Information” (100%), Pain Education” (100%) and “Behavioral Therapy” (100%) were unanimously considered useful. So we now only ask you this: “Do you agree that in process of patient management and shared decision making throughout the different stages (acute, sub-acute, and chronic) of lumbar radiculopathy, both “Providing Information,” “Pain Education” as well as Behavioral Therapy” are necessary treatment modalities or interventions?”
For the acute stage, agreement on deletion of interventions was reached with regards to one proposed physiotherapy treatment (“General strength training”), all but one (lumbar mobilization) in the spinal manipulative group, as well as “manual traction.” In the “Miscellaneous” section, none of the proposed interventions were carried forward to Round 3. Four proposed medication categories (“anti-epileptic drug” and combinations of “NSAIDs and anti-epileptic drugs,” “opioids and anti-epileptic drugs,” and “NSAIDs and opioids and anti-epileptic drugs” were deleted, leaving only NSAIDs to be carried forward to Round 3. Of the additional interventions suggested by panelists in Round 1, only “Foraminal opening exercises,” “Transforaminal (epidural) and/or interlaminar/caudal corticosteroids (injections)” and “combination of foraminal opening and neurodynamic slider exercises” were agreed upon.
Round 3
Additional feedback from panelists on Round 3 was minimal and consisted of three individual comments, two of them on the need for personalized choices of medication. There was near perfect agreement that in the process of patient management and shared decision-making throughout the different stages (acute, sub-acute, and chronic) of lumbar radiculopathy, both “Providing Information,” “Pain Education” as well as Behavioral Therapy” are necessary treatment modalities or interventions.
For the acute stage, agreement on interventions was reached with regards to only two proposed physiotherapy treatments: “Individualized Physical Activity” and “Directional Preference (MDT) Exercise,” and only for NSAIDS from the Medication treatment group. For the subacute stage agreement was reached for “Focused/Targeted Strength Training,” “Individualized Physical Activity,” and “Neurodynamic mobilization (NM)” as effective physiotherapy interventions and NSAID’s as proposed medication. From the additional interventions suggested by panelists in Round 1, the agreement was reached for “Transforaminal and/or epidural corticosteroids.” More physiotherapy interventions were deemed effective in the chronic stage: “General Aerobic Exercise,” “General Strength Training,” “Focused/Targeted Strength Training,” “Individualized Physical Activity,” “Supervised Exercise,” “Motor Control Exercise,” and “SMT combined with specific exercise.” From the additional interventions suggested by panelists in Round 1, the agreement was reached for “Vocational, ergonomic and postural advice” and “Biomechanical training for functional and work activities” ().
Summary of findings after Round 3
In the acute stage, management should focus on providing the patient with information about the condition including pain education, together with individualized physical activity, directional preference (MDT) exercises, and supported with NSAIDs. In the sub-acute phase, management should then include strength training and neurodynamic mobilization and transforaminal and/or epidural injections should also be considered. In the chronic stage, spinal manipulative therapy combined with specific (motor control) exercise and physical training (including general strength training and aerobic fitness) should focus on restoring functional capacity for the patient’s activities of daily life, combined with individualized vocational, ergonomic and postural advice.
Discussion
This is the first study establishing consensus from international experts on effective conservative treatment modalities for patients in different stages (i.e., acute, sub-acute, and chronic) of LR. Experts agreed that different interventions are needed in different stages of LR. Based on an understanding of (neuro)physiology, interventions should take into consideration the potential of an irritable nerve root inflammation, intra neural oedema, encroachment of the nerve root by a herniated or sequestered disc, or degenerative spondylotic changes in the neuro foramen. Especially in the chronic stage, the development of (aspects of) central sensitization should also be considered, as it has a significant impact on the choice of treatment modalities [Citation52,Citation53]. Research on central sensitization and cervical radiculopathy is recent but ongoing [Citation54].
The results from this study will assist clinicians and researchers in formulating an individualized management plan for patients with LR in clinical practice and future RCTs. By grouping separate effective treatment modalities with respect to the stage of recovery and through the evolution of the disorder, clinicians will be better able to tailor management plans to the individual patient through their course of recovery, instead of using a standardized “one size fits all” approach. The results from this study will also serve a need both clinically and within the contemporary literature to inform further research methodology on the effectiveness of conservative management of patients with LR.
The Danish Health Authority LR guideline suggests offering supervised exercise, motor control exercise, directional (MDT) exercise, and SMT to patients with recent onset LR [Citation20]. It also advises not to use acupuncture [Citation20]. In contrast, the recently updated Dutch treatment guideline published by the Royal Dutch Society for Physical Therapy (KNGF) for patients with LR ((which is incorporated in the treatment guideline for patients with non-specific low back pain) [Citation55]) solely promotes the use of active exercise therapy for patients with LR irrespective of which stage, but does suggest to assess for reactivity. It also advises not to use massage, manipulations, mobilizations, IFC, or TENS [Citation55]. The National Institute for Health and Clinical Excellence (NICE) guideline “low back pain and sciatica” generally does not differentiate in non-invasive treatment options between low back pain and LR [Citation14]. For the pharmacological management of LR, they propose to not offer gabapentinoids, other antiepileptics, oral corticosteroids, or benzodiazepines for managing sciatica in any stage and not to offer opioids for managing chronic LR [Citation14]. The Academy of Orthopaedic Physical Therapy (AOPT) recently updated their clinical practice guidelines for acute and chronic low back pain, but did not specifically mention of LR [Citation56]. In clinical practice and in clinical practice guidelines, non-specific low back pain is often differentiated from LR as prognosis and management differ [Citation20,Citation55,Citation57]. A recent systematic review of clinical practice guidelines concluded that consistent and common therapeutic recommendations for “should do” are: providing educational care and physical activity and referral to a specialist when conservative therapy fails or when steppage gait is present [Citation58]. They also reported none or inconsistent evidence for manipulation/mobilization/soft-tissue techniques and massage [Citation58].
In the current study, it was interesting to see that some of the proposed interventions for which there is evidence of effectiveness did not reach the required level of consensus to be included in the panel’s final recommendations. Spinal manipulative therapy has been reported in both RCTs and systematic reviews to be effective in reducing pain in the acute and subacute stages of LR [Citation59–63], but the panelists in our study only considered it to be useful in the chronic stage. “Individualized Physical Activity” was deemed to be an effective intervention throughout all three stages. Even though there is evidence to suggest wasting of muscles (e.g., transverse abdominus and multifidus) within the first 6 weeks of symptoms as expressed through fat infiltration in patients with localized lumbar disc or nerve root pathology [Citation64,Citation65], the majority of panelists concluded motor control exercises were useful only in the chronic stage. And while LR is considered a different condition from non-specific low back pain [Citation20,Citation55,Citation57,Citation66], consensus on this is in line with evidence from robust systematic reviews concerning non-specific low back pain [Citation67–69]. Similar to the reported ineffectiveness of traction for cervical radiculopathy [Citation70], our panel also agreed traction was not effective for LR. In line with recent evidence [Citation71] and treatment guidelines [Citation20,Citation55,Citation56], our panelists also almost unanimously agreed that providing the patient with adequate information on the condition, etiology, prognosis, self-management, pain management, and education and to assess if and when “Behavioral Therapy” should be considered, is essential across all stages.
Recently, a Delphi survey consensus was published on the timing of conservative management for cervical radiculopathy (CR) [Citation28]. Although nearly similar interventions were suggested across the stages for LR and CR, there are some notable differences. These differences might be explained by the fact that there are biomechanical and (patho)anatomical differences in the lumbar and cervical spine and respective causes for LR and CR [Citation2,Citation72,Citation73]. For the acute stage of CR, “spinal manipulative therapy combined with specific exercise” was also suggested as an effective intervention. For the sub-acute stage of CR, an additional number of specific types of exercise (“directional preference (MDT),” “motor control,” and “supervised” exercise were suggested, as well as “spinal manipulative therapy (manipulation and/or mobilization) combined with (specific) exercise and/or neurodynamic mobilization” [Citation28]. And “workplace, vocational and ergonomic assessment” was also already suggested for the sub-acute stage of CR, whereas for LR it was suggested for the chronic stage. And while “spinal manipulative therapy combined with specific exercise” was suggested for the chronic stage of LR, it was no longer suggested for CR [Citation28].
Implications for clinical practice and future research
The results from this study are meant to be used as a framework from which an individualized management plan can be designed. It is not meant to be prescriptive or to exclude specific treatments on an individual basis, and patient’s responses to treatment should be monitored to avoid aggravation, especially in the acute and sub-acute stages. As always, patient management needs to be tailored to the individual.
Current literature provides the clinician with only a list of potentially effective individual treatment modalities derived from RCTs and CCTs. It does not allow for individualized management plans tailored to the stage of recovery patients might be in. Future clinical trials assessing the effectiveness of conservative management of patients with LR could include more homogeneous groups of patients with respect to acute/early onset, sub-acute, or chronic patients, to elicit a better understanding of which type of management might be more effective for which group of patients. Additionally, in research protocols, management strategies should evolve over time, taking into consideration the different stages of LR. Alternatively, researchers could report a subgroup analysis to that effect.
It would also be of interest to expand on this study with in-depth interviews with the experts as to their rationale for scoring interventions and treatment modalities across the different stages in the way they did and (if and when they did so) their rationale for having a different clinical opinion as opposed to the currently available evidence.
Strengths and limitations of this study
One of the strengths of this study was the spread of panelists across different continents and different professions, from both a medical/neurosurgical as well as a physiotherapy and chiropractic background. Also, all panelists completed all three rounds of the Delphi survey. Unfortunately, less than the advised 20 panelists could be included in this study, which has an impact on the generalizability of the results. The consensus from a larger number of experts could have provided readers with a more robust framework of effective treatment modalities across the different stages and through the evolution of the disorder. Specifically, as in a smaller sample size, one strongly opinionated panelist has a potentially larger effect on the percentage of agreement. However, if the heterogeneity of the group is sufficient, this has a smaller impact on the result [Citation74].
This study was reported in line with CREDES recommendations [Citation30] and utilized both qualitative and quantitative data. However, the views of the Delphi panelist may differ from those of experts that declined to participate and so may not fully represent the opinion of all experts in the field. The evidence-based opinion of the panelists was based on a mixture of clinical experience, patient’s perspectives, and scientific evidence [Citation75–77].
Not all panelists had the direct ability to prescribe medication but were able to confer with prescribing colleagues. This resulted in many panelists feeling competent to form an opinion on the preferred prescription of the different types of medication.
The panelists repeatedly mentioned the lack of evidence from high-quality RCTs, which was also mentioned in previously published guidelines and systematic reviews. Therefore, panelists also mentioned their opinion being based on a mixture of clinical experience, patient’s perspectives, and scientific evidence.
Conclusion
The consensus of this Delphi study is that, in the acute stage, the focus of management should consist in providing the patient with information about the condition, pain education with positive reinforcing and non-nocebo content, individualized physical activity, directional preference (MDT) exercises, and NSAIDs. In the sub-acute phase, strength training targeting afflicted muscles could be added along with neurodynamic mobilization, again supported by NSAIDs. The use of transforaminal and/or epidural corticosteroid injections should be also considered at the sub-acute stage. In the chronic stage, spinal manipulative therapy combined with specific (motor control) exercises and physical training could be effective. Physical training should include general strength training and aerobic fitness and should focus on restoring functional capacity for the patient’s activities of daily life, combined with individualized vocational, ergonomic and postural advice.
Author contributions
MTdG and ET devised the focus of this Delphi study. ET is a PGR student, DF is the lead supervisor, AG is the co-supervisor, JAC and MTdG are co-researchers. ET drafted the initial protocol manuscript with lead and co-supervisors providing guidance on methodological decisions and proposed analyses. All authors have contributed subject-specific expertise. ET recruited participants into the study. All authors contributed to data interpretation, conclusions, and dissemination. All authors have read, contributed to, and agreed to the final manuscript. DF is the guarantor of the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ropper AH, Zafonte RD. Sciatica. N Engl J Med. 2015;372(13):1240–1248.
- Bogduk N. On the definitions and physiology of back pain, referred pain, and radicular pain. Pain. 2009;147(1–3):17–19.
- Merskey HBN, editor. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. 2nd ed. Seattle (WA): IASP Press; 1994.
- Porchet F, Wietlisbach V, Burnand B, et al. Relationship between severity of lumbar disc disease and disability scores in sciatica patients. Neurosurgery. 2002;50(6):1253.
- Koes BW, van Tulder MW, Peul WC. Diagnosis and treatment of sciatica. BMJ. 2007;334(7607):1313–1317.
- Hoy D, Brooks P, Blyth F, et al. The epidemiology of low back pain. Best Pract Res Clin Rheumatol. 2010;24(6):769–781.
- Lambeek LC, van Tulder MW, Swinkels IC, et al. The trend in total cost of back pain in The Netherlands in the period 2002 to 2007. Spine. 2011;36(13):1050–1058.
- Frymoyer JW. Back pain and sciatica. N Engl J Med. 1988;318(5):291–300.
- Younes M, Béjia I, Aguir Z, et al. Prevalence and risk factors of disk-related sciatica in an urban population in Tunisia. Joint Bone Spine. 2006;73(5):538–542.
- van Tulder MW, Koes BW, Bouter LM. A cost-of-illness study of back pain in The Netherlands. Pain. 1995;62(2):233–240.
- Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain. 2000;84(1):95–103.
- Weber H, Holme I, Amlie E. The natural course of acute sciatica with nerve root symptoms in a double-blind placebo-controlled trial evaluating the effect of piroxicam. Spine. 1993;18(11):1433.
- Vroomen PC, de Krom MC, Slofstra PD, et al. Conservative treatment of sciatica: a systematic review. J Spinal Disord. 2000;13(6):463–469.
- National Institute for Health and Care Excellence: Clinical Guidelines. Low back pain and sciatica in over 16s: assessment and management. London: National Institute for Health and Care Excellence (UK); 2020.
- van Wambeke P, Desomer A, Jonckheer P, et al. The belgian national guideline on low back pain and radicular pain: key roles for rehabilitation, assessment of rehabilitation potential and the PRM specialist. Eur J Phys Rehabil Med. 2020;56(2):220–227.
- Lee J, Gupta S, Price C, et al. Low back and radicular pain: a pathway for care developed by the british pain society. Br J Anaesth. 2013;111(1):112–120.
- Chou R, Atlas SJ, Stanos SP, et al. Nonsurgical interventional therapies for low back pain: a review of the evidence for an American pain society clinical practice guideline. Spine. 2009;34(10):1078–1093.
- Lequin MB, Verbaan D, Jacobs WC, et al. Surgery versus prolonged conservative treatment for sciatica: 5-year results of a randomised controlled trial. BMJ Open. 2013;3(5):e002534.
- Machado GC, Witzleb AJ, Fritsch C, et al. Patients with sciatica still experience pain and disability 5 years after surgery: a systematic review with Meta-analysis of cohort studies. Eur J Pain. 2016;20(10):1700–1709.
- Stochkendahl MJ, Kjaer P, Hartvigsen J, et al. National clinical guidelines for non-surgical treatment of patients with recent onset low back pain or lumbar radiculopathy. Eur Spine J. 2018;27(1):60–75.
- Lewis R, Williams N, Matar HE, et al. The clinical effectiveness and cost-effectiveness of management strategies for sciatica: systematic review and economic model. Health Technol Assess. 2011;15(39):1–578.
- Sibbald B, Roland M. Understanding controlled trials. Why are randomised controlled trials important? BMJ. 1998;316(7126):201.
- Alentado VJ, Lubelski D, Steinmetz MP, et al. Optimal duration of conservative management prior to surgery for cervical and lumbar radiculopathy: a literature review. Global Spine J. 2014;4(4):279–286.
- Meadows JR, Finnoff JT. Lower extremity nerve entrapments in athletes. Curr Sports Med Rep. 2014;13(5):299–306.
- Albert HB, Manniche C. The efficacy of systematic active conservative treatment for patients with severe sciatica: a single-blind, randomized, clinical, controlled trial. Spine. 2012;37(7):531–542.
- Luijsterburg PA, Verhagen AP, Ostelo RW, et al. Physical therapy plus general practitioners’ care versus general practitioners’ care alone for sciatica: a randomised clinical trial with a 12-month follow-up. Eur Spine J. 2008;17(4):509–517.
- Luijsterburg PA, Verhagen AP, Ostelo RW, et al. Effectiveness of conservative treatments for the lumbosacral radicular syndrome: a systematic review. Eur Spine J. 2007;16(7):881–899.
- Thoomes E, Graaf MT, Cleland JA, et al. Timing of evidence-based nonsurgical interventions as part of multimodal treatment guidelines for the management of cervical radiculopathy: a Delphi study. Phys Ther. 2022;13.
- Dalkey N, Helmer O. An experimental application of the DELPHI method to the use of experts. Manage Sci. 1963;9(3):458–467.
- Jünger S, Payne SA, Brine J, et al. Guidance on conducting and REporting DElphi studies (CREDES) in palliative care: recommendations based on a methodological systematic review. Palliat Med. 2017;31(8):684–706.
- Keeney S, Hasson F, McKenna H. Consulting the oracle: ten lessons from using the delphi technique in nursing research. J Adv Nurs. 2006;53(2):205–212.
- Vanti C, Panizzolo A, Turone L, et al. Effectiveness of mechanical traction for lumbar radiculopathy: a systematic review and meta-analysis. Phys Ther. 2021;101(3).
- Fernandez M, Hartvigsen J, Ferreira ML, et al. Advice to stay active or structured exercise in the management of sciatica: a systematic review and meta-analysis. Spine. 2015;40(18):1457–1466.
- Hahne AJ, Ford JJ, McMeeken JM. Conservative management of lumbar disc herniation with associated radiculopathy: a systematic review. Spine. 2010;35(11):E488–E504.
- Mistry J, Falla D, Noblet T, et al. Clinical indicators to identify neuropathic pain in low back-related leg pain: protocol for a modified delphi study. BMJ Open. 2020;10(2):e033547.
- Rushton AB, Fawkes CA, Carnes D, et al. A modified delphi consensus study to identify UK osteopathic profession research priorities. Man Ther. 2014;19(5):445–452.
- Wiangkham T, Duda J, Haque MS, et al. Development of an active behavioural physiotherapy intervention (ABPI) for acute whiplash-associated disorder (WAD) II management: a modified delphi study. BMJ Open. 2016;6(9):e011764.
- Zambaldi M, Beasley I, Rushton A. Return to play criteria after hamstring muscle injury in professional football: a delphi consensus study. Br J Sports Med. 2017;51(16):1221–1226.
- Gill FJ, Leslie GD, Grech C, et al. Using a web-based survey tool to undertake a Delphi study: application for nurse education research. Nurse Educ Today. 2013;33(11):1322–1328.
- McKenna H. The delphi technique: a worthwhile research approach for nursing? J Adv Nurs. 1994;19(6):1221–1225.
- Helms C, Gardner A, McInnes E. The use of advanced web-based survey design in delphi research. J Adv Nurs. 2017;73(12):3168–3177.
- Merskey HE. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. Pain. 1986;11(Suppl 3):226–226.
- IASP. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. Philadelphia: IASP Press; 1994.
- van Tulder MW, Koes BW, Bouter LM. Conservative treatment of acute and chronic nonspecific low back pain. A systematic review of randomized controlled trials of the most common interventions. Spine. 1997;22(18):2128–2156.
- Armstrong D, Marshall JK, Chiba N, et al. Canadian consensus conference on the management of gastroesophageal reflux disease in adults – update 2004. Can J Gastroenterol. 2005;19(1):15–35.
- Akins RB, Tolson H, Cole BR. Stability of response characteristics of a Delphi panel: application of bootstrap data expansion. BMC Med Res Methodol. 2005;5:37.
- Jairath N, Weinstein J. The Delphi methodology (part one): a useful administrative approach. Can J Nurs Adm. 1994;7(3):29–42.
- De Vet HC, Terwee CB, Mokkink LB, et al. Measurement in medicine: a practical guide. Amersfoort: Cambridge University Press; 2011.
- Norman G. Likert scales, levels of measurement and the “laws” of statistics. Adv Health Sci Educ Theory Pract. 2010;15(5):625–632.
- Allen IE, Seaman CA. Likert scales and data analyses. Qual Prog. 2007;40(7):64–65.
- Rayens MK, Hahn EJ. Building consensus using the policy delphi method. Policy Polit Nurs Pract. 2000;1(4):308–315.
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–S15.
- Roldán-Jiménez C, Pérez-Cruzado D, Neblett R, et al. Central sensitization in chronic musculoskeletal pain disorders in different populations: a cross-sectional study. Pain Med. 2020;21(11):2958–2963.
- Kapitza C, Lüdtke K, Tampin B, et al. Application and utility of a clinical framework for spinally referred neck-arm pain: a cross-sectional and longitudinal study protocol. PLOS One. 2020;15(12):e0244137.
- Koninklijk Nederlands Genootschap voor Fysiotherapie (KNGF) VvOCeMV. KNGF-richtlijn Lage rugpijn en lumbosacraal radiculair syndroom. Amersfoort; 2021.
- George SZ, Fritz JM, Silfies SP, et al. Interventions for the management of acute and chronic low back pain: revision 2021. J Orthop Sports Phys Ther. 2021;51(11):CPG1–CPG60.
- Oliveira CB, Maher CG, Pinto RZ, et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J. 2018;27(11):2791–2803.
- Khorami AK, Oliveira CB, Maher CG, et al. Recommendations for diagnosis and treatment of lumbosacral radicular pain: a systematic review of clinical practice guidelines. JCM. 2021;10(11):2482.
- Peterson CK, Pfirrmann CW, Hodler J, et al. Symptomatic, magnetic resonance imaging-confirmed cervical disk herniation patients: a comparative-effectiveness prospective observational study of 2 age- and sex-matched cohorts treated with either imaging-guided indirect cervical nerve root injections or spinal manipulative therapy. J Manipulative Physiol Ther. 2016;39(3):210–217.
- Wong JJ, Côté P, Sutton DA, et al. Clinical practice guidelines for the noninvasive management of low back pain: a systematic review by the Ontario protocol for traffic injury management (OPTIMa) collaboration. Eur J Pain. 2017;21(2):201–216.
- Leemann S, Peterson CK, Schmid C, et al. Outcomes of acute and chronic patients with magnetic resonance imaging-confirmed symptomatic lumbar disc herniations receiving high-velocity, low-amplitude, spinal manipulative therapy: a prospective observational cohort study with one-year follow-up. J Manipul Physiol Ther. 2014;37(3):155–163.
- Santilli V, Beghi E, Finucci S. Chiropractic manipulation in the treatment of acute back pain and sciatica with disc protrusion: a randomized double-blind clinical trial of active and simulated spinal manipulations. Spine J. 2006;6(2):131–137.
- Bronfort G, Haas M, Evans RL, et al. Efficacy of spinal manipulation and mobilization for low back pain and neck pain: a systematic review and best evidence synthesis. Spine J. 2004;4(3):335–356.
- Battié MC, Niemelainen R, Gibbons LE, et al. Is level- and side-specific multifidus asymmetry a marker for lumbar disc pathology? Spine J. 2012;12(10):932–939.
- Fortin M, Lazáry À, Varga PP, et al. Paraspinal muscle asymmetry and fat infiltration in patients with symptomatic disc herniation. Eur Spine J. 2016;25(5):1452–1459.
- Konstantinou K, Hider SL, Jordan JL, et al. The impact of low back-related leg pain on outcomes as compared with low back pain alone: a systematic review of the literature. Clin J Pain. 2013;29(7):644–654.
- Macedo LG, Saragiotto BT, Yamato TP, et al. Motor control exercise for acute non-specific low back pain. Cochrane Database Syst Rev. 2016;2(2):CD012085.
- Saragiotto BT, Maher CG, Yamato TP, et al. Motor control exercise for nonspecific low back pain: a cochrane review. Spine. 2016;41(16):1284–1295.
- Hayden JA, Ellis J, Ogilvie R, et al. Exercise therapy for chronic low back pain. Cochrane Database Syst Rev. 2021;9(9):CD009790.
- Thoomes EJ, Scholten-Peeters W, Koes B, et al. The effectiveness of conservative treatment for patients with cervical radiculopathy: a systematic review. Clin J Pain. 2013;29(12):1073–1086.
- Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain. Cochrane Database Syst Rev. 2014;2(9):CD000963.
- Fakhoury J, Dowling TJ. Cervical degenerative disc disease. Treasure Island (FL): StatPearls Publishing LLC; 2022.
- Bogduk N. The anatomy and pathophysiology of neck pain. Phys Med Rehabil Clin N Am. 2011;22(3):367–382, vii.
- Nasa P, Jain R, Juneja D. Delphi methodology in healthcare research: how to decide its appropriateness. World J Methodol. 2021;11(4):116–129.
- Haynes RB, Devereaux PJ, Guyatt GH. Physicians’ and patients’ choices in evidence based practice. BMJ. 2002;324(7350):1350.
- Sackett DL. Evidence-based medicine and treatment choices. Lancet. 1997;349(9051):570.
- Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312(7023):71–72.
