Abstract
Purpose
To determine the safety, feasibility, and potential effect of an 18-week exercise intervention for adults with primary brain cancer.
Materials and methods
Eligible patients were 12-26-weeks post-radiotherapy for brain cancer. The individually-prescribed weekly exercise was ≥150-minutes of moderate-intensity exercise, including two resistance-training sessions. The intervention was deemed “safe” if exercise-related, serious adverse events (SAE) were experienced by <10% of participants, and feasible if recruitment, retention, and adherence rates were ≥75%, and ≥75% compliance rates were achieved in ≥75% of weeks. Patient-reported and objectively-measured outcomes were assessed at baseline, mid-intervention, end-intervention, and 6-month follow-up, using generalized estimating equations.
Results
Twelve participants enrolled (51 ± 19.5 years, 5 females). There were no exercise-related SAEs. The intervention was feasible (recruitment:80%, retention:92%, adherence:83%). Participants completed a median of 172.8 (min:77.5, max:560.8) minutes of physical activity per week. 17% met the compliance outcome threshold for ≥75% of the intervention. Improvements in quality of life (mean change (95% CI): 7.9 units (1.9, 13.8)), functional well-being (4.3 units (1.4, 7.2)), depression (−2.0 units (−3.8, −0.2)), activity (112.8 min (42.1, 183.4)), fitness (56.4 meters (20.4, 92.5)), balance (4.9 s (0.9, 9.0)), and lower-body strength (15.2 kg (9.3, 21.1)) were observed end-intervention.
Conclusion
Preliminary evidence support that exercise is safe and beneficial to the quality of life and functional outcomes for people with brain cancer.Registration: ACTRN12617001577303
IMPLICATIONS FOR REHABILITATION
The BRAin Cancer and Exercise (BRACE) study highlights the need for regular monitoring of disease- and treatment-related side effects which may present as barriers to exercise.
Exercise prescription should be modified according to the presence and severity of disease- and treatment-related barriers.
Adverse events observed, such as dizziness, highlight the importance of supervised exercise for people with brain cancer.
If supervision is not possible, then exercise modes with low risk of harm from falls are recommended (e.g., walking, machine-based resistance training).
Introduction
Brain cancer and other central nervous system (CNS) cancers are responsible for substantial morbidity and mortality worldwide [Citation1]. Although malignant brain tumors are rare, the disease burden is high with a 5-year survival rate of 22% [Citation2], which is markedly lower when compared to all cancers combined (69%) [Citation3]. Brain cancer and its treatment can result in physical, cognitive and psychological impairments, which negatively impact quality of life (QOL) [Citation4]. Side-effects specific to brain cancer and its treatment may include neurological deficits impacting balance, motor skills and vision, as well as fatigue, headaches, seizures, and memory and speech loss [Citation5].
The American College of Sports Medicine (ACSM) roundtable [Citation6] in 2019 concluded that there was strong evidence for exercise in improving physical function and health-related QOL, and managing anxiety and depression, fatigue and lymphoedema, and moderate evidence for bone health and sleep in cancer survivors both during and after treatment [Citation6]. Further, higher physical activity levels post-diagnosis have been associated with improved all-cause mortality for a range of cancers including, gliomas [Citation7]. This evidence has contributed to the development of international (ACSM) and national (Exercise Sports Science Australia) exercise guidelines for people living with and after cancer which encourage avoiding inactivity and aiming towards participating in at least 150-min of moderate-intensity or 75-min of vigorous-intensity activities, including two resistance training sessions per week [Citation6,Citation8].
Data used to support exercise oncology prescription guidelines have most typically been drawn from studies involving patients with cancer types, such as breast, prostate, or colorectal cancer, that are associated with 5-year survival rates equal to or exceeding the 69% 5-year survival rate observed for all-cancers combined [Citation9]. While findings from a systematic review, including data from nine exercise trials (only one of which was a randomized, controlled trial) specifically involving people with brain cancer, suggested that exercise leads to improvements in aerobic capacity, strength, functional and patient-reported outcomes, the overall grade of this evidence base was rated as weak[Citation10]. Further, it was identified that the safety and feasibility of exercise for people with brain cancer have been poorly assessed and described to date [Citation10]. Given the adverse impacts to physical and mental function from brain cancer and its associated treatment, there is a clear need to better understand the safety of exercise for people with brain cancer, as well as what exercise people with brain cancer are able and willing to do [Citation10,Citation11]. Therefore, the aims of the BRAin Cancer and Exercise (BRACE) study were to: 1) assess the safety and feasibility of an 18-week exercise intervention for adults with primary brain cancer, and 2) explore the effect of the intervention on patient-relevant, self-reported and objectively-assessed outcomes.
Methods
Design and participants
BRACE was a pre-post, phase II exercise intervention. Participants were eligible if they: were ≥ 18 years, diagnosed with primary or recurrent brain cancer (if they had been continuously disease-free for ≥ two years after primary treatment), 12–26 weeks post-radiotherapy, had an Eastern Cooperative Oncology Group[Citation12] status between 0–2 and had an anticipated survival of ≥12 months (as determined by their treating oncologist). Participants were excluded if they had a diagnosis of metastatic brain cancer, a history of another malignancy within two years or serious medical or psychiatric condition that could limit participation with the protocol.
Nurses or oncologists identified potential participants from Brisbane-based hospitals during a routine appointment and introduced the study via a flyer and discussion. This appointment typically occurred following surgery but before adjuvant treatment or four weeks post-radiotherapy. Participants could also self-refer in response to social media. All interested patients were contacted by the study coordinator who provided further information, confirmed eligibility, and ascertained consent. The study was prospectively registered on the Australian and New Zealand Clinical Trials Registry (ACTRN12617001577303). Ethical approval was obtained from Bellberry Human Research Ethics Committee, and participating hospitals in Brisbane, Australia.
Exercise intervention
The intervention involved 18 weeks of individually-prescribed moderate-intensity, mixed-mode (aerobic and resistance) exercise by an accredited exercise physiologist (AEP). The weekly exercise target dosage was based on cancer and exercise guidelines at the time of protocol development: 150 min of moderate-intensity exercise, including two resistance training sessions per week, the equivalent of 600 metabolic equivalent minutes (MET-mins) [Citation13,Citation14]. Participant characteristics, treatment-related side effects, and baseline assessment outcomes influenced the starting exercise dosage. Exercise prescription typically included a combination of walking (or other modes of aerobic exercise) and resistance exercises using body weight, free weights or therabands, while following the exercise principle of gradual progression. Participants were scheduled to attend supervised sessions with an AEP at least once per week for weeks one to eight and once per fortnight for weeks nine to 18 (≥13 sessions). During the face-to-face (supervised) sessions, the AEP prescribed exercise for the following week and encouraged participants to exercise outside of supervised sessions in order to meet the weekly exercise target dosage. The prescription was modified according to participant symptoms, side effects, and other needs. More details relating to the exercise intervention, reported in line with the Template for Intervention Description and Replication (TIDieR) checklist, can be found in Supplementary Table S1.
Data collection
Participant baseline characteristics were collected during screening by the study coordinator, and disease and treatment characteristics were confirmed with the treatment team. The primary outcomes, safety and feasibility, were collected systematically throughout the intervention. Participants were asked to record details of any adverse events (AE) and all exercise completed (e.g., type, intensity, duration) in a study-specific logbook; these data were then recorded in case management folders by the AEP during each supervised session. Safety data were assessed by monitoring AE occurrences, defined as any untoward medical occurrence, which may or may not have a causal relationship with the intervention (exercise). AEs were classified according to their severity (Grade 1 -5) using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0[15], as well as their relationship to the intervention (unrelated, unlikely, possible, likely, and certain). A serious adverse event (SAE) was defined as a fracture, hospitalization, or death (CTCAE grade 3–5) [Citation15]. Designated a priori, the intervention was deemed safe if exercise-related SAEs were experienced by <10% of participants. This threshold was chosen following discussion between the investigator team, and consideration of thresholds used in previous exercise oncology trials with different cancer cohorts and modes of intervention delivery [Citation16], and of the frequency and severity of adverse events associated with brain cancer and its treatment.
Feasibility was assessed via participant recruitment (number consented/number referred and eligible x 100%); retention (number completed end-intervention assessment/number completed baseline assessment, x 100%); adherence (number of attended/scheduled exercise sessions (n = 13), x 100%); and compliance (completed exercise per week compared with weekly exercise target). Study records and case management folders were used for the collection of recruitment and retention data. Adherence and compliance data were collected by the AEP, with type of participant contact (e.g., face-to-face, telephone, email), prescribed and completed exercise type, intensity using the Borg 6-20 Rate of Perceived Exertional (RPE) scale [Citation17], and duration (minutes) recorded in the case management folders. MET-mins for each session were calculated as minutes of exercise, multiplied by the MET-value equivalent to the reported RPE. Conversion of RPE to MET-value was extrapolated from Norton et al. [Citation18]. The intervention was deemed feasible if recruitment, retention, and adherence rates were ≥75%, and ≥75% compliance rates were achieved in ≥75% of weeks (i.e., ≥14 of 18 weeks).
The secondary outcomes of interest were patient-reported and objectively-assessed outcomes measured at baseline, mid-intervention (week-9), end-intervention (week-18) and at 6-month follow-up intervention (6-month post-end intervention).
QOL was assessed by the Functional Assessment of Cancer Therapy–Brain questionnaire (FACT-Br total score; range 0–200)[Citation19]. The FACT-Br includes the FACT-General (FACT-G, range 0–108)[Citation20], which includes the following subscale domains: physical-wellbeing (range 0–28), social-wellbeing (range 0–28), emotional-wellbeing (range 0–24), and functional-wellbeing (0–28), plus the brain cancer specific additional concerns (Brain cancer subscale (BrCS, range: 0–92)) [Citation19]. A higher score indicates better QOL. Fatigue was assessed by the Functional Assessment of Chronic Illness fatigue subscale (FACIT-F; score 0-52), where a higher score indicates less fatigue [Citation21]. The 14-item Hospital Anxiety and Depression Scale (HADS) was used to measure depression and anxiety, with a higher score indicating greater symptoms [Citation22,Citation23]. Physical activity levels were assessed using the Godin Leisure-Time Exercise Questionnaire [Citation24].
Objectively-measured outcomes included: body mass index, aerobic fitness (distance covered in the 6-min walk test) [Citation25], physical function (single leg balance (seconds), and the 12-item Short Physical Performance Battery, which is an assessment of lower extremity functioning (range: 0 indicates worst performance, to 12 indicating best performance) [Citation26], and muscular strength (hand-grip strength - Smedley handheld dynamometer [Citation26], and upper-body (supported row) and lower-body (leg press) 10-repetition maximum)).
Statistical analysis
Descriptive statistics were used to analyze the primary outcomes of safety and feasibility, with categorical variables described using counts and proportions and continuous variables described using means and standard deviations (SD) when normally distributed (i.e., parametric data) (or medians, minimums, and maximums for non-parametric data). Generalized estimating equations were used to determine the effect of the exercise intervention over time (baseline, mid-intervention, end-intervention, and follow-up) for secondary outcomes. Estimated means and p-values are reported for each estimate and mean difference. Sensitivity analyses, using data from only those with complete data were also undertaken for each of the self-reported and objectively-measured outcomes to assess for potential for participant bias. Clinically-relevant changes were determined a priori for each outcome based on values previously reported in the literature including: change of five units, FACT-G total score [Citation20]; two units, FACT-G subscale domains [Citation20]; three units, FACIT-Fatigue [Citation27]; 1.5 units, HADS[22]; 54 meters, 6-min walk test[Citation28], and ≥20 min, total physical activity per week [Citation29]. In the absence of established thresholds, clinically-relevant changes for all other outcomes were based on a ½ SD improvement from baseline [Citation30,Citation31]. Data were analyzed using IBM SPSS (Version 26, Armonk, NY: IBM Corp).
Results
Over a 24-month recruitment period, 18 patients were referred to BRACE, 15 were eligible and 12 of these consented to participate. The characteristics of the sample are described in . The mean age of participants was 51 years (range: 21–74), and 42% (n = 5) were female. Glioblastoma multiforme was the most common tumor diagnosis (n = 5, 42%). Most participants received surgery, radiotherapy, and oral chemotherapy. The mean time from the end of radiotherapy to the commencement of the intervention was 15 weeks.
Table 1. Baseline characteristics of BRACE participants.
Safety
Five participants reported no AEs and the remaining seven (58%) reported a total of 27 AEs (). Five AEs (19%) were characterized as “serious” (Grades 3-5), although none were exercise-related. Nine AEs reported by five participants were classified as exercise-related, all of which were “non-serious” (grades 1-2), with 17% and 83% considered a “possible” and “likely” result of exercise, respectively. A remaining 13 AEs were classified as both non-serious and not exercise-related ().
Table 2. Exercise and non-exercise related adverse events reported during the BRACE study.
Feasibility
The recruitment rate was 80% (12 of 15 eligible participants identified over a 24-month recruitment period consented) and the retention rate was 92% with 11 participants completing the baseline and end-intervention assessments (). The average number of supervised exercise sessions attended across the cohort was 21 ± 8, with 10 participants attending ≥75% (nine of 13 scheduled sessions) of scheduled supervised exercise sessions (83% adherence). Of the remaining two participants, one withdrew from the intervention and the other did not attend due to medical complications, but did complete eight sessions. The median dose of exercise completed was 172.8 (min:77.5, max:560.8) minutes/week ( and Supplementary Figure 1). Across the intervention, 10 participants (83%) achieved an average of ≥150-min of exercise/week, with five participants (42%) achieving this in ≥75% (≥14 weeks) of intervention weeks (Supplementary Figure 1). The average number of resistance training sessions completed/week was 2.6 ± 1.2 (). Two participants (17%) met the compliance criteria of ≥150 min of combined exercise, including ≥ two resistance training sessions per week for ≥75% of intervention weeks. Reasons for being unable to meet the weekly exercise target included fatigue, nausea, acute illnesses (e.g., urinary tract infection), lower back pain, lack of motivation, or being too busy.
Figure 1. Brain Cancer and Exercise (BRACE) Study flowchart.
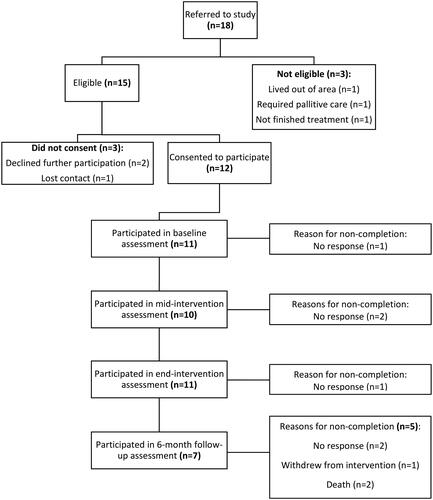
Table 3. Individual participant results of weekly exercise completed (median minutes of exercise, number of exercise sessions and volume of exercise).
Efficacy
Improvements in patient-reported outcomes including the FACT functional well-being subscale, FACT-G, FACT-Br total score, depression, and weekly physical activity were observed between baseline and end-intervention and were sustained at 6-month follow-up (). From baseline to end-intervention, improvements in objectively-measured outcomes including 6-min walk test, single leg balance (eyes closed) and lower-body strength were also observed (). These improvements were maintained at 6-month follow-up. No worsening in any outcome was observed.
Table 4. Patient-reported and objectively-measured outcomes at baseline, mid-intervention, end-intervention, and 6-month follow-up post-intervention.
When analyses were restricted to include data from participants with available data at all four time points, patient-reported findings remained consistent, with an additional improvement observed in the Brain cancer-specific subscale (Supplementary Table S2). For the objectively-assessed outcomes, the findings were also consistent and additional clinically-relevant improvements were observed in the short physical performance battery, and upper-body strength (Supplementary Table S3), although this was driven by a sample of 4 and 2 participants, respectively.
Supplementary Figure S2a-e shows health outcome results for each participant over time and demonstrates that contributing to the mean changes were some participants who experienced little-to-no change (e.g., between 1%-6% change in 6-min walk test), some who experienced improvements (e.g., 13%–100% change in 6-min walk test, for those who changed a clinically-relevant amount) and between −29% to −62% in participants who showed declines.
Discussion
Findings from BRACE suggest that an individually-prescribed 18-week exercise intervention, commencing 13 to 21 weeks post-radiotherapy for brain cancer was safe, feasible, and potentially beneficial for a range of health outcomes including QOL, functional well-being, depression, physical activity levels, lower-body strength, aerobic fitness, and balance. While exercise-related AEs were reported by 42% of the BRACE sample, none of these were deemed serious. Of note, the nine grade 1 and 2 adverse events reported by five participants, is higher than that previously reported by others. Specifically, in a systematic review of previous exercise intervention studies (n = 9) [Citation10] involving people with brain cancer (total sample size, n = 137), only one adverse event (soreness to the head as a consequence of a fall) was reported [Citation32]. Given the frequency and severity of known side effects associated with brain cancer and its treatment, the lack of adverse events reported in previous trials is more likely related to a lack of comprehensive adverse event assessment and the absence of standardized reporting methods in the exercise oncology field [Citation33,Citation34], rather than a reflection of safety differences between previous interventions and BRACE. The need to improve harms assessment and reporting in exercise oncology has been identified in previous reviews of studies involving stage II + breast and colorectal cancer where exercise harms were not reported in 34%–47% of studies [Citation35,Citation36]. With a comprehensive adverse event assessment and reporting framework such as that used in BRACE, all undesirable medical occurrences that occur during the intervention period are captured, whether or not they are related to the intervention. This allows for a full understanding of the harms that occurred (i.e. those adverse events believed to have a causal relationship with exercise), as well as non-exercise-related adverse events (such as disease-related side effects) that were not caused by the intervention but that reflect important considerations for exercise prescription in this population [Citation33]. Future studies are encouraged to similarly report both exercise-related and all-cause adverse events that occur during the intervention period so that a comprehensive safety profile can be established and relative contraindications that are necessary to inform exercise prescription can be described.
Although BRACE was deemed “safe” according to our a priori safety criteria, caution with respect to exercise safety in this unique cancer group remains warranted. Specific exercise-related AEs observed (in particular, dizziness and falls) highlight the importance of exercise supervision for those with brain cancer. If supervision is not possible, then prescription of exercise modes with less risk of harm from falls should be encouraged (e.g., walking) while other exercise modes, such as challenging balance exercises might be completed under supervised conditions. Further, while most AEs reflected common side-effects of brain cancer and treatment (in particular, fatigue) [Citation5], the regular occurrence during BRACE highlights the need for (i) routine and regular monitoring of persistent disease- and treatment-related side-effects, (ii) understanding the extent to which side-effects present as a barrier to exercise and identifying ways to overcome these barriers, and (iii) ensuring the exercise prescription is modifiable according to the presence and severity of disease- and treatment-related barriers. The Exercise and Sports Science Australia exercise and cancer guidelines [Citation8] articulate a process for exercise prescription for people with cancer which promotes symptom assessment and monitoring, identifying and overcoming barriers and ensuring a flexible prescription. Our experiences with BRACE suggest that actioning this process will likely require more frequent contact between the Exercise Professional and patient, when compared with the frequency of contact that would be required when working with someone with early-stage disease and diagnosis of a more commonly studied cancer type, such as breast or prostate.
The intervention was deemed feasible according to our predefined recruitment, retention, and adherence targets, but not according to compliance data, with only the minority (17%) able to meet weekly exercise targets ≥75% of the weeks. Our adherence findings were similar to findings of previous pre-post exercise trials following a brain cancer diagnosis (61%–100%) [Citation32,Citation37–42]. By far, our greatest challenge was recruitment – identifying only 15 eligible patients over a recruitment period of 24 months was time and resource-intensive. This problem was neither unique to BRACE, with recruitment outcomes reported in the previous exercise and brain cancer trials ranging from 25%–80% [Citation10,Citation32,Citation37,Citation38,Citation41], nor was it surprising, given brain cancer is rare cancer. However, our observations also indicate that having a complex survivorship journey (e.g., poor prognosis, invasive treatment leading to physical and psychosocial acute and persistent side-effects), lack of evidence confirming exercise safety and/or feasibility, and added burden with accessing clinic-based exercise, likely influenced who was invited to participate in this study beyond the application of our defined eligibility criteria, and contributed to our recruitment challenges. As such, it is plausible that our recruitment rate of 80% overstates the proportion of people who consented to participate in the study compared with the number of “true” eligible patients.
Burden associated with attending clinic-based exercise, alongside symptom management issues, also contributed to the wide variation observed in exercise compliance within and between BRACE participants and represented reasons participants withdrew or were unable to complete all assessments. Our comprehensive reporting of compliance data (that is, beyond presenting group averages and individual averages), clearly highlights that consistently meeting internationally-endorsed exercise targets recommended for people with cancer was not possible for the majority of participants in BRACE. Thus, BRACE compliance findings support the need for (i) individually-prescribed exercise that can accommodate a fluctuating/changing symptom profile, and (ii) exercise delivery using wide-reaching modes (e.g., telehealth delivery) that can overcome access barriers while concurrently supporting benefits through exercise therapy[Citation43–46]. Use of digital-health technology that facilitates live-streaming of an exercise session and/or live feedback of exercise response information such as, heart rate, has the potential to overcome barriers related to access (e.g., inability to drive 6 months after surgery), while also providing a means for some degree of exercise supervision.
The preliminary effect findings from BRACE show that exercise is associated with improvements in patient-reported (quality of life, functional well-being, depression) and objectively measured (fitness, balance, lower-body strength) outcomes. These positive pilot findings indicate that exercise post-brain cancer could help patients live better, and add to the evidence base (strength: level III) reported in a systematic review of previous exercise trials which also showed benefits to fitness, mental health, and quality of life[Citation10]. It is noteworthy though that we did not observe improvements in the brain cancer subscale, which asks questions specifically related to brain cancer concerns (e.g., seizures, concentration, headaches). This finding is in contrast to previous pilot findings in brain cancer studies[Citation10], as well as those shown in other cancer types, including breast, prostate, and colorectal cancers, whereby exercise therapy has been found to reduce the number and severity of cancer-specific concerns [Citation6]. As such, it is plausible that exercise is ineffective at influencing brain cancer-specific side effects or that the magnitude of the effect of exercise is influenced by the timing of the intervention with respect to timing of brain cancer treatment. Alternatively, a larger or more representative sample may show differing results.
Strengths, limitations, and conclusions
Strength of the BRACE findings is drawn from our comprehensive assessment and reporting of the safety and feasibility of an intervention that included supervised and unsupervised mixed-mode exercise [Citation33], and prospective sampling of participants. Key limitations of the BRACE study include its phase II, pre-post study design, and small, urban-based sample, with potential for participant bias (with more well patient with brain cancer likely agreeing to participate), which influence the strength of findings and limit the generalizability of findings to the wider brain cancer population. Nonetheless, these findings add some weight to international exercise oncology guidelines which endorse that exercise therapy should be incorporated among care for all cancers, including for more rare types associated with poorer prognosis [Citation6,Citation11]. However, for benefits to be accrued, exercise needs to be accessible, with the potential for the use of technology and telehealth to facilitate wide-spread, equitable access, and exercise prescription (including mode, intensity, duration and overall target dosage) needs to be flexible to accommodate the presence of, and changing, disease and treatment-related side-effects. BRACE findings suggest that these needs are particularly relevant for people with brain cancer.
Author contributions
Conceived and designed the study: SCH, CXS, JB, PE, DW, DP, MS, VB, BM, FJ. Data collection and analysis: CXS, GCG, RRS, TLJ, PE, DW, AA, CB, JK, P, MS, VB, BM, MF, JB, SCH. Interpretation of results and drafted the manuscript: CXS, GCG, RRS, TLJ, SCH. All authors provided a review of the manuscript draft.
Supplemental Material
Download PDF (271.7 KB)Acknowledgments
This study was a collaboration between the ICON Cancer Foundation, Queensland Sports Medicine Centre and the Improving Health Outcomes Research Group (improvinghealth.com.au) but was only made possible as a consequence of the people who agreed to participate.
Disclosure statement
The authors report no conflicts of interest.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- GBD 2016 Brain and Other CNS Cancer Collaborators Global, regional, and national burden of brain and other CNS cancer, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(4):376–393.
- Australian Institute of Health Welfare. Brain and other Central nervous system cancers. Canberra: AIHW; 2017.
- Australian Institute of Health Welfare. Cancer in Australia. Canberra: AIHW; 2020.
- Sterckx W, Coolbrandt A, de Casterlé BD, et al. The impact of a high-grade glioma on everyday life: a systematic review from the patient’s and caregiver’s perspective. Eur J Oncol Nurs. 2013;17(1):107–117.
- Chandana SR, Movva S, Arora M, et al. Primary brain tumors in adults. American Family Physician. 2008;77(10):1423–1430.
- Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375–2390.
- Friedenreich CM, Stone CR, Cheung WY, et al. Physical activity and mortality in cancer survivors: a systematic review and meta-analysis. JNCI Cancer Spectr. 2020;4(1):pkz080.
- Hayes SC, Newton RU, Spence RR, et al. The exercise and sports science Australia position statement: exercise medicine in cancer management. J Sci Med Sport. 2019;22(11):1175–1199.
- Singh B, Spence RR, Hayes SC. Is exercise really safe, feasible, and effective for all people diagnosed with cancer? Asia Pac J Clin Oncol. 2022;18(1):156–157.
- Sandler CX, Matsuyama M, Jones TL, et al. Physical activity and exercise in adults diagnosed with primary brain cancer: a systematic review. J Neurooncol. 2021;153(1):1–14.
- Spence RR, Sandler CX, Newton RU, et al. Physical activity and exercise guidelines for people with cancer: why are they needed, who should use them, and when? Semin Oncol Nurs. 2020;36(5):151075.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. 1982;5(6):649–656.
- Brown WJ, Bauman AE, Bull F, et al. Development of Evidence-based Physical Activity Recommendations for Adults (18–64 years). Report prepared for the Australian Government Department of Health, August 2012 2013.
- Hayes SC, Spence RR, Galvão DA, et al. Australian association for exercise and sport science position stand: optimising cancer outcomes through exercise. J Sci Med Sport. 2009;12(4):428–434.
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). 2017.
- Spence RR, Sandler CX, Singh B, et al. A randomised, comparative, effectiveness trial evaluating low- versus high-level supervision of an exercise intervention for women with breast cancer: the SAFE trial. Cancers. 2022;14(6):1528.
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381.
- Norton K, Norton L. Pre-exercise screening. Guide to the Australian adult pre-exercise screening system exercise and sports science Australia. 2011.
- Weitzner MA, Meyers CA, Gelke CK, et al. The functional assessment of cancer therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer. 1995;75(5):1151–1161.
- Brucker PS, Yost K, Cashy J, et al. General population and cancer patient norms for the functional assessment of cancer therapy-general (FACT-G). Eval Health Prof. 2005;28(2):192–211.
- Yellen SB, Cella DF, Webster K, et al. Measuring fatigue and other anemia-related symptoms with the functional assessment of cancer therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63–74.
- Puhan MA, Frey M, Büchi S, et al. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2008;6(1):46.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370.
- Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985; 10(3):141–146.
- Schmidt K, Vogt L, Thiel C, et al. Validity of the six-minute walk test in cancer patients. Int J Sports Med. 2013;34(7):631–636.
- Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94.
- Cella D, Eton DT, Lai J-S, et al. Combining anchor and distribution-based methods to derive minimal clinically important differences on the functional assessment of cancer therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002; 24(6):547–561.
- Rasekaba T, Lee AL, Naughton MT. The six-minute walk test: a useful metric for the cardiopulmonary patient. Internal Medicine Journal. 2009 2009/08/01;39(8):495-501.
- Li T, Wei S, Shi Y, et al. The dose-response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med. 2016;50(6):339–345.
- Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592.
- Sloan JA, Cella D, Hays RD. Clinical significance of patient-reported questionnaire data: another step toward consensus. J Clin Epidemiol. 2005;58(12):1217–1219.
- Capozzi LC, Boldt KR, Easaw J, et al. Evaluating a 12-week exercise program for brain cancer patients. Psychooncology. 2016;25(3):354–358.
- Spence RR, Sandler CX, Jones TL, et al. Practical suggestions for harms reporting in exercise oncology: the exercise harms reporting method (ExHaRM). BMJ Open. 2022;12(12):e067998.
- Thomsen SN, Lahart IM, Thomsen LM, et al. Harms of exercise training in patients with cancer undergoing systemic treatment: a systematic review and meta-analysis of published and unpublished controlled trials. EClinicalMedicine. 2023;59:101937.
- Singh B, Hayes SC, Spence RR, et al. Exercise and colorectal cancer: a systematic review and meta-analysis of exercise safety, feasibility and effectiveness. Int J Behav Nutr Phys Act. 2020;17(1):122.
- Singh B, Spence RR, Steele ML, et al. A systematic review and meta-analysis of the safety, feasibility, and effect of exercise in women with stage II + breast cancer. Arch Phys Med Rehabil. 2018;99(12):2621–2636.
- Ayotte SL, Harro CC. Effects of an individualized aerobic exercise program in individuals with a brain tumor undergoing inpatient rehabilitation [article.] Rehabilitation Oncology. 2017;35(4):163–171.
- Gehring K, Kloek CJ, Aaronson NK, et al. Feasibility of a home-based exercise intervention with remote guidance for patients with stable grade II and III gliomas: a pilot randomized controlled trial. Clin Rehabil. 2018;32(3):352–366.
- Hansen A, Søgaard K, Minet LR. Development of an exercise intervention as part of rehabilitation in a glioblastoma multiforme survivor during irradiation treatment: a case report. Disabil Rehabil. 2019;41(13):1608–1614.
- Levin GT, Greenwood KM, Singh F, et al. Exercise improves physical function and mental health of brain cancer survivors: two exploratory case studies. Integr Cancer Ther. 2016;15(2):190–196.
- Milbury K, Liao Z, Shannon V, et al. Dyadic yoga program for patients undergoing thoracic radiotherapy and their family caregivers: results of a pilot randomized controlled trial. Psychooncology. 2019;28(3):615–621.
- Milbury K, Mallaiah S, Mahajan A, et al. Yoga program for high-grade glioma patients undergoing radiotherapy and their family caregivers. Integr Cancer Ther. 2018; 17(2):332–336.
- Eakin EG, Hayes SC, Haas MR, et al. Healthy living after cancer: a dissemination and implementation study evaluating a telephone-delivered healthy lifestyle program for cancer survivors. BMC Cancer. 2015;15:992.
- Eakin EG, Lawler SP, Winkler EA, et al. A randomized trial of a telephone-delivered exercise intervention for non-urban dwelling women newly diagnosed with breast cancer: exercise for health. Ann Behav Med. 2012; 43(2):229–238.
- Hayes SC, Rye S, DiSipio T, et al. Exercise for health: a randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment-related side effects following breast cancer. Breast Cancer Res Treat. 2013;137(1):175–186.
- Paterson C, Bacon R, Dwyer R, et al. The role of telehealth during the COVID-19 pandemic across the interdisciplinary cancer team: implications for practice. Semin Oncol Nurs. 2020;36(6):151090.
- Bjelland I, Dahl AA, Haug TT, et al. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77.
- Amireault S, Godin G. The Godin-Shephard leisure-time physical activity questionnaire: validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept Mot Skills. 2015;120(2):604–622.
- Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561.
- Freire AN, Guerra RO, Alvarado B, et al. Validity and reliability of the short physical performance battery in two diverse older adult populations in Quebec and Brazil. J Aging Health. 2012;24(5):863–878.
