Abstract
Purpose
Cancer-related fatigue (CRF) is unrelenting. As neither rest nor sleep ameliorates cognitive, emotional, and physical symptoms, quality of life is diminished. This study examines resistance training (RT) effectiveness on CRF in cancer survivors. The secondary aims were to identify the dose-response relationship of RT frequency, intensity, and volume on CRF in different cancer survivor populations.
Materials and methods
Systematic searches via numerous databases for RCTs were performed in June 2022. Patient-reported outcome measures (PROM), were analysed, pre-to-post intervention, using a random-effects model. The Physiotherapy Evidence Database (PEDro) scale informed methodological quality assessment.
Results
Eight studies were included (cancer survivors: breast (BCS) = 5; endometrial (ECS) = 1; prostate (PCS) = 2). Overall, RT interventions ≥ 6 weeks elicited large significant reductions in CRF for FACIT-F (SMD = 0.932, p = <0.001) and moderate significant reductions in CRF for PFS-R (SMD = −0.622, p = 0.004).
Conclusion
Main findings indicate that RT ameliorates CRF, especially in BCS; however, individualised approaches should be advocated. Supervised training elicited the greatest positive outcomes, thus should be a pivotal part of the cancer rehabilitation pathway. Future studies should be adequately powered, undertake discrete analyses of different cancer types, and investigate chronic RT effects.
IMPLICATIONS FOR REHABILITATION
Cancer-related fatigue (CRF) is debilitating and distressing, leading to reduced quality of life and function in cancer survivors.
Considerable heterogeneity exists in disease histology and clinical patient presentation.
Individualised resistance training (RT) is an effective, safe, and accessible intervention to mitigat:e fatigue levels, thus aid function, most notably in breast cancer survivors
Introduction
Cancer is a leading cause of death in >100 countries [Citation1]. Advancements in medicine are responsible for greater prognosis and survivorship rates [Citation2]; however, the encompassing burden associated with the disease and survivorship is widely known and published [Citation3–5]. Acutely distressing, and relentless in nature, cancer related fatigue (CRF) [Citation6,Citation7] is often associated with exhaustion inordinate with recent activity levels that is not relieved with rest or sleep, severely diminishing QOL; however, cognitive, and emotional fatigue also manifests [Citation8,Citation9]. Approximately 30–90% of individuals on the cancer spectrum suffer with CRF [Citation10–12]. Conversely, Campos et al. [Citation13] and Horneber et al. [Citation12] suggest CRF may be underdiagnosed, underreported, and overlooked, as it is commonly seen as a normal part of treatment by patients; yet it is a common factor in treatment cessation [Citation14]. A reduction in the ability to complete activities of daily living through diminishing physical function [Citation15]; CRF was reported in the top 3 most-adverse symptoms post-treatment, 1 year on, by survivors [Citation16,Citation17]. Significant associations between increased all-cause mortality and reduced survivorship in individuals with a CRF diagnosis are reported [Citation18]; however, when stress-related psychosocial symptoms of fatigue are present, the association is greater [Citation19].
A stepwise approach to diagnosis is common practice [Citation20]; including exclusion or treatment of fatigue-inducing comorbidities and/or pathologies (e.g., anaemia, hyperthyroidism, and medication effects), followed by comprehensive clinical history taking, physical examination, laboratory measures, and PROMs [Citation11,Citation13]. Bower [Citation21] highlighted the importance of PROMs (e.g., PFS-R) in distinguishing whether fatigue is related to cancer, and associated therapies, or secondary to depression, as this would alter treatment options. Furthermore, PROMs also allow for the assessment of individual dimensions of fatigue (e.g., emotional, psychological, physical, or social), thus providing the opportunity for tailored care and treatments [Citation22,Citation23].
Often characterised as a systemic inflammation dysfunction, it is proposed that CRF occurs as a result of interrupted protein and energy balance facilitated by various mechanisms [Citation7]; however, the association exists with cancer cachexia, a multifactorial inflammatory syndrome characterised by loss of skeletal muscle and fat mass [Citation24]. Gentile et al. [Citation25] proposed that the complex aetiology could be divided into two categories; central and peripheral fatigue. O’Higgings et al. [Citation26] suggested the true pathogenesis is still unknown, yet Bower [Citation14] and Yang et al. [Citation7] proposed inflammatory pathways, and mitochondrial dysfunction, are the most pragmatic rationales.
Cancer-related fatigue is often managed utilising a biopsychosocial approach [Citation27]; however, exercise is regularly prescribed [Citation28]. Mechanistically, there is ample evidence to suggest exercise, and specifically RT, can ameliorate the negative effects of CRF [Citation29,Citation30]. Resistance training has been shown to attenuate muscle cell apoptosis and degradation, promote mitochondrial biogenesis and muscle protein synthesis, and positively effect skeletal muscle contractile properties in cancer populations [Citation31,Citation32]. Resistance training advantageously influences inflammation [Citation24,Citation33]; with a substantial reduction in CRP and IL-6 levels being noted [Citation34,Citation35]. Additionally, RT positively influences mental health and reduces depressive symptoms [Citation36], a key aspect of holistic cancer care, and the amelioration of fatigue [Citation37], through the enhancement of the neurotransmitters, dopamine, norepinephrine, and serotonin [Citation38]. In spite of RT’s known beneficial effects on CRF, the optimal prescription is still unknown and often vague, attributed to the vast heterogeneity between cancer survivors [Citation39,Citation40]. The American College of Sports Medicine (ACSM) propose 150 min/week of moderate, or 75 min/week of vigorous aerobic exercise, and 2 d/week of moderate intensity RT for cancer survivors; however, this mirrors the recommendations proposed for healthy individuals [Citation41]. More recently, Stout et al. [Citation42] proposed specific exercise guidelines for CRF, however, these again mirrors the aforementioned. Conversely, Strasser et al. [Citation43] proposed optimal parameters for alleviation of CRF through RT were twice weekly sessions, consisting of 2–6 sets of 12–17 reps at 60–70% 1 repetition maximum (RM), per major muscle group. Supporting the aforementioned, Campbell et al. [Citation44] suggested twice-weekly RT sessions, consisting of 2 sets of 12–15 reps at 60% 1RM or 12 BORG, with an unspecified number of exercises, for 12 weeks was also adequate at ameliorating CRF. However, Brown et al. [Citation45] reported that cancer survivors got greater amelioration of fatigue, when RT intensity exceeded 60% of 1RM compared to lower intensities, as a result of greater stimulus driving greater physiological adaptions.
The primary purpose of this systematic review and meta-analysis is to evaluate the effect of RT on CRF in cancer survivors utilising PROMs. Secondary purposes include exploring the dose-response relationship of RT frequency, intensity, and volume, and CRF, and the effects of RT on CFR on different cancer survivor populations.
Materials and methods
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA was used (), which highlights the process of inclusion and exclusion of studies through a flow chart diagram. Articles were retrieved from MEDLINE and CINAHL via Ebsco, EMBASE, and the International Clinical Trials Registry Platform via the Cochrane Library, PubMed, and Scholar from inception to June 2022, in June 2022. Only studies published in English with human participants were included. The following search strategy was used for all database searches (cancer) AND (fatigue) AND (resistance training) OR (strength training) OR (weight training). An independent literature search of previous, related, systematic reviews and meta-analyses was also undertaken.
Inclusion and exclusion criteria
Types of studies
Only RCT’s were considered for inclusion within this systematic review and meta-analysis.
Types of participants
Only studies where participants were aged 18 and over, and who had completed primary treatment for cancer were eligible. Studies where participants were classified as advanced or non-curative were excluded. There were no limitations on gender, ethnicity, or tumour type, location, or treatment.
Types of intervention
Only studies that included a form of resistance, strength, or weight training lasting >6 weeks were included within this systematic review and meta-analysis.
Types of outcomes
Only studies which investigated the pre-post change in fatigue through resistance training intervention were eligible for inclusion. Pre- and post-intervention measurements of fatigue from resistance training and control groups were extracted for use within the meta-analysis. The PROMs included within the qualitative and quantitative analysis were the FACIT-F and PFS-R.
Selection of studies
The reviewers independently screened study titles and abstracts identified through the search results against pre-determined eligibility criteria. If eligibility from initial screening could not be determined, full-text screening was utilised. A manual search of relevant systematic reviews and meta-analysis reference lists was also conducted.
Data extraction
Data extraction included study design, population (cancer type) and participant characteristics (mean age, gender), intervention type (type of resistance training i.e., free weights), intensity (e.g., rate of perceived exertion), volume (e.g., 3 sets of 10 repetitions), frequency (e.g., 3× per week for 12 weeks), comparator/control group, primary outcome (measure of fatigue), timepoint, and methodological quality score.
Methodological quality assessment
The PEDro scale was utilised to evaluate the methodological quality of the studies. Established as a valid measure of methodological assessment [Citation46,Citation47], the PEDro scale assesses for internal validity and is scored out of 10 (criteria 2–11), as criterion 1 is a measure of external validity. Each criterion was graded by the reviewer as; 1 = yes; 0 = no which was determined by the information analysed within each study. Studies scoring 3 or below, 4 or 5, 6 to 8, or 9 and above were deemed to have “poor”, “fair”, “good” or “excellent” methodological quality, respectively [Citation48]. The scale assesses for random and concealed allocation, similarity of groups at baseline, subject, therapist, and assessor blinding, >85% follow-up for at least one key outcome measure, intention-to-treat analysis, between-group statistical comparison for at least one key outcome measure, and point measures and measures of variability.
Measures of treatment effect
The meta-analysis was conducted using Comprehensive Meta-Analysis V3 (NIH) software. Standardised mean differences (SMD) and confidence intervals (CI) were calculated as summary statistics and determined for the evaluation of resistance training interventions, and respective control groups. Forest plots were utilised to display the individual study SMD and CI along with the pooled overall SMD to give a description of the intervention effectiveness. The direction of the SMD (positive or negative) being indicative of “effect” and zero being no effect. Sensitivity analysis utilising the leave-one-out method was also completed to assess result robustness. As per Cohen [Citation49], an SMD of 0.8, 0.5, and 0.2 are indicated as “large”, “moderate” and “small” effects, respectively.
Data synthesis
Pooled effect estimates from studies comparing RT in a pre-post intervention design with comparable PROMs were completed using the Comprehensive Meta-Analysis V3 software. As a result of clinical and methodological heterogeneity, a random-effects model was used. Both, the Chi2 (if p < 0.05) and I2 statistics were included to further explore the true effect of the interventions. Rodseth and Marais [Citation50] indicate boundaries for the I2 statistic and heterogeneity; 0–40% equals “non-importance”, 30–60% shows “moderate”, 50–90% indicates “substantial” and 75–100% is deemed as “significant”.
Results
Search results
Searches through electronic databases identified 30 548 articles, duplicates were removed returning 29 369 studies. After screening titles and abstracts, and exclusion of non-related studies (e.g., not cancer, mixed intervention), 30 articles were assessed for eligibility (see ). A further 22 studies were excluded as a result of no measures of fatigue (11), mixed exercise intervention (5), and required data being unavailable (6). This left 8 studies, which were included within the quantitative and qualitative synthesis.
Participant characteristics
The characteristics of participants and included studies are displayed in . Overall, 325 participants (96 males and 229 females) were included in this review. Participant cohorts included female-only BCS [Citation52–54,Citation56,Citation58], ECS [Citation51], and PCS [Citation55,Citation57]. Sample sizes ranged from 14 to 66, while age ranged from 35 to 77 years old. The countries or region of publication were as follows; Australia (n = 1), Brazil (n = 1), Canada (n = 2), India (n = 1), Iran (n = 1), and the United States of America (n = 2). Hagstrom et al. [Citation52], Moraes et al. [Citation54], Norris et al. [Citation55] and Santa Mina et al. [Citation57] included participants on adjuvant therapies.
Table 1. Characteristics of population and interventions of included studies.
Resistance training interventions and control groups
The RT interventions () included in this review lasted from 6 to 26 weeks, the frequency of sessions ranged from 1 to 5 times per week, with the number of exercises varying between 5 and 10 per session. All studies promoted progressive overload through sessional volume, with sets ranging from 1 to 3, and reps varying between 8 and 12. Two studies did not report intensity [Citation53,Citation58]; however, the remaining studies utilised relative intensity scales (e.g., %1RM, BORG), with four studies applying progressive overload through intensity [Citation51,Citation55–57]. Moraes et al. [Citation54] and Norris et al. [Citation55] provided supervised sessions, whereas the rest did not. Five studies asked control groups to remain physically inactive or maintain usual care pathways, while Khan et al. [Citation53] and Santa Mina et al. [Citation57] utilised walking exercise control groups (frequency and session duration matched). Norris et al. [Citation55] altered the frequency only of his control group, reducing the RT intervention to twice weekly. Only Hagstrom et al. [Citation52] and Moraes et al. [Citation54] mentioned offering the experimental intervention to the control group after the study completion.
Resistance training and FACIT-F
Post-intervention changes in fatigue significantly favoured the RT in comparison to the controls (5 comparison groups, 120 participants, 115 controls; SMD = 0.932, CI = 0.111–1.753, p = 0.026; ). However, significant heterogeneity was found within the analysis (I2 = 88.167, p = <0.001; ) indicating a large variability within the sample. The sensitivity analysis highlighted the exclusion of Gorzelitz et al. [Citation51] and Norris et al. [Citation55] still resulted in significant statistical results (SMD = 1.142, CI = 0.249–2.044, p = 0.013 and SMD = 1.118, CI = 0.182–2.053, p = 0.019, respectively; ); whereas, when Hagstrom et al. [Citation52] (SMD = 1.022, CI = −0.005 to 2.049, p = 0.051), Khan et al. [Citation53] (SMD = 0.596, CI = −0.078 to 1.270, p = 0.083) and Santa Mina et al. [Citation57] (SMD = 0.788, CI = −0.238 to 1.814, p = 0.132) were removed, as part of the leave-one-out analysis, pooled results became non-significant ().
Resistance training and PFS-R
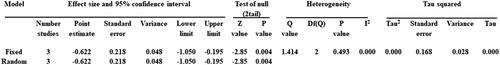
Post-intervention changes in fatigue significantly favoured RT versus control groups, with non-important heterogeneity (3 comparison groups, 45 participants, 45 controls; SMD = −0.622, CI = −1.050 to −0.195, p = 0.004; I2 = 0.000, p = 0.493; and ). During the sensitivity analysis, each study was individually removed and the pooled SMD was recalculated to assess the significance of each study; it can be seen from that the exclusion of each study did not change the significance or direction of SMD.
Methodological quality of included studies
Details and results of the methodological quality assessment can be found in . Methodological scoring ranged from 3 to 7, with a mean of 5.63 ± 1.51. One study was deemed of having “poor” [Citation56], while two had “fair” [Citation51,Citation54] methodological quality; finally, the remaining 5 had “good” methodological quality [Citation52,Citation53,Citation55,Citation57,Citation58].
Table 2. Methodological quality assessment [PEDro].
All 8 studies were at risk of performance and detection bias, due to not fulfilling participant and assessor blinding criterion, respectively; however, the blinding of exercise intervention is impossible thus justifiable. Only Hagstrom et al. [Citation52], Norris et al. [Citation55], and Santa Mina et al. [Citation57] satisfied criterion 3, leaving the remaining studies at risk of selection bias; additionally, Nouri et al. [Citation56] didn’t explicitly state randomisation procedure thereby heightening the risk further. Attrition bias was highlighted in Gorzelitz et al. [Citation51] and Nouri et al. [Citation56] as neither criterion 8 nor 9 was satisfied, whereas Moraes et al. [Citation54] satisfied criterion 8 only.
Discussion
This systematic review and meta-analysis investigated the effects of RT on CRF in cancer survivors, who had completed primary treatment. The initial findings from this review indicate that there is a statistically significant reduction in CRF following RT; however, the sensitivity analysis raises questions regarding the efficacy and heterogeneity of within-group and -population data.
Outcome measures
Both FACIT-F and PFS-R outcome measures had a Cronbach’s α value greater than 0.90, indicating excellent internal consistency [Citation59–61]; however, values above 0.90 can suggest redundant questions [Citation62].
Minimally clinically important differences (MCID) indicate the smallest beneficial change to a patient’s symptoms and should be considered when interpreting the effectiveness of an intervention [Citation63,Citation64]. Amarsheda and Bhise [Citation65] reported a 2-point reduction was the MCID for improvement of PFS-R in breast cancer populations and a 2.8–6.8 increase is required for amelioration of symptoms for the FACIT-F measure across all cancer populations [Citation66].
Cancer histology, resistance training and cancer-related fatigue
This systematic review and meta-analysis, with sensitivity analysis, provides some evidence that RT is beneficial for ameliorating CRF. The individual presentation of CRF through different fatigue constructs and severity potentially explains the variance in intervention effectiveness.
Heterogeneity within the included studies was always going to be pronounced with three different cancer types being studied. Additionally, exercise prescription varied across trials in terms of duration of intervention, RT modality, frequency, volume, and intensity, amongst other variables, making specific exercise recommendations difficult. However, supervised RT seemed to elicit a greater effect on CRF than unsupervised studies, as the ability to control training variables that help promote physiological and morphological adaptions to attenuate CRF are greater [Citation67]. Furthermore, when exercise is within a group-setting, emotional, mental, and physical QOL is improved greater than in volume- and intensity-matched individual training in numerous populations [Citation68], including cancer patients [Citation69–71].
From the included studies, where comparable, BCS showed greater improvement to fatigue levels following RT versus other cancers, which supports previous literature [Citation72,Citation73]; however, research suggests the amelioration of fatigue is possible in numerous cancer populations [Citation29,Citation30,Citation74,Citation75] adding substance to the effect of significant heterogeneity between and within cancer survivors. With skeletal muscle atrophy being common following primary treatment in BCS, the likelihood of CRF increases [Citation76,Citation77]; thus, placing RT in an advantageous position due to its innate ability to positively change body composition [Citation78]. Furthermore, Leite et al. [Citation79] indicated low RT volume and longer recovery intervals were more advantageous in BCS; supported by Lopez et al. [Citation80] who reported a greater magnitude of physical recovery and exercise adherence following low-volume RT.
A critical factor to consider within the analysis is age; the PCS within the current meta-analysis were generally older than those in other trials, thus reportedly having less potential for hypertrophic changes as per Lee [Citation81]. Despite hypertrophic changes are often blunted in older adults [Citation82], supervised RT can attenuate cancer-related losses of muscle mass in older populations [Citation83]. However, Lee [Citation81] also reported that RT may not be effective at ameliorating CRF specifically in older PCS; especially when survivors are utilising androgen deprivation therapy as it induces metabolic dysfunction, thus promoting muscle wastage [Citation84,Citation85]. Conversely, clinically meaningful, improvements to body composition were reported in Clifford et al.’s [Citation86] meta-analysis following RT after primary treatment in PCS. Furthermore, Hanson et al. [Citation87] noted significant increases in muscle volume and strength (p < 0.001), and reduction in fatigue (p < 0.05) following thrice weekly RT to volitional failure in older black prostate cancer patients on ADT. Overall, this suggests that RT when supervised, and meets minimal effective dose, it is plausible to ameliorate fatigue in PCS.
In ECS the co-morbidity burden is greater than with other histological types as age-related deterioration in health-related QOL can be more prevalent as diagnosis is usually made in over 65s [Citation88,Citation89]. Additionally, as stated earlier, in older individuals the potential for hypertrophic changes following RT is diminished [Citation81]. Metabolic dysfunction, presenting as type 2 diabetes mellitus and obesity, is often prevalent in ECS and can influence CRF as independent fatigue generator’s [Citation68,Citation90,Citation91]. Fortunately, RT participation promotes unique metabolic adaptions, thus attenuating some of the subsequent side effects of metabolic dysfunction, that may also drive CRF [Citation92–95]. Even with a lack of evidence suggesting the utility of RT with ECS, Maqbali et al. [Citation96] suggests it is effective at attenuating CRF; however, Zhang et al. [Citation97] suggested ∼80% of ECS require supervision while exercising, for it to be effective, adding an additional barrier to effectiveness.
Limitations of the review
The limitations of this study are as follows; significant between- and within-group heterogeneity reduces the generalisability and ecological validity of the results. Additionally, the small number of studies across different histological diagnoses further decreases generalisability. Reduced precision was present as wide confidence intervals were reported, due to relatively small sample sizes. Additionally, study durations were typically short. Future research should endeavour to have greater sample sizes and study duration, across a wider range of histologically diagnosed cancer types; and include a follow-up to investigate the long-term effectiveness of RT intervention of CRF.
Conclusion
The findings of this review indicate that RT is effective at attenuating CRF; with BCS having the greatest response. Although specific RT prescription cannot be derived due to significant between- and within-group heterogeneity; studies with supervised elements typically had greater reductions versus home-based or non-supervised. This is likely due to the social elements and subsequent ability to control the training variables that help promote the physiological and morphological adaptions required for the amelioration of CRF.
Disclosure statement
No conflicts of interest have been declared by the authors.
Additional information
Funding
References
- World Health Orginization. Global health estimates 2020: deaths by cause, age, sex, by country and by region, 2000–2019. Geneva: WHO; 2020.
- Shapiro CL. Cancer survivorship. N Engl J Med. 2018;379(25):2438–2450. doi: 10.1056/NEJMra1712502.
- Caruso R, Breitbart W. Mental health care in oncology. Contemporary perspective on the psychosocial burden of cancer and evidence-based interventions. Epidemiol Psychiatr Sci. 2020;29:e86. doi: 10.1017/S2045796019000866.
- Nurgali K, Jagoe RT, Abalo R. Editorial: adverse effects of cancer chemotherapy: anything new to improve tolerance and reduce sequelae? Front Pharmacol. 2018;9:245. doi: 10.3389/fphar.2018.00245.
- Schirrmacher V. From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment (review). Int J Oncol. 2019;54(2):407–419. doi: 10.3892/ijo.2018.4661.
- Savina S, Zaydiner B. Cancer-related fatigue: some clinical aspects. Asia Pac J Oncol Nurs. 2019;6(1):7–9. doi: 10.4103/apjon.apjon_45_18.
- Yang S, Chu S, Gao Y, et al. A narrative review of cancer-related fatigue (CRF) and its possible pathogenesis. Cells. 2019;8(7):738. doi: 10.3390/cells8070738.
- Jones JM, Olson K, Catton P, et al. Cancer-related fatigue and associated disability in post-treatment cancer survivors. J Cancer Surviv. 2016;10(1):51–61. doi: 10.1007/s11764-015-0450-2.
- Ma Y, He B, Jiang M, et al. Prevalence and risk factors of cancer-related fatigue: a systematic review and meta-analysis. Int J Nurs Stud. 2020;111:103707. doi: 10.1016/j.ijnurstu.2020.103707.
- Christen S, Roser K, Mulder RL, et al. Recommendations for the surveillance of cancer-related fatigue in childhood, adolescent, and young adult cancer survivors: a report from the international late effects of childhood cancer guideline harmonization group. J Cancer Surviv. 2020;14(6):923–938. doi: 10.1007/s11764-020-00904-9.
- Fabi A, Bhargava R, Fatigoni S, et al. Cancer-related fatigue: ESMO clinical practice guidelines for diagnosis and treatment. Ann Oncol. 2020;31(6):713–723. doi: 10.1016/j.annonc.2020.02.016.
- Horneber M, Fischer I, Dimeo F, et al. Cancer-related fatigue: epidemiology, pathogenesis, diagnosis, and treatment. Dtsch Arztebl Int. 2012;109(9):161–171.
- Campos MP, de O, Hassan BJ, et al. Cancer-related fatigue: a review. Rev Assoc Med Bras (1992). 2011;57(2):211–219. doi: 10.1590/s0104-42302011000200021.
- Bower JE. The role of neuro-immune interactions in cancer-related fatigue: biobehavioral risk factors and mechanisms. Cancer. 2019;125(3):353–364. doi: 10.1002/cncr.31790.
- Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4.
- Shi Q, Smith TG, Michonski JD, et al. Symptom burden in cancer survivors 1 year after diagnosis: a report from the American Cancer Society’s studies of cancer survivors. Cancer. 2011;117(12):2779–2790. doi: 10.1002/cncr.26146.
- Wang XS, Woodruff JF. Cancer-related and treatment-related fatigue. Gynecol Oncol. 2015;136(3):446–452. doi: 10.1016/j.ygyno.2014.10.013.
- Adam S, van de Poll-Franse LV, Mols F, et al. The association of cancer-related fatigue with all-cause mortality of colorectal and endometrial cancer survivors: results from the population-based PROFILES registry. Cancer Med. 2019;8(6):3227–3236. doi: 10.1002/cam4.2166.
- Weber D, O’Brien K. Cancer and cancer-related fatigue and the interrelationships with depression, stress, and inflammation. J Evid Based Complementary Altern Med. 2017;22(3):502–512. doi: 10.1177/2156587216676122.
- Donovan KA, McGinty HL, Jacobsen PB. A systematic review of research using the diagnostic criteria for cancer-related fatigue. Psychooncology. 2013;22(4):737–744. doi: 10.1002/pon.3085.
- Bower JE. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. doi: 10.1038/nrclinonc.2014.127.
- Ahlberg K, Ekman T, Gaston-Johansson F, et al. Assessment and management of cancer-related fatigue in adults. Lancet. 2003;362(9384):640–650. doi: 10.1016/S0140-6736(03)14186-4.
- Minton O, Berger A, Barsevick A, et al. Cancer-related fatigue and its impact on functioning. Cancer. 2013;119 Suppl 11:2124–2130. doi: 10.1002/cncr.28058.
- Leal LG, Lopes MA, Peres SB, et al. Exercise training as therapeutic approach in cancer cachexia: a review of potential anti-inflammatory effect on muscle wasting. Front Physiol. 2020;11:570170. doi: 10.3389/fphys.2020.570170.
- Gentile D, Beeler D, Wang XS, et al. Cancer-related fatigue outcome measures in integrative oncology: evidence for practice and research recommendations. Oncology. 2022;36(5):276–287.
- O’Higgins CM, Brady B, O’Connor B, et al. The pathophysiology of cancer-related fatigue: current controversies. Support Care Cancer. 2018;26(10):3353–3364. doi: 10.1007/s00520-018-4318-7.
- Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-related fatigue, version 2.2015. J Natl Compr Canc Netw. 2015;13(8):1012–1039. doi: 10.6004/jnccn.2015.0122.
- Hilfiker R, Meichtry A, Eicher M, et al. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med. 2018;52(10):651–658. doi: 10.1136/bjsports-2016-096422.
- LaVoy ECP, Fagundes CP, Dantzer R. Exercise, inflammation, and fatigue in cancer survivors. Exerc Immunol Rev. 2016;22:82–93.
- Mustian KM, Sprod LK, Janelsins M, et al. Exercise recommendations for cancer-related fatigue, cognitive impairment, sleep problems, depression, pain, anxiety, and physical dysfunction: a review. Oncol Hematol Rev. 2012;8(2):81–88. doi: 10.17925/ohr.2012.08.2.81.
- Beltrà M, Pin F, Ballarò R, et al. Mitochondrial dysfunction in cancer cachexia: impact on muscle health and regeneration. Cells. 2021;10(11):3150. doi: 10.3390/cells10113150.
- Buffart LM, Sweegers MG, Ruijter CJ, et al. Muscle contractile properties of cancer patients receiving chemotherapy: assessment of feasibility and exercise effects. Scand J Med Sci Sports. 2020;30(10):1918–1929. Oct 2doi: 10.1111/sms.13758.
- Rose GL, Skinner TL, Mielke GI, et al. The effect of exercise intensity on chronic inflammation: a systematic review and meta-analysis. J Sci Med Sport. 2021;24(4):345–351. Aprdoi: 10.1016/j.jsams.2020.10.004.
- Galvão DA, Taaffe DR, Spry N, et al. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28(2):340–347. doi: 10.1200/JCO.2009.23.2488.
- Sardeli AV, Tomeleri CM, Cyrino ES, et al. Effect of resistance training on inflammatory markers of older adults: a meta-analysis. Exp Gerontol. 2018;111:188–196. doi: 10.1016/j.exger.2018.07.021.
- Westcott WL. Resistance training is medicine: effects of strength training on health. Curr Sports Med Rep. 2012;11(4):209–216. doi: 10.1249/JSR.0b013e31825dabb8.
- Brown LF, Kroenke K. Cancer-related fatigue and its associations with depression and anxiety: a systematic review. Psychosomatics. 2009;50(5):440–447. doi: 10.1016/S0033-3182(09)70835-7.
- Smith PJ, Merwin RM. The role of exercise in management of mental health disorders: an integrative review. Annu Rev Med. 2021;72:45–62. doi: 10.1146/annurev-med-060619-022943.
- Fairman CM, Hyde PN, Focht BC. Resistance training interventions across the cancer control continuum: a systematic review of the implementation of resistance training principles. Br J Sports Med. 2017;51(8):677–685. doi: 10.1136/bjsports-2016-096537.
- Fairman CM, Zourdos MC, Helms ER, et al. A scientific rationale to improve resistance training prescription in exercise oncology. Sports Med. 2017;47(8):1457–1465. doi: 10.1007/s40279-017-0673-7.
- Wolin KY, Schwartz AL, Matthews CE, et al. Implementing the exercise guidelines for cancer survivors. J Support Oncol. 2012;10(5):171–177. doi: 10.1016/j.suponc.2012.02.001.
- Stout NL, Santa Mina D, Lyons KD, et al. A systematic review of rehabilitation and exercise recommendations in oncology guidelines. CA Cancer J Clin. 2021;71(2):149–175. doi: 10.3322/caac.21639.
- Strasser B, Steindorf K, Wiskemann J, et al. Impact of resistance training in cancer survivors: a meta-analysis. Med Sci Sports Exerc. 2013;45(11):2080–2090. doi: 10.1249/MSS.0b013e31829a3b63.
- Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375–2390. doi: 10.1249/MSS.0000000000002116.
- Brown JC, Huedo-Medina TB, Pescatello LS, et al. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20(1):123–133. doi: 10.1158/1055-9965.EPI-10-0988.
- de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129–133. doi: 10.1016/s0004-9514(09)70043-1.
- Elkins MR, Moseley AM, Sherrington C, et al. Growth in the physiotherapy evidence database (PEDro) and use of the PEDro scale. Br J Sports Med. 2013;47(4):188–189. doi: 10.1136/bjsports-2012-091804.
- Hariohm K, Prakash V, Saravankumar J. Quantity and quality of randomized controlled trials published by Indian physiotherapists. Perspect Clin Res. 2015;6(2):91–97. doi: 10.4103/2229-3485.154007.
- Cohen J. Statistical power analysis. Curr Dir Psychol Sci. 1992;1(3):98–101. doi: 10.1111/1467-8721.ep10768783.
- Rodseth R, Marais L. Meta-analysis: Everything you wanted to know but were afraid to ask. SA Orthop J. 2016;15(4):31–36. doi: 10.17159/2309-8309/2016/v15n4a4.
- Gorzelitz JS, Stoller S, Costanzo E, et al. Improvements in strength and agility measures of functional fitness following a telehealth-delivered home-based exercise intervention in endometrial cancer survivors. Support Care Cancer. 2022;30(1):447–455. doi: 10.1007/s00520-021-06415-2.
- Hagstrom AD, Marshall PWM, Lonsdale C, et al. Resistance training improves fatigue and quality of life in previously sedentary breast cancer survivors: a randomised controlled trial. Eur J Cancer Care. 2016;25(5):784–794. doi: 10.1111/ecc.12422.
- Khan S, Agrawal R, Shaikh S, et al. Comparison of effect of aerobic training versus resistance training on cancer-related fatigue and quality of life in breast cancer survivors. Indian J Public Health Res Dev. 2020;11:827–833.
- Moraes RF, Ferreira-Júnior JB, Marques VA, et al. Resistance training, fatigue, quality of life, anxiety in breast cancer survivors. J Strength Cond Res. 2021;35(5):1350–1356. doi: 10.1519/JSC.0000000000003817.
- Norris MK, Bell GJ, North S, et al. Effects of resistance training frequency on physical functioning and quality of life in prostate cancer survivors: a pilot randomized controlled trial. Prostate Cancer Prostatic Dis. 2015;18(3):281–287. doi: 10.1038/pcan.2015.28.
- Nouri R, Braumann KM, Mahmoudieh ChamPiri B, et al. Cancer related fatigue and upper limb disabilities cannot improve after 6 weeks resistance training with Thera-Band in breast cancer survivors. Int J Appl Exerc Physiol. 2018;7(2):76–84. doi: 10.22631/ijaep.v7i2.263.
- Santa Mina D, Alibhai S MH, Matthew AG, et al. A randomized trial of aerobic versus resistance exercise in prostate cancer survivors. J Aging Phys Act. 2013;21(4):455–478. doi: 10.1123/japa.21.4.455.
- Yuen HK, Sword D. Home-based exercise to alleviate fatigue and improve functional capacity among breast cancer survivors. J Allied Health. 2007;36(4):e257-75.
- Al Maqbali M, Hughes C, Gracey J, et al. Quality assessment criteria: psychometric properties of measurement tools for cancer related fatigue. Acta Oncol. 2019;58(9):1286–1297. doi: 10.1080/0284186X.2019.1622773.
- Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. doi: 10.1016/j.jcm.2016.02.012.
- Minton O, Stone P. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF). Ann Oncol. 2009;20(1):17–25. doi: 10.1093/annonc/mdn537.
- Tavakol M, Dennick R. Making sense of Cronbach’s alpha. Int J Med Educ. 2011;2:53–55. doi: 10.5116/ijme.4dfb.8dfd.
- Cook CE. Clinimetrics corner: the minimal clinically important change score (MCID): a necessary pretense. J Man Manip Ther. 2008;16(4):E82–3.
- Copay AG, Subach BR, Glassman SD, et al. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541–546. doi: 10.1016/j.spinee.2007.01.008.
- Amarsheda S, Bhise AR. Systematic review of cancer-related fatigue instruments in breast cancer patients. Palliat Support Care. 2022;20(1):122–128. doi: 10.1017/S1478951521000444.
- Nordin Å, Taft C, Lundgren-Nilsson Å, et al. Minimal important differences for fatigue patient reported outcome measures-a systematic review. BMC Med Res Methodol. 2016;16:62. doi: 10.1186/s12874-016-0167-6.
- Ramos-Campo DJ, Andreu Caravaca L, Martínez-Rodríguez A, et al. Effects of resistance Circuit-Based training on body composition, strength and cardiorespiratory fitness: a systematic review and meta-analysis. Biology. 2021;10(5):377. doi: 10.3390/biology10050377.
- Maffiuletti NA, Ratel S, Sartorio A, et al. The impact of obesity on in vivo human skeletal muscle function. Curr Obes Rep. 2013;2(3):251–260. doi: 10.1007/s13679-013-0066-7.
- Yorks DM, Frothingham CA, Schuenke MD. Effects of group fitness classes on stress and quality of life of medical students. J Am Osteopath Assoc. 2017;117(11):e17–25–e25. doi: 10.7556/jaoa.2017.140.
- Losito J, Murphy S, Thomas M. The effects of group exercise on fatigue and quality of life during cancer treatment. Oncol Nurs Forum. 2006;33(4):821–825. doi: 10.1188/06.ONF.821-825.
- Paltiel H, Solvoll E, Loge JH, et al. “The healthy me appears”: palliative cancer patients’ experiences of participation in a physical group exercise program. Palliat Support Care. 2009;7(4):459–467. doi: 10.1017/S1478951509990460.
- Battaglini CL, Mills RC, Phillips BL, et al. Twenty-five years of research on the effects of exercise training in breast cancer survivors: a systematic review of the literature. World J Clin Oncol. 2014;5(2):177–190. doi: 10.5306/wjco.v5.i2.177.
- Dos Santos WDN, Gentil P, de Moraes RF, et al. Chronic effects of resistance training in breast cancer survivors. Biomed Res Int. 2017;2017:8367803. doi: 10.1155/2017/8367803.
- Cramp F, James A, Lambert J. The effects of resistance training on quality of life in cancer: a systematic literature review and meta-analysis. Support Care Cancer. 2010;18(11):1367–1376. doi: 10.1007/s00520-010-0904-z.
- Fuller JT, Hartland MC, Maloney LT, et al. Therapeutic effects of aerobic and resistance exercises for cancer survivors: a systematic review of meta-analyses of clinical trials. Br J Sports Med. 2018;52(20):1311. doi: 10.1136/bjsports-2017-098285.
- Guigni BA, Callahan DM, Tourville TW, et al. Skeletal muscle atrophy and dysfunction in breast cancer patients: role for chemotherapy-derived oxidant stress. Am J Physiol Cell Physiol. 2018;315(5):C744–C756. doi: 10.1152/ajpcell.00002.2018.
- Song EJ, Lee CW, Jung SY, et al. Prognostic impact of skeletal muscle volume derived from cross-sectional computed tomography images in breast cancer. Breast Cancer Res Treat. 2018;172(2):425–436. doi: 10.1007/s10549-018-4915-7.
- Paoli A, Moro T, Bianco A. Lift weights to fight overweight. Clin Physiol Funct Imaging. 2015;35(1):1–6. doi: 10.1111/cpf.12136.
- de Jesus Leite MAF, Puga GM, Arantes FJ, et al. Effects of combined and resistance training on the inflammatory profile in breast cancer survivors: a systematic review. Complement Ther Med. 2018;36:73–81. doi: 10.1016/j.ctim.2017.11.023.
- Lopez P, Galvão DA, Taaffe DR, et al. Resistance training in breast cancer patients undergoing primary treatment: a systematic review and meta-regression of exercise dosage. Breast Cancer. 2021;28(1):16–24. doi: 10.1007/s12282-020-01147-3.
- Lee J. The effects of resistance training on muscular strength and hypertrophy in elderly cancer patients: a systematic review and meta-analysis. J Sport Health Sci. 2022;11(2):194–201. doi: 10.1016/j.jshs.2021.02.002.
- Straight CR, Fedewa MV, Toth MJ, et al. Improvements in skeletal muscle fiber size with resistance training are age-dependent in older adults: a systematic review and meta-analysis. J Appl Physiol (1985). 2020;129(2):392–403. doi: 10.1152/japplphysiol.00170.2020.
- Koeppel M, Mathis K, Schmitz KH, et al. Muscle hypertrophy in cancer patients and survivors via strength training. A meta-analysis and meta-regression. Crit Rev Oncol Hematol. 2021;163:103371. doi: 10.1016/j.critrevonc.2021.103371.
- Storey DJ, McLaren DB, Atkinson MA, et al. Clinically relevant fatigue in men with hormone-sensitive prostate cancer on long-term androgen deprivation therapy. Ann Oncol. 2012;23(6):1542–1549. doi: 10.1093/annonc/mdr447.
- Yunfeng G, Weiyang H, Xueyang H, et al. Exercise overcome adverse effects among prostate cancer patients receiving androgen deprivation therapy: an update meta-analysis. Medicine. 2017;96(27):e7368. doi: 10.1097/MD.0000000000007368.
- Clifford B, Koizumi S, Wewege MA, et al. The effect of resistance training on body composition during and after cancer treatment: a systematic review and meta-analysis. Sports Med. 2021;51(12):2527–2546. doi: 10.1007/s40279-021-01542-6.
- Hanson ED, Sheaff AK, Sood S, et al. Strength training induces muscle hypertrophy and functional gains in black prostate cancer patients despite androgen deprivation therapy. J Gerontol A Biol Sci Med Sci. 2013;68(4):490–498. doi: 10.1093/gerona/gls206.
- Lukowski J, Gil KM, Jenison E, et al. Endometrial cancer survivors’ assessment of the benefits of exercise. Gynecol Oncol. 2012;124(3):426–430. doi: 10.1016/j.ygyno.2011.11.002.
- van Walree IC, Hamaker ME, de Rooij BH, et al. Do age and comorbidity impair recovery during two years after treatment for endometrial cancer? J Geriatr Oncol. 2020;11(7):1078–1086. doi: 10.1016/j.jgo.2020.02.012.
- Fader AN, Arriba LN, Frasure HE, et al. Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol. 2009;114(1):121–127. doi: 10.1016/j.ygyno.2009.03.039.
- Fritschi C, Quinn L. Fatigue in patients with diabetes: a review. J Psychosom Res. 2010;69(1):33–41. doi: 10.1016/j.jpsychores.2010.01.021.
- Courneya KS, Karvinen KH, Campbell KL, et al. Associations among exercise, body weight, and quality of life in a population-based sample of endometrial cancer survivors. Gynecol Oncol. 2005;97(2):422–430. doi: 10.1016/j.ygyno.2005.01.007.
- Koutoukidis DA, Knobf MT, Lanceley A. Obesity, diet, physical activity, and health-related quality of life in endometrial cancer survivors. Nutr Rev. 2015;73(6):399–408. doi: 10.1093/nutrit/nuu063.
- Anzuini F, Battistella A, Izzotti A. Physical activity and cancer prevention: a review of current evidence and biological mechanisms. J Prev Med Hyg. 2011;52(4):174–180.
- Hills AP, Shultz SP, Soares MJ, et al. Resistance training for obese, type 2 diabetic adults: a review of the evidence. Obes Rev. 2010;11(10):740–749. doi: 10.1111/j.1467-789X.2009.00692.x.
- Maqbali M, Hughes C, Dunwoody L, et al. Exercise interventions to manage fatigue in women with gynecologic cancer: a systematic review. Oncol Nurs Forum. 2019;46(1):71–82.
- Zhang X, Haggerty AF, Brown JC, et al. The prescription or proscription of exercise in endometrial cancer care. Gynecol Oncol. 2015;139(1):155–159. doi: 10.1016/j.ygyno.2015.08.007.

![Figure 2. FACIT-F Forest Plot [above zero favours RT; below zero favours control].](/cms/asset/c7732972-4e22-43c8-a811-0747ffbbb0b8/idre_a_2226408_f0002_b.jpg)

![Figure 4. FACIT-F Sensitivity analysis [above zero favours RT; below zero favours control].](/cms/asset/5b98d286-086c-4e42-9bc6-e9bcfb594c60/idre_a_2226408_f0004_b.jpg)
![Figure 5. PFS-R Forest Plot [below zero favours RT; above zero favours control].](/cms/asset/52b41cf4-ec09-40ff-9dea-28c63646001c/idre_a_2226408_f0005_b.jpg)
![Figure 7. PFS-R Sensitivity analysis [below zero favours RT; above zero favours control].](/cms/asset/f54a2120-c562-4254-8084-f5c0f2b77523/idre_a_2226408_f0007_b.jpg)