Abstract
Purpose
After a total knee arthroplasty (TKA), ensuring rehabilitation is continued at home is essential for a successful recovery. The aim of this randomized clinical trial (NCT04155957) was to demonstrate the safety and efficacy of an interactive telerehabilitation system (ReHub®) to guide and provide feedback during exercise in the postoperative period of a fast-track TKA program.
Methods
Fifty-two patients who underwent TKA were randomized to intervention (N = 26) or control (N = 26). Upon discharge, they followed a 4-week plan of 5 daily exercises and up to 10 physiotherapy home visits. The intervention group performed exercises with ReHub® autonomously, control did not use any auxiliary device. Data were collected 1) on the day of discharge, 2) after 2 weeks and 3) after 4 weeks.
Results
Telerehabilitation patients showed higher adherence to exercise (p = 0.002) and greater quadriceps strength (p = 0.028). No significant differences between groups were found in other outcomes. Only 1 adverse event was linked to ReHub®. Patients gave the platform high System Usability Scale scores (83/100).
Conclusion
Interactive telerehabilitation with ReHub® during a post-TKA exercise program is effective, safe, and well-received by patients. It provides real-time performance feedback and ensures communication. Quadriceps strength and adherence to the exercise plan are improved with ReHub®.
IMPLICATIONS FOR REHABILITATION
Telerehabilitation platforms can be introduced into fast-track total knee arthroplasty protocols to monitor home-based exercise programmes without compromising efficacy and safety.
Telerehabilitation and remote patient monitoring contribute to obtaining high levels of adherence to exercise plans, which is a current challenge faced by rehabilitation professionals.
Real-time biofeedback on exercise performance facilitates correct exercise performance and motivates the patient.
This technology allows professionals to monitor and adjust the patient’s therapy remotely and avoid unnecessary travel.
Introduction
The knee is the joint most commonly affected by osteoarthritis (OA), accounting for 17% of cases among people aged 50–75 years [Citation1]. In its end-stage, total knee arthroplasty (TKA) may be indicated, which aims to reduce pain and improve function, thereby increasing the patient’s quality of life [Citation2].
TKA indications have been continuously increasing during the last years all over the world [Citation3–5]. The ageing population, the increasing functional demands of elderly people, and the good clinical outcomes of TKA interventions support the idea that these indications will continue growing. Projections in Germany [Citation6] expect a 55% increase in the TKA incidence rate from 2016 to 2040, while the United States is expected to see a 69% increase from 2015 to 2050 [Citation7].
To face these forecasts, sustainable and cost-effective solutions for hospitalization and rehabilitation are required. Fast-Track strategies have demonstrated their efficacy and efficiency during the last ten years, changing the standard surgery management procedure of TKA in a great number of hospitals [Citation8–10]. These strategies are focused on achieving early mobilisation and reducing pain and postoperative complications [Citation10], thereby reducing the length of stay (LOS) and associated hospital costs [Citation11]. Once the patient is discharged, continuing the rehabilitation process is crucial to optimise functional knee outcomes and patient quality of life and avoid overcrowding healthcare with unnecessary outpatient visits [Citation2,Citation12,Citation13].
The post-discharge strategy in several hospitals is to combine visits to the patient’s home by physiotherapists with a daily exercise plan to be followed by the patient in an unsupervised manner. However, a reported low compliance rate with such guidelines seems to be a major barrier to a successful recovery [Citation14]. To monitor patients’ activities, rehabilitation professionals only rely on the information provided by the patients themselves and/or their families. This makes them unaware of the patient’s adherence to the guidelines, which may significantly delay any necessary intervention and have an impact on the patient’s recovery.
Within a context of growing indications, more affordable technologies and more technically literate patients, telerehabilitation—remotely monitored home-based rehabilitation—can play a very important role in improving patient performance and adherence to postoperative physiotherapy programs in a cost containment environment. Telerehabilitation provides patients with a guide to performing their personalized rehabilitation exercises correctly in terms of timing, quantity and quality. At the same time, it allows professionals to remotely monitor their progress, which encourages patients to increase their adherence to the treatment, thereby reducing healthcare costs [Citation15]. These technological solutions also empower patients and facilitate their self-management to achieve their goals [Citation16]. In terms of patient experience, studies show that telerehabilitation is well received by patients [Citation17]. However, a more in-depth study of the use of telerehabilitation in Fast Track program contexts is needed.
The main objective of this study is to demonstrate the safety and efficacy of an interactive telerehabilitation system for guiding and giving real-time feedback to patients during rehabilitation exercises in the postoperative period of a fast-track TKA. For comparison, a group followed the same type of program without a telerehabilitation device, autonomously.
Methods
Study design
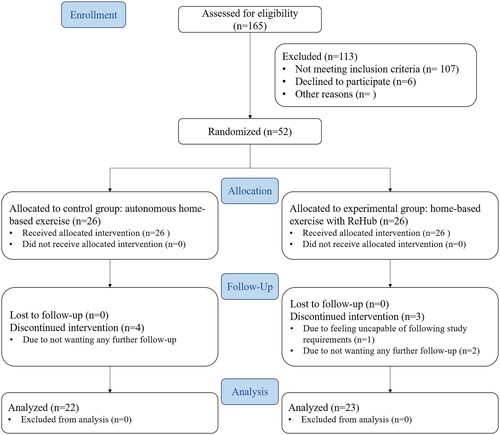
This study was a prospective parallel-group randomized controlled trial. The CONSORT diagram of the study design is shown in . It was carried out by researchers at Hospital Clínic of Barcelona, Spain. The study conformed to the guidelines of the Declaration of Helsinki, was approved by the hospital’s ethics committee (“Comité Ético de Investigación Clínica del HCB,” reg. HCB/2019/0571), and was registered at the ClinicalTrials.gov website (identifier NCT04155957).
Figure 1. CONSORT diagram of the REHAPT trial.
Participants
All patients admitted to a primary TKA, without exception, are systematically allocated on a Fast-Track protocol that includes prehabilitation, surgery under spine and local intraarticular anaesthesia (LIA) and early mobilization a few hours after the surgery. During the prehabilitation phase, patients are taught about the physiotherapy exercises they should do in the preoperative and immediate postoperative periods. Patients were screened for eligibility in the perioperative period. Subjects were included if they met the inclusion and exclusion criteria (). A total of fifty-two patients (n = 52) were recruited between October 2019 and December 2020.
Table 1. Inclusion and exclusion criteria for patient recruitment.
Randomization and blinding
Following informed consent, patients were randomized with a 1:1 allocation ratio to the control group (CTL) or the telerehabilitation group (TRH). An investigator who did not carry out recruitment or assessment duties generated a randomization list with an Internet-based randomization tool (www.randomization.com) and kept it secret from other investigators. The allocation of newly recruited patients to one of the two groups was disclosed sequentially upon notification.
Due to the nature of the telerehabilitation intervention, it was not possible to blind the patients as to the intervention they were receiving. The investigator physicians and physiotherapists were not blinded either because they had access to a dashboard to supervise the frequency and quality of exercise done with ReHub® by TRH. The outcome assessors were the only ones blinded.
Interventions
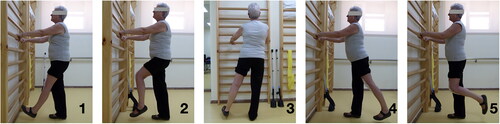
Patients in both groups were discharged approximately 48 hours after the TKA and were given a leaflet detailing a program of 5 exercises, each focusing on the isotonic contraction of a different lower limb muscle: quadriceps, iliopsoas, gluteus medius, gluteus maximus, and hamstring (). The patient was expected to complete 4 sets of 10 repetitions of each exercise at home daily, with an approximate time interval of 2 hours between sets. Each repetition consisted in a 6 second contraction phase and a 3 second rest phase. The physiotherapist made sure that all patients understood the exercises and would perform them correctly. The exercise program was kept unchanged for the whole duration of the study for each patient. Additionally, the leaflet also provided instructions to carry out active knee kinesiotherapy with a rolling board called Flexet.
Figure 2. Exercise plan contained in the leaflet provided to patients. Each exercise focuses on the isotonic contraction of 1) quadriceps, 2) iliopsoas, 3) gluteus medius, 4) gluteus maximus, and 5) hamstring.
TRH used the telerehabilitation system ReHub® ( and ) to perform their exercises, receive real-time feedback and automatically record their adherence to the program, while CTL did not have any auxiliary devices and manually recorded their adherence in a diary. Starting 12–15 days after discharge, both groups received 2–3 domiciliary visits per week by an external physiotherapist for the standard home treatment protocol, up to a total of 10 non-consecutive visits.
Figure 3. Patient performing one of the exercises in the daily program with the help of ReHub®.

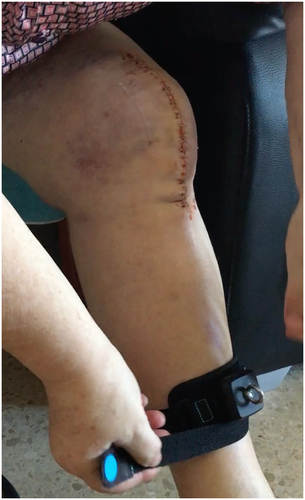
Figure 4. Patient wearing the ReHub® sensor on the shin to prepare for another exercise.
TRH patients received a home training session by a site team physiotherapist and a ReHub® technician 24h after hospital discharge. The site team physiotherapist repeated the visit on the following day and after 2 weeks for a quick check-up on the use of the system. In special cases where the patient required additional training, another visit could be arranged. The TRH patients’ adherence to the program was asynchronously monitored daily through the platform by site team physiotherapists, who could communicate with patients using the platform to talk about their difficulties, solve doubts or provide encouragement.
The rehabilitation program had a duration of 4 weeks (30 days) after hospital discharge. Outcome assessment was performed by trained blinded nurses just before discharge (baseline), and at 2 and 4 weeks after discharge.
The telerehabilitation system
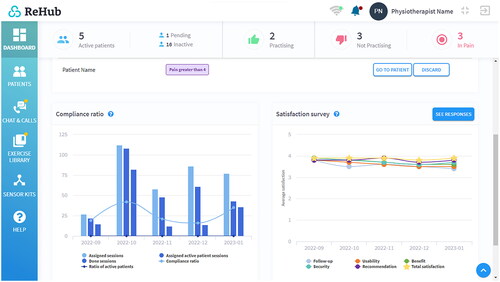
The telerehabilitation system used, ReHub® (DyCare, Barcelona, Spain) was composed of two main parts: a web platform and an inertial motion sensor (IMU) that incorporated an accelerometer, a gyroscope, a magnetometer and, as a unique feature among these types of sensors, also a strain gauge to measure forces exerted by resistance bands attached to the sensor. The sensor could be strapped around a body segment and calculate orientation from a reference position with a ± 6° precision. The platform () allowed physiotherapists to create a rehabilitation exercise program specifically tailored to each patient’s condition. In this case, all patients had to perform the same 5 exercises, but the range and speed of motion were adjusted for each patient.
Figure 5. Telerehabilitation system (ReHub®), professional dashboard.
Patients used the platform to receive visual and written instructions on where they should wear the sensor to capture motion data correctly and how to perform the exercise. Then, the user interface represented the patient’s movement with a moving ball that could go along a curved or straight path, depending on the type of motion it was meant to represent. A visual cue moving along the same path, coupled with an auditory cue, marked the pace at which the patient was asked to perform the exercise. ReHub®’s algorithms delivered real-time biofeedback through an animated talking coach to suggest corrections and motivate the patient.
Outcome measures
Range of Motion
The primary outcome measure was the change from baseline in the active Range of Motion (ROM) of the operated knee in flexion (AFROM). ROM is one of the main indicators of success after a TKA [Citation18] and is directly related to a person’s ability to carry out functional activities in their daily life normally [Citation19]. Secondary outcomes also included other ROM measures: the active ROM in extension (AEROM), the passive ROM in flexion (PFROM) and the passive ROM in extension (PEROM). All ROM tests were conducted using a manual, plastic, 2-arm goniometer with 1-degree increments, centred on the knee joint. The distal reference used was the peroneal malleolus and the proximal reference point was the greater trochanter in the hip. To make sure that the goniometer was centred on the knee axis point, patients were asked to bend and extend the knee a couple of times. For AFROM, patients had to sit in a chair and were asked to bend their knee as much as possible. For AEROM, patients had to be in the supine position on a bed with legs stretched out and were asked to push their knee against the bed as much as possible, causing the extension of the knee. For calculation purposes, full knee extension was defined as 0° of flexion and lack of extension was represented by negative values. The passive counterparts of these outcomes were carried out in the same way, but an assistant helped the patients bend or extend until the patient declared they had reached their maximum point.
Walking ability and maximal isometric strength
Additional secondary outcomes were measured: the change from baseline in the Timed Up-and-Go test (TUG), the maximal isometric quadriceps strength of the operated leg (QS) and the maximal isometric hamstring strength of the same leg (HS). For TUG, the assessor used a chronometer to measure the time the patient needed for standing up from a chair, walking straight for 3 meters, turning, walking back to the chair, and sitting down [Citation20]. To obtain QS and HS, participants were placed in a sitting position with their feet on the floor and with the hip at 90° of flexion and were asked to extend or bend the knee (for QS and HS, respectively) with as much strength as possible against the handheld dynamometer (Lafayette Manual Muscle Tester) held by the assessor [Citation21].
Patient-reported outcome measures
Another set of secondary outcomes was related to the patient-reported quality of life: the change from baseline in pain level (Pain), the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and the EuroQol-5D-5L scores. To assess the pain level, the patient was asked to rate the pain they felt at that moment on a Visual Analogue Scale ranging from 0 to 10 (0 being "no pain" and 10 being the "worst pain imaginable") [Citation22]. WOMAC is a self-administered questionnaire consisting of 24 items divided into 3 subscales: 5 items for pain, 2 for stiffness and 17 for physical function [Citation23,Citation24]. EuroQol-5D-5L [Citation25] is a self-administered evaluation tool that consists of a questionnaire (EQ5-Q) with 5 items (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) and a visual analogue scale in which the patient self-rates their health (EQ5-VAS).
Adherence to the exercise program
The adherence to the home exercise program was measured by obtaining the percentage of exercises done from each group’s corresponding source: the ReHub® database automatically generated by TRH patients and the exercise diary manually filled by CTL patients.
Usability, satisfaction and safety
The usability of ReHub® and satisfaction with the system perceived by TRH patients was collected at the 4-week outcome assessment with the System Usability Scale [Citation26], a self-administered Likert scale with 10 questions.
Safety was evaluated based on the frequency, severity and causality of adverse events reported during the study (e.g., cutaneous injuries, incidents on soft body parts, software failures). The severity could be labelled as light, medium or severe.
Statistical analysis
Sample size estimation was performed considering the active knee flexion Range of Motion as the primary outcome measure based on a previous clinical trial [Citation27]. Considering a power of 80%, a two-sided significance level of 0.05 and a dropout rate of 10%, it was estimated that a sample size of 52 patients would be needed to detect a 10° difference between the two groups. A standard deviation of 12° was determined according to existing literature [Citation27,Citation28].
The homogeneity of the clinical and demographic characteristics of the two study groups at baseline was investigated with a one-way ANOVA in the case of continuous variables and a chi-squared test in the case of categorical variables. The main effects of the group on the change of the clinical outcomes from the baseline to the final visit were investigated with a two-way ANOVA; the interaction effects were not investigated. The threshold for statistical significance was set to p = 0.05. Before conducting the two-way ANOVA, the homogeneity, homoscedasticity and normality of the data was tested with Bartlett’s test, Levene’s test and the Shapiro-Wilk test respectively.
Calculations were performed using MATLAB data analysis software (The MathWorks, Inc., Natick, Massachusetts, United States).
Results
A total of 165 patients were assessed for eligibility during the study period. 107 of them did not meet the inclusion criteria, the main reason being residency outside of the hospital’s area of influence, and 6 patients declined to join the study. 52 patients were randomized into the two groups with a 1:1 ratio (26 patients each).
During the study, 7 participants withdrew consent to continue, 3 in TRH (11.54% dropout rate) and 4 in CTL (15.38% dropout rate). 6 participants (2 TRH, 4 CTL) stated they did not want to attend further follow-up visits, and 1 TRH participant dropped out because they did not feel capable of following the study requirements. These participants were excluded from the analysis. A total of 45 patients who complied with the protocol (23 TRH, 22 CTL) were analysed. Due to the state-mandated lockdown due to the COVID-19 pandemic, the last visit of 9 of these 45 patients (4 TRH, 5 CTL) was conducted via telephone, where only Patient-Reported Outcomes were collected.
Study population characteristics
There were no differences at baseline between the two groups in demographic variables with potential impact on outcomes (). The mean age of the participants was 68.26 ± 5.42 (TRH) and 68.82 ± 4.41 (CTL).
Table 2. Demographics, hospital stay information and outcomes at baseline of experimental and control groups.
Clinical characteristics at baseline also showed no significant differences except for one variable (9.09%), the self-perceived health status as indicated by the EQ5-VAS (TRH: 68.30 ± 16.63, CTL: 57.18 ± 18.6, p = 0.044).
Outcome measures
The evaluation of all outcome measures at the three different time points can be found in .
Table 3. Evaluation of outcome measures at different time points.
Range of motion
No significant differences in active flexion ROM (the primary outcome measure) or passive flexion ROM were found between the groups (p = 0.535; 0.680). In both groups, ROM gains achieved during the 4-week period put the final ROMs over 100° (actively, TRH: 105.22 ± 8.46°, CTL: 102.27° ± 13.16°; passively, TRH: 106.09 ± 8.78°, CTL: 105.23 ± 10.63°).
The changes found in active and passive extension ROM were smaller, especially passively, and the differences found between the groups were not statistically significant either (p = 0.240; 0.424).
Walking ability and maximal isometric strength
Significant reductions in the time to complete the Timed Up-and-Go Test were observed in both groups after 4 weeks (TRH: −30.56 ± 13.16 s; CTL: −36.37 ± 23.15 s), but no significant differences were found between the groups (p = 0.523).
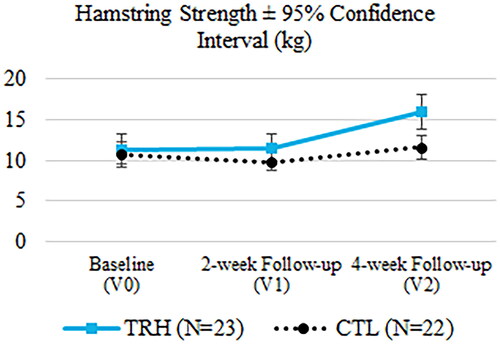
In the case of maximal isometric quadriceps strength, significant differences were found in the change from baseline to the last visit in favour of TRH (TRH: 6.73 ± 6.62 kg, CTL: 1.67 ± 5.43 kg, p = 0.028). The gain in maximal isometric hamstring strength was also notable in TRH relative to CTL, but the difference was not statistically different enough to reach the 0.05 p-value threshold (TRH: 4.69 ± 6.18 kg, CTL: 0.91 ± 4.92 kg, p = 0.081). The evolution of the mean quadriceps strength and mean hamstring strength over time can be observed in and .
Figure 6. Evaluation of the quadriceps strength at the three time points.
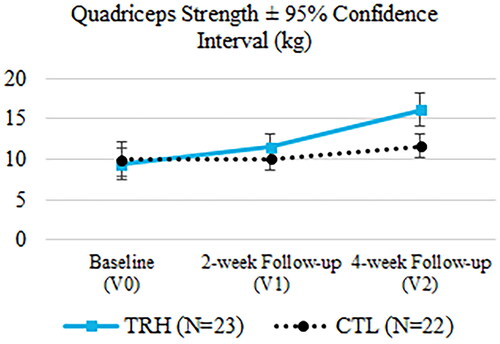
Figure 7. Evaluation of the hamstring strength at the three time points.
Patient-reported outcome measures
Though displaced by 1 point in the 11-point scale between each other, the pain VAS patterns observed are very similar for both groups: pain reduced by half after 2 weeks and then slightly increased after 4 weeks, which resulted in an overall reduction of roughly 1.5 points (TRH: −1.45 ± 1.6; −1.5 ± 2.15). It is likely for this reason that the ANOVA yields a particularly high p-value (p = 0.971).
WOMAC scores improved substantially at 4 weeks in both groups (TRH: −26.9 ± 17.17; CTL: −22.27 ± 13.82), but the differences between them were not significant (p = 0.647). In the TRH group, one sample was discarded due to missing data at baseline.
Regarding the EQ-5D-5L questionnaire, again both groups improved similarly (TRH: 0.22 ± 0.12; CTL: 0.23 ± 0.21) with no significant statistical differences (p = 0.664). Two samples from the TRH group and one sample from the CTL group were discarded due to a lack of baseline responses. In the VAS part of EQ-5D-5L, greater but still not statistically different improvements were found in CTL (HRT: 10.17 ± 11.92; CTL: 18.5 ± 15.05; p = 0.396), likely due to the superior health state perceived by TRH at baseline.
Adherence
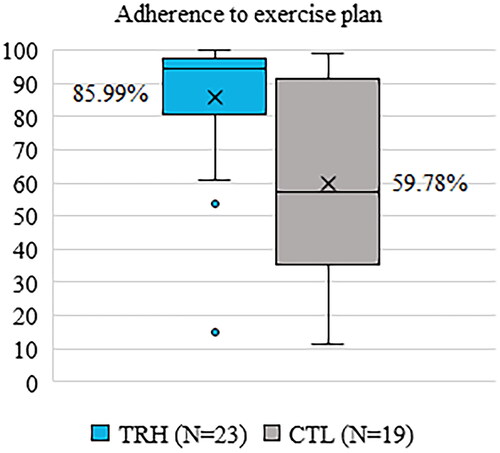
The adherence rate in TRH was significantly higher than in CTL (TRH: 85.99 ± 19.48%, CTL: 59.78 ± 29.78%, p = 0.002). Boxplots for each group can be observed in .
Figure 8. Adherence to the exercise plan.
Usability and satisfaction
The mean satisfaction and usability score indicated with the System Usability Scale by TRH patients was 82.83 ± 15.68 out of 100. The most frequent scores belonged to the Grade A category (>80.3), representing 69.56% (16/23) of the respondents, followed by Grade B (68–80.3, 21.74%, 5/23), Grade D (51–68, 4.35%, 1/23) and Grade F (<51, 4.35%, 1/23). Of the 10 statements in the questionnaire, the responses patients were most likely to agree or disagree with were "I felt confident using the system" (Strongly Agree, 9.46/10), "I thought the system was easy to use" (Strongly Agree, 9.13/10) and "I found the system very cumbersome to use" (Strongly Disagree, 1.09/10).
Safety
A total of 6 adverse events were reported during the study, 3 in the control group and 3 in the experimental group, all of them occurring in different patients. The investigators only found a link between ReHub® and one adverse event, a medium-severity functional overload with pain and swelling. Two TRH participants, including the one that experienced this adverse event, abandoned the study after the onset of their respective adverse events.
Discussion
To our knowledge, this is the first prospective study on the effect of an interactive telerehabilitation system for guiding and providing patients with real-time feedback on their exercise performance during a post-TKA exercise plan within a Fast-Track protocol and asynchronously supervising them. Our results demonstrate that the investigated telerehabilitation system, ReHub®, is safe and effective in reducing patient disability, thus confirming our main hypothesis. The data also show the device is easy to use and is well accepted by a standard TKA patient population.
Because patient discharge in a Fast-Track protocol occurs approximately 48–72h after surgery, most of the functional recovery period takes place at the patients’ home. While there is currently a lack of consensus on the most effective post-TKA physiotherapy strategies in terms of mode, frequency, intensity and duration of exercise [Citation29–33], there are studies linking strengthening [Citation34, Citation35] and mobility exercises with improved functional performance and quality of life [Citation36, Citation37]. The exercise plan used in this study belonged to the Fast-Track protocol established for TKA in this hospital. This plan aims to strengthen the muscle groups that the surgery procedure weakens and that are essential to regain a good gait pattern and function of the lower extremity—the quadriceps, the hamstrings, the psoas and the hip abductors [Citation38–41]—, as recommended by other authors in literature [Citation34, Citation42–44].
After 4 weeks of treatment, TRH showed a greater quadriceps strength relative to the baseline compared to CTL patients (p = 0.028). Mizner et al. concluded in their study that quadriceps strength was more related to functional outcomes than knee flexion ROM and can be reduced by 30.7% immediately after TKA [Citation42]. The improvement in quadriceps strength found in this study illustrates the promising effect of ReHub® in these patients. Furthermore, although the requirement to reject the null hypothesis was not met, it is noteworthy that hamstring strength was the second outcome measure with the largest statistical differences between groups, in favour of TRH (p = 0.081). The hamstrings are often overlooked in the TKA recovery process, resulting in a significant residual functional deficit [Citation34], so this result needs to be highlighted.
Given that patients in both groups received the same physiotherapy exercise instructions, type and frequency, the superior muscle strength found in the TRH group of this study could be attributable to greater adherence to the exercise plan. The use of ReHub® is related to increased adherence, as evidenced by the higher rate of adherence to the exercise plan observed among platform users (TRH: 85.99 ± 19.48%, CTL: 59.78 ± 29.78%, p = 0.002). The reason for this increase could be rooted in the positive effect on adherence that supervised physiotherapy has over unsupervised physiotherapy [Citation45–47]: it is possible that patients felt motivated to do exercise with ReHub® knowing that a physiotherapist would review the results of the exercises, even asynchronously, and with whom he/she could communicate via chat. Another possible cause of the observed greater muscle strength is improved exercise execution. ReHub® provides feedback to patients through a virtual coach for each repetition performed, which is intended to help the patient perform the exercises according to the reference set by the therapist.
Regardless of the nature of the cause for the superior muscle strength seen among TRH patients, it is unclear why similar improvements are not replicated in other outcome measures (TUG, other ROMs, pain VAS, WOMAC, EQ-Q and EQ-VAS). A possible explanation to this phenomenon is that the brief follow-up period prevented us from observing improvements in clinical outcomes that would be observed several weeks after following the program with that level of adherence. Another study with a longer follow-up period would help us shed some light into the matter. Despite the lack of significant differences between TRH and CTL in outcomes other than muscle strength, it should be remarked that all patients experienced a statistically significant improvement at 4 weeks from baseline, on average. This, in practice, translates into functional and quality-of-life improvements.
It is difficult to compare our findings on functional outcomes and PROMs with those of other published studies, as most of their experimental interventions replace face-to-face home-based physiotherapy (HBPT) sessions with telerehabilitation sessions [Citation48–54]. However, in our study, we introduced the platform within a well-established Fast-Track program that we wanted to change as little as possible because of its history of good results. Both TRH and CTL were instructed to do exercise in the preoperative period and the immediate postoperative period and were scheduled for HBPT, which started approximately two weeks after surgery. In the period between hospital discharge and the start of HBPT, TRH had the platform and CTL only could follow the exercise plan conventionally. Both groups were recommended to continue with the autonomous exercise regimen once HBPT had begun, with TRH having the platform as an auxiliary device.
The most similar published study in this regard is that of Eichler et al. 2019, in which both groups were offered a face-to-face physiotherapy program (though optional, unlike in our study) and the experimental group included telephone therapy sessions with a platform. In this case, the Eichler control group was not instructed to exercise autonomously. This point is a distinguishing feature of our study: to the best of our knowledge, we are the only ones to have monitored the adherence of all patients, experimental and control, to an autonomous exercise program. In the other studies, either the control group did not perform autonomous exercises or the authors did not consider it relevant to mention this aspect for their publication. The percentage of use of the platform by Eichler’s telerehabilitation group during the first 4 weeks remains slightly above 80% [Citation55], similar to the adherence rate of TRH in our study, 85.99 ± 19.48% over the 4 weeks. This confirms the ability of telerehabilitation systems to motivate patients to exercise.
One aspect to consider is the fact that, when marking sessions as completed, CTL patients may have marked extra sessions to get more recognition from the hospital team. As it was not possible to make this measure objective, it could be masking the real adherence rate of patients following unsupervised rehabilitation exercises without a telerehabilitation system. For reference, in the Eichler et al. study, the telerehabilitation group was asked to complete an exercise diary, in which they reported higher activity than the activity recorded on the platform. However, we concluded that it was necessary to include the patient diary in the CTL group to make a comparison possible.
Functional outcomes are difficult to compare due to differences in study design. The Eichler 2019 study [Citation55] comprises 12 weeks of treatment, and the only functional outcomes were collected after discharge and at the end of week 12. The only overlapping outcome with our study is the Timed Up-and-Go Test, for which no intergroup differences were found in their study or ours [Citation55]. In the results collected by Piqueras et al. [Citation48], after 10 days of treatment with telerehabilitation, there was a significant difference in quadriceps strength between the two groups that favours the telerehabilitation group (1.64 kg vs. 2.98 kg, p = 0.011), similar to our study. It should be noted that, in this case, telerehabilitation did replace traditional physiotherapy. Piqueras also observed significant results in active knee extension, something that has not been replicated in this study.
Regarding satisfaction with the telerehabilitation system, TRH patients expressed high satisfaction with the platform, indicating that they would like to use this rehabilitation method again in the future and would recommend it to their acquaintances. Although other studies in the literature use methods other than the System Usability Scale to investigate satisfaction with their corresponding telerehabilitation systems, we can consider that the high satisfaction obtained in our study is in line with that of similar systems (91.30% of the respondents, 21 patients from the TRH group, reported scores of "Good" or "Excellent"). The high levels of satisfaction and usability of the system are particularly interesting considering the average age of the patients in the study, which initially suggested that they might have difficulties with technology. These results, together with the improved adherence of patients in the telerehabilitation group, are very promising for the implementation of telerehabilitation of older patients in other programs.
We must consider some limitations concerning our study. For obvious reasons, patients were not blinded to the treatment due to the nature of the intervention, but it should be noted that the evaluators and the data analyst were blinded to the protocol received by the patient. Another limitation is the length of the study, which was limited to four weeks for operational reasons. A longer follow-up could shed light on whether the practice of therapeutic exercise using the ReHub® platform produces long-term clinical benefits that are not observable in our study period. But undoubtedly, the biggest unanticipated limitation that occurred during the study was the onset of the COVID-19 pandemic, which affected two major aspects of our study. On the one hand, the hospital’s elective surgical activity was abruptly halted, which greatly impacted the speed of recruitment, and on the other hand, the ability to interact with patients already enrolled in the study was impaired due to physical limitations in conducting control visits. This situation delayed the study and caused some patients to drop out of the study due to their reluctance to visit the medical centre or to contact the investigators in such a situation. The visits of already recruited patients that could not be made in person were partially replaced by telephone visits. Nevertheless, this unusual circumstance gave us an extra opportunity to appreciate the value of TRH in monitoring patient activity and interacting with patients. Due to the inability to collect the borrowed ReHub® device from patients because of the lockdown and the impossibility of receiving face-to-face physiotherapy, several of the patients requested to continue the use of the platform beyond the four weeks foreseen by the study.
As a counterpoint, a great strength of the study is that patients in both groups received the same physiotherapy exercise instructions and guidelines in terms of type, dose and intensity, whereas the exercise programs of other studies in the literature are much more variable. This unique feature of our study makes comparisons between groups more robust.
The results observed in the present study are encouraging for the role of telerehabilitation platforms in recovery processes. The fact that TKA patients can receive this type of intervention opens the door for telerehabilitation to be used in more indications and thus for more patients to benefit from the associated improvements. The use of a virtual coach to provide real-time feedback encourages patients to do their exercises autonomously and makes the technology more user-friendly, thus improving patient involvement, who feel a direct reaction to their movement when they receive real-time feedback. This stimulates the patient to reach their goals independently—without losing the possibility of contact with the healthcare professionals—and makes the patient deeply involved in their recovery. In this way, patient empowerment and self-management of their recovery are greatly enhanced. It should be noted that the use of the sensor has not posed any physical difficulties for patients, including patients with very high BMI and/or advanced age. For rehabilitation professionals, a platform such as ReHub® provides valuable information on the patient’s recovery at home after discharge from the hospital, in particular on their adherence to the therapeutic exercise program and the evolution of functional parameters such as range of motion. This facilitates a timely follow-up of the patient’s recovery, acting when the patient needs it most. This bilateral feedback could have benefits for both therapists and patients: improved therapeutic adherence could decrease complications after surgery [Citation56], while the possibility of targeting patients with the greatest difficulties could increase the operational capacity of each professional and lead to financial savings. Additionally, easier access to data analysis would increase the potential for scientific investigation of rehabilitation processes.
In conclusion, our results indicate that telerehabilitation with ReHub® is a safe and effective system for monitoring exercise programs in post-TKA patients within a Fast-Track program after hospital discharge. Therapeutic adherence to the exercise program and quadriceps strength improved more in the telerehabilitation group and patients were highly satisfied with the usability of the system. In light of our results, we can consider a future modification of the face-to-face home program to increase the presence of telerehabilitation, which would optimise follow-up and patient interaction.
Acknowledgements
The authors thank all members of the investigation team and the patients that have made the study possible.
Disclosure statement
RJ and MC have a shareholder position at Bio-Sensing Solutions S.L. DR is an employee of Bio-Sensing Solutions S.L., but does not have shareholder positions. The rest of the authors declare no competing interest.
Additional information
Funding
References
- Briggs AM, Cross MJ, Hoy DG, et al. Musculoskeletal health conditions represent a global threat to healthy aging: a report for the 2015 world health organization world report on ageing and health. GERONT. 2016;56(Suppl 2): s243–S255. doi: 10.1093/geront/gnw002.
- Akbaba YA, Yeldan I, Guney N, et al. Intensive supervision of rehabilitation programme improves balance and functionality in the short term after bilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2016;24(1):26–33. doi: 10.1007/s00167-014-3179-y.
- Leitner L, Türk S, Heidinger M, et al. Trends and economic impact of hip and knee arthroplasty in Central Europe: findings from the Austrian national database. Sci Rep. 2018;8(1):6–10. doi: 10.1038/s41598-018-23266-w.
- NiemeläInen MJ, MäKelä KT, Robertsson O, et al. Different incidences of knee arthroplasty in the nordic countries: a population-based study from the nordic arthroplasty register association. Acta Orthop. 2017;88(2):173–178. doi: 10.1080/17453674.2016.1275200.
- Spekenbrink-Spooren A, Van Steenbergen LN, Denissen GAW, et al. Higher mid-term revision rates of posterior stabilized compared with cruciate retaining total knee arthroplasties: 133,841 cemented arthroplasties for osteoarthritis in The Netherlands in 2007–2016. Acta Orthop. 2018;89(6):640–645. doi: 10.1080/17453674.2018.1518570.
- Rupp M, Lau E, Kurtz SM, et al. Projections of primary TKA and THA in Germany from 2016 through 2040. Clin Orthop Relat Res. 2020;478(7):1622–1633. doi: 10.1097/CORR.0000000000001214.
- Inacio MCS, Paxton EW, Graves SE, et al. Projected increase in total knee arthroplasty in the United States – an alternative projection model. Osteoarthritis Cartilage. 2017;25(11):1797–1803. doi: 10.1016/j.joca.2017.07.022.
- Ascione F, Braile A, Romano AM, et al. Experience-optimised fast track improves outcomes and decreases complications in total knee arthroplasty. Knee. 2020;27(2):500–508. doi: 10.1016/j.knee.2019.11.002.
- Kehlet H, Thienpont E. Fast-track knee arthroplasty - status and future challenges. Knee [Internet]. 2013;20:S29–S33. doi: 10.1016/S0968-0160(13)70006-1.
- Pujol O, García B, Faura T, et al. Results of a fast-track knee arthroplasty according to the experience of a multidisciplinary team. J Orthop. 2019;16(3):201–205. doi: 10.1016/j.jor.2019.02.020.
- Wainwright TW, Gill M, McDonald DA, et al. Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: enhanced recovery after surgery (ERAS®) society recommendations. Acta Orthop. 2020;91(1):3–19. doi: 10.1080/17453674.2019.1683790.
- Falvey JR, Bade MJ, Forster JE, et al. Home-health-care physical therapy improves early functional recovery of medicare beneficiaries after total knee arthroplasty. J Bone Joint Surg Am. 2018;100(20)Vol. :1728–1734. doi: 10.2106/JBJS.17.01667.
- Kubota M, Kokubo Y, Miyazaki T, et al. Effects of knee extension exercise starting within 4 h after total knee arthroplasty. Eur J Orthop Surg Traumatol. 2022;32(5):803–809. doi: 10.1007/s00590-021-03042-9.
- Groen JW, Stevens M, Kersten RFMR, et al. After total knee arthroplasty, many people are not active enough to maintain their health and fitness: an observational study. J Physiother. 2012;58(2):113–116. doi: 10.1016/S1836-9553(12)70091-7.
- Busso C, Castorina G, Di Monaco M, et al. Effectiveness of a home-based telerehabilitation system in patients after total hip arthroplasty: study protocol of a randomized controlled trial. Trials. 2020;21(1):438. doi: 10.1186/s13063-020-04791-4.
- Spindler H, Leerskov K, Joensson K, et al. Conventional rehabilitation therapy versus telerehabilitation in cardiac patients: a comparison of motivation, psychological distress, and quality of life. Int J Environ Res Public Health. 2019;16:1–15.
- Galea MD. Telemedicine in rehabilitation. Phys Med Rehabil Clin N Am. 2019;30(2):473–483. doi: 10.1016/j.pmr.2018.12.002.
- Ritter MA, Campbell ED. Effect of range of motion on the success of a total knee arthroplasty. J Arthroplasty. 1987;2(2):95–97. doi: 10.1016/s0883-5403(87)80015-3.
- Rowe PJ, Myles CM, Walker C, et al. Knee joint kinematics in gait and other functional activities measured using flexible electrogoniometry: how much knee motion is sufficient for normal daily life? Gait Posture. 2000;12(2):143–155. doi: 10.1016/s0966-6362(00)00060-6.
- Podsiadlo D, Richardson S. The timed “up&go”: a best of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x.
- Mentiplay BF, Perraton LG, Bower KJ, et al. Assessment of lower limb muscle strength and power using Hand-Held and fixed dynamometry : a reliability and validity study. PLoS ONE. 2015;10(10):e0140822. doi: 10.1371/journal.pone.0140822.
- Boeckstyns M, Backer M. Reliability and validity of the evaluation of pain in patients with total knee replacement. Pain. 1989;38(1):29–33. doi: 10.1016/0304-3959(89)90069-9.
- Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840.
- Batlle-Gualda E, Esteve-Vives J, Piera Riera MC, et al. Traducción y adaptación al español del cuestionario WOMAC específico Para artrosis de rodilla y cadera. Rev Española Reumatol [Internet]. 1999;26:0. https://www.elsevier.es/es-revista-revista-espanola-reumatologia-29-articulo-traduccion-adaptacion-al-espanol-del-7745
- Cabasés JM. El EQ-5D como medida de resultados en salud the EQ-5D as a measure of health outcomes. Gac Sanit. 2015;29(6):401–403. doi: 10.1016/j.gaceta.2015.08.007.
- Brooke J. SUS: a “quick and dirty” usability scale. In: Jordan PW, Thomas B, McClelland IL, et al. editors. Usability evaluation in industry. 1st ed. London: Taylor & Francis; 1996. pp. 207–212.
- Mau-Moeller A, Behrens M, Finze S, et al. The effect of continuous passive motion and sling exercise training on clinical and functional outcomes following total knee arthroplasty: a randomized active-controlled clinical study. Health Qual Life Outcomes. 2014;12(1):68. doi: 10.1186/1477-7525-12-68.
- Denis M, Moffet H, Caron F, et al. Effectiveness of continuous passive motion and conventional physical therapy after total knee arthroplasty: a randomized clinical trial. Phys Ther. 2006;86(2):174–185. doi: 10.1093/ptj/86.2.174.
- Dávila Castrodad IM, Recai TM, Abraham MM, et al. Rehabilitation protocols following total knee arthroplasty : a review of study designs and outcome measures. Ann Transl Med. 2019;7(Suppl 7):S255. doi: 10.21037/atm.2019.08.15.
- Hamilton DF, Loth FC, MacDonald DJ, et al. Exploring variation in patient access of post-discharge physiotherapy following total hip and knee arthroplasty under a choice based system in the UK: an observational cohort study. BMJ Open. 2019;9(2):e021614. doi: 10.1136/bmjopen-2018-021614.
- Artz N, Elvers KT, Lowe CM, et al. Effectiveness of physiotherapy exercise following total knee replacement: systematic review and meta-analysis. BMC Musculoskelet Disord. 2015;16:15. doi: 10.1186/s12891-015-0469-6.
- L. Snell D, Hipango J, Sinnott KA, et al. Rehabilitation after total joint replacement: a scoping study. Disabil Rehabil. 2018;40(14):1718–1731. doi: 10.1080/09638288.2017.1300947.
- Bandholm T, Kehlet H. Physiotherapy exercise after fast-track total hip and knee arthroplasty: time for reconsideration? Arch Phys Med Rehabil. 2012;93(7):1292–1294. doi: 10.1016/j.apmr.2012.02.014.
- Oktas B, Vergili O. The effect of intensive exercise program and kinesiotaping following total knee arthroplasty on functional recovery of patients. J Orthop Surg Res. 2018;13(1):7. doi: 10.1186/s13018-018-0924-9.
- Pozzi F, White DK, Snyder-Mackler L, et al. Restoring physical function after knee replacement: a cross sectional comparison of progressive strengthening vs standard physical therapy. Physiother Theory Pract. 2020;36(1):122–133. doi: 10.1080/09593985.2018.1479475.
- Petterson SC, Mizner RL, Stevens JE, et al. Improved function from progressive strengthening interventions after total knee arthroplasty : a randomized clinical trial with an imbedded prospective cohort. Arthritis Rheum. 2009;61(2):174–183. doi: 10.1002/art.24167.
- Minns Lowe CJ, Barker KL, Dewey M, et al. Effectiveness of physiotherapy exercise after knee arthroplasty for osteoarthritis: systematic review and meta-analysis of randomised controlled trials. BMJ. 2007;335(7624):812–815. doi: 10.1136/bmj.39311.460093.BE.
- Piva SR, Teixeira PEP, Almeida GJM, et al. Contribution of hip abductor strength to physical function in patients with total knee arthroplasty. Phys Ther. 2011;91(2):225–233. doi: 10.2522/ptj.20100122.
- Bade MJ, Stevens-Lapsley JE. Early high-intensity rehabilitation following total knee arthroplasty improves outcomes. J Orthop Sports Phys Ther. 2011;41(12):932–941. doi: 10.2519/jospt.2011.3734.
- Coulter CL, Weber JM, Scarvell JM. Group physiotherapy provides similar outcomes for participants after joint replacement surgery as 1-to-1 physiotherapy: a sequential cohort study. Arch Phys Med Rehabil. 2009;90(10):1727–1733. doi: 10.1016/j.apmr.2009.04.019.
- Moffet H, Collet JP, Shapiro SH, et al. Effectiveness of intensive rehabilitation on functional ability and quality of life after first total knee arthroplasty: a single-blind randomized controlled trial. Arch Phys Med Rehabil. 2004;85(4):546–556. doi: 10.1016/j.apmr.2003.08.080.
- Mizner RL, Petterson SC, Stevens JE, et al. Preoperative quadriceps strength predicts functional ability one year after total knee arthroplasty. J Rheumatol. 2005;32:1533–1539.
- Alnahdi AH, Zeni JA, Snyder-Mackler L. Hip abductor strength reliability and association with physical function after unilateral total knee arthroplasty: a cross-sectional study. Phys Ther. 2014;94(8):1154–1162. doi: 10.2522/ptj.20130335.
- Takahashi K, Takahashi HE, Nakadaira H, et al. Different changes of quantity due to aging in the psoas major and quadriceps femoris muscles in women. J Musculoskelet Neuronal Interact. 2006;6:201–205.
- Picha KJ, Howell DM. A model to increase rehabilitation adherence to home exercise programmes in patients with varying levels of self-efficacy. Musculoskeletal Care. 2018;16(1):233–237. doi: 10.1002/msc.1194.
- Pisters MF, Veenhof C, Schellevis FG, et al. Exercise adherence improving long-term patient outcome in patients with osteoarthritis of the hip and/or knee. Arthritis Care Res (Hoboken). 2010;62(8):1087–1094. doi: 10.1002/acr.20182.
- Picorelli AMA, Pereira LSM, Pereira DS, et al. Adherence to exercise programs for older people is influenced by program characteristics and personal factors: a systematic review. J Physiother. 2014;60(3):151–156. doi: 10.1016/j.jphys.2014.06.012.
- Piqueras M, Marco E, Coll M, et al. Effectiveness of an interactive virtual telerehabilitation system in patients after total knee arthroplasty: a randomized controlled trial. J Rehabil Med. 2013;45(4):392–396. doi: 10.2340/16501977-1119.
- Russell TG, Buttrum P, Wootton R, et al. Internet-Based outpatient telerehabilitation for patients following total knee arthroplasty. J Bone Joint Surg Am. 2011;93(2):113–120. doi: 10.2106/JBJS.I.01375.
- Correia FD, Nogueira A, Magalhães I, et al. Home-based rehabilitation with a novel digital bieedback system versus conventional in-person rehabilitation after total knee replacement : a feasibility study. Sci Rep. 2018;8(1):12. doi: 10.1038/s41598-018-29668-0.
- Bettger JP, Green CL, Holmes DN, et al. Effects of virtual exercise rehabilitation in-home therapy compared with traditional care after total knee arthroplasty. VERITAS, a randomized controlled trial. J Bone Joint Surg Am. 2020;102(2):101–109. doi: 10.2106/JBJS.19.00695.
- Bini SA, Mahajan J. Clinical outcomes of remote asynchronous telerehabilitation are equivalent to traditional therapy following total knee arthroplasty : a randomized control study. J Telemed Telecare. 2017;23(2):239–247. doi: 10.1177/1357633X16634518.
- Kuether J, Moore A, Kahan J, et al. Telerehabilitation for total hip and knee arthroplasty patients: a pilot series with high patient satisfaction. Hss J. 2019;15(3):221–225. doi: 10.1007/s11420-019-09715-w.
- Moffet H, Tousignant M, Nadeau S, et al. In-Home telerehabilitation compared with face-to-Face rehabilitation after total knee arthroplasty. J Bone Joint Surg Am. 2015;97(14):1129–1141. doi: 10.2106/JBJS.N.01066.
- Eichler S, Salzwedel A, Rabe S, et al. The effectiveness of telerehabilitation as a supplement to rehabilitation in patients after total knee or hip replacement : randomized controlled trial. JMIR Rehabil Assist Technol. 2019;6(2):e14236. doi: 10.2196/14236.
- Losina E, Katz JN. Total joint replacement outcomes in patients with concomitant Comorbidities-A glass half empty or half full? Arthritis Rheum. 2013;65:157–1159.
