Abstract
Purpose
The aim of this review is to assess the efficacy and safety of using heat and cold therapy for adults with lymphoedema.
Methods
A multi-database search was undertaken. Only studies which included adults with lymphoedema who were treated with heat or cold therapy reporting any outcome were included. Screening, data extraction, and assessment of bias were undertaken by a single reviewer and verified by a second. Due to the substantial heterogeneity, a descriptive synthesis was undertaken.
Results
Eighteen studies were included. All nine studies which assessed the effects of heat-therapy on changes in limb circumference reported a point estimate indicating some reduction from baseline to end of study. Similarly, the five studies evaluating the use of heat-therapy on limb volume demonstrated a reduction in limb volume from baseline to end-of-study. Only four studies reported adverse events of which all were deemed to be minor. Only two studies explored the effects of cold therapy on lymphoedema.
Conclusions
Tentative evidence suggests heat-therapy may have some benefit in treating lymphoedema with minimal side effects. However, further high-quality randomised controlled trials are required, with a particular focus on moderating factors and assessment of adverse events.
This review highlights the potential benefit that heat therapy may have on reducing limb circumference and volume for adults with lymphoedema.
There was no evidence that controlled localised heat therapy was unsafe.
The current evidence-base is at a point where no specific clinical recommendations can be made.
The use of heat therapy should only be applied as part of a methodologically robust study to treat lymphoedema.
Implications for rehabilitation
Introduction
Lymphoedema is a chronic disease that occurs when the lymphatic system fails, resulting in the accumulation of excess fluid in the body’s tissue [Citation1]. Approximately, 140 million to 250 million people worldwide have a form of lymphoedema, although this is considered an underestimate [Citation2,Citation3]. Lymphoedema typically presents as either primary or secondary lymphoedema [Citation1]. Primary lymphedema is a rare inherited or congenital condition that causes a malformation of the lymphatics system, more often because of genetic mutation [Citation1,Citation4]. Secondary lymphoedema is most common and typically results from an injury, or obstruction to the lymphatic system [Citation1,Citation4,Citation5]. The causes of lymphoedema are wide-ranging but are often the result of obstructive lesions (e.g., tumour) within the lymphatic system, infections, and complications of surgical procedures [Citation1]. Globally, the single primary cause of lymphoedema is nematode infection (known as filariasis), which, despite recent advances, is associated with more than 16 million cases worldwide [Citation6].
Once diagnosed and classified, the treatment and management of lymphoedema begins with conservative strategies [Citation7]. Conservative treatments typically involve an intensive phase of treatment known as decongestive lymphatic therapy (DLT) and consists of centripetal massage of the lymphatics, manual lymph drainage (MLD) [Citation8], multi-layer lymphoedema bandaging (MLLB) [Citation9], and the use of pneumatic compression devices followed by a maintenance phase, which supports the patient to self-manage their condition with compression garments, exercise, skin hygiene, and care [Citation10,Citation11]. Surgical approaches involve restoring lymphatic flow to the limb (either by reconstruction of lymphatic channels or by bridging lymphoedematous tissue with normal lymphatics), or removing excess tissue to reduce the limb to a functional size (skin grafting or subcutaneous excision) [Citation12]. For lymphedema treatments to be effective, early diagnosis, treatment, and patient compliance are necessary [Citation13].
Literature and international guidelines suggest that patients should take preventative measures which can help reduce lymphedema symptoms and prevent complications [Citation1,Citation13,Citation14]. Some of these preventative strategies include, avoiding limb constriction, needles, air travel, vigorous exercise, and extreme temperatures [Citation15,Citation16]. Although many of these strategies are evidence based, guidance on the effects of temperature appears less certain given the conflicting evidence on the risks posed [Citation17–19]. Historically, research has recommended that patients avoid prolonged exposure to heat and cold, as it is thought that it may increase blood flow, intensifying lymphatic load [Citation14,Citation15,Citation20]. In contrast, recent evidence shows a beneficial effect, with heat exposure reducing lymphedema [Citation17]. Given the uncertainties, we conducted a systematic review of the evidence to assess the effectiveness of heat and cold therapy for adults with lymphoedema.
Aims
The aim of this review was to assess the efficacy and safety of using heat and cold therapy for adults with lymphoedema.
Design and methods
Prior to commencing this systematic review, a protocol was registered on Prospero (registration number: CRD42022309475). This systematic review has been reported in accordance with Reporting Items for Systematic Reviews and Meta-Analyses [Citation21].
Searches
The following electronic bibliographic databases were searched on 22 March 2023: MEDLINE (Ovid), Embase (Ovid), CINAHL Complete (EBSCOhost), AMED (EBSCOhost), Cochrane Library via Wiley (all databases), and Web of Science (indexes: SCI-EXPANDED; SSCI; AHCI; CPCI-S; CPCI-SSH; ESCI) using the terms identified by the review team (see Appendix 1 for full search strategies for each database). No language or other limits were applied to the searches. Additional snowball sampling of all included studies was undertaken. Duplicate removal was undertaken using EndNote (version 9.3) and then in Rayyan [Citation22].
Study selection
Only uncontrolled before-and-after studies, controlled trials both randomised and nonrandomised were included. Studies had to include adults with primary or secondary lymphoedema defined by the author from any clinical setting. The intervention had to use heat or cold therapy (thermotherapy or cryotherapy) using any modality with no minimum or maximum number of exposures (e.g., full, or partial body cryotherapy, cold water, ice packs, heat packs, hot water, ultrasound, microwave, and laser therapy). Low level laser therapy was excluded as producing heat is not its primary purpose. No comparator was specified (e.g., usual care, placebo, exercise, massage/physiotherapy, MLD, compression bandages/garments, sequential pneumatic compression, etc.). No specified inclusion criteria were set for outcome of included studies.
Data extraction and quality assessment
Abstract and title screening were carried out by a single reviewer using Rayyan (NS and JH). This selection process was piloted with 10% of abstracts and titles being screened by a second reviewer independently (JW). A Kappa score was calculated for this piloted screening process. Substantial agreement (0.61–0.80) was required before continuation. If the agreement level was unable to be achieved, this process was repeated until substantial agreement was achieved.
Full paper screening was carried out by a single reviewer and verified by a second reviewer (NS & JW or JH & OH). Reasons for exclusion were recorded and reported for full paper screening. Data extraction was undertaken by a single reviewer and verified by a second reviewer using a pre-piloted form (NS, JW, or JH). Discrepancies within abstract and title, full paper screening and data extraction were resolved by discussion, if consensus was unable to be achieved arbitration was carried out by a third reviewer (JH and JW).
The data items extracted were: study type, population description, condition, clinical setting, country, age, gender, location of lymphoedema, lymphoedema type, time of diagnosis, intervention duration, temperature, mode of heat or cold, control group description, all outcomes, number of participants and number of adverse events, adverse event type, number of patients as a ratio completed treatment, conflicts of interest and funding.
Quality assessment
Study level quality assessment was undertaken by a single reviewer and verified by a second reviewer (NS, JH, or JW). Discrepancies within the quality assessment were resolved by discussion, if consensus was unable to be achieved, arbitration was carried out by a third reviewer (JH or JW). Depending on the study design, either the methodological index for non-randomized studies (comparative or non-comparative) (MINORS) [Citation23] or the randomised control trial risk of bias in randomized trials (RoB-1) was used [Citation24]. Both tools were selected due to them being deemed to be valid, reliable [Citation23,Citation25] and the review team having experience of using the tools.
Data synthesis
Due to the expected wide variation in study design, interventions, and outcomes, a narrative synthesis approach was used to assess effectiveness and types of adverse events. Study findings were clustered around two broad categories of interventions of heat and cold therapies and sub-sectioned into varying modalities. Due to the wide variation in outcome type, comparison of outcomes and unit of measurement, a “vote counting” method was applied. This approach was used as it was felt that pooling estimates of individual studies may be misleading due to the inherent heterogeneity [Citation24]. For each modality, the number of positive studies for every outcome and the number of studies reporting a statistically significant positive outcome (p ≥ 0.05) were reported. A positive study was classified as an improvement in the outcome being measured from baseline to end of study within the intervention group. For the interpretation of the findings, the number of positive studies was the primary measure. We did not interpret the number of studies demonstrating a statistically significant difference as an indication of degree of improvement, rather that we reported it as an indication of the probability of the improvement occurring by chance within these individual studies. Any comparisons between groups were described using a similar method. Therefore, these findings will answer the question if there is any evidence of efficacy rather than giving a specific estimate of efficacy [Citation26].
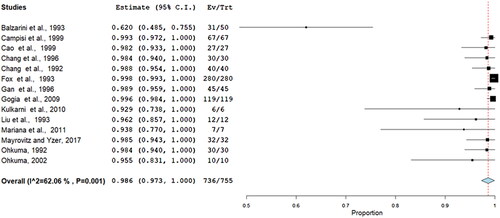
Acceptability of treatment for heat and cold therapies was meta-analysed using a random effects model (DerSimonian–Laird) of the proportion of people who completed the intervention (number of participants reported in the results section) compared to those who started the study (number of participants reported in methods) [Citation27]. Heterogeneity was assessed through visual inspection of forest plots and the I-squared statistic [Citation24]. Meta-analysis was undertaken using OpenMeta [Analyst] [Citation28].
Results
Study identification and characteristics
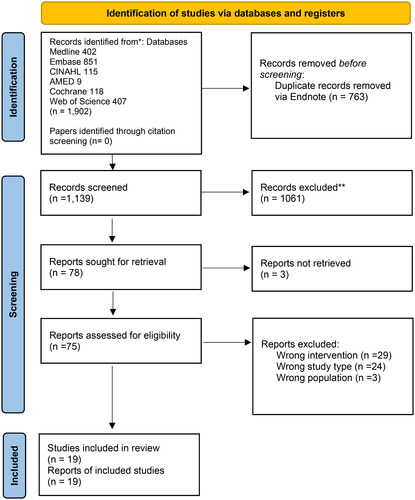
After duplicate removal, 1139 citations were identified (see for full paper screening and reasons for exclusion). A Kappa score of 0.75 (substantial agreement) with 97% agreement was achieved during abstract and title screening between the two reviewers using a 10% sample. After title and abstract screening, 78 full papers were retrieved, of which 18 studies were included (19 citations). Of these 19 included studies, 11 studies used a before-and-after design [Citation18,Citation19,Citation29–37], four used a controlled before-and-after study design [Citation38–41], three were randomised controlled trials (RCTs) [Citation41–43], and one study used a cross-over randomized study design [Citation44]. They were published between 1986 and 2022 [Citation37,Citation43]. These studies took place in various countries, of which seven were from China [Citation30,Citation32,Citation34,Citation36,Citation37,Citation40,Citation41], four from Italy [Citation19,Citation29,Citation31,Citation38], three studies from Japan [Citation35,Citation42,Citation45], two studies from India [Citation18,Citation39], and one study each from Brazil, Egypt, and USA [Citation33,Citation43,Citation44].
Figure 1. PRISMA flow diagram.
Across the 18 included studies, a total of 1137 people with lymphoedema were included (see for study characteristics). The majority of studies included adults with secondary lymphoedema of the legs [Citation35,Citation36,Citation40,Citation41,Citation45], arms [Citation32,Citation33,Citation38,Citation43], or both arms and legs [Citation19,Citation29,Citation30]. Included studies took place in a range of clinical environments of which nine were within a hospital [Citation32,Citation34–37,Citation39–42], six in outpatients [Citation30,Citation31,Citation33,Citation38,Citation43,Citation45], three in a lab [Citation19,Citation29,Citation44], and one at home [Citation18]. Three studies included a mixed adult sample [Citation30,Citation34,Citation37]. Out of the 18 included studies, only two studies assessed the effectiveness of cold therapy on lymphoedema using ethanol–water [Citation33] and cold air therapy [Citation43]. One study aimed to reduce the surface temperature of the skin between 32.4 and 33.8 °C [Citation33]. One study did not report exact skin temperature but stated using cold air at a temperature of −32 °C [Citation43].
Table 1. Study characteristics.
The remaining 17 studies used localised heat therapy provided through 20 modalities, of which five studies used microwave therapy [Citation32,Citation34,Citation37,Citation40,Citation41]; four studies used microwave plus bandaging [Citation30,Citation42]; with two including vibration [Citation40,Citation45]; three studies used hot water [Citation19,Citation29,Citation40]; two studies used light therapy [Citation18,Citation39]; one study used light therapy plus interferential therapy [Citation39]; light therapy plus interferential therapy plus compression [Citation39]; infrared [Citation36]; electric blanket plus mechanical lymph drainage [Citation44]; ultrasound [Citation38] and electromagnetic therapy [Citation31]. The majority of these studies that reported skin temperature set a target tissue temperature of between 39 and 42 °C [Citation19,Citation29,Citation31,Citation34,Citation36,Citation37] and the majority of studies which reported total intervention treatment time provided treatments between 1200 up to 3600 min of treatment [Citation19,Citation34–36,Citation40–42].
Quality assessment
Nine out of 11 before-and-after studies were deemed to be of low quality (overall score less than 13) [Citation18,Citation29–32,Citation34–37] (see and for quality assessment of all included studies). With the three main issues being lack of blinding of endpoints [Citation18,Citation29–32,Citation34,Citation36], prospective calculation of the sample size [Citation18,Citation30,Citation32,Citation34–37] and appropriate follow-up period [Citation18,Citation29,Citation31,Citation34–36]. One out of the four non-randomized controlled trials was judged to be of low quality (less than 16) [Citation45]. With the main issues being a lack of blinding of endpoints [Citation38,Citation39,Citation45], inappropriate control group [Citation19,Citation39,Citation45], and lack of clarity regarding if the control and an intervention group were treated at the same time [Citation19,Citation39,Citation45]. Three out of the four RCTs were judged to be at high risk of bias [Citation35,Citation43,Citation44]. All four RCTs had methodological limitations with randomisation sequence generation, lack of allocation concealment and selective reporting bias due to the lack of protocol registration [Citation41–44]. The majority of issues within the before and after studies, randomised and non-randomised studies were caused by poor reporting standards.
Table 2. Quality assessment using methodological index for non-randomized studies (comparative or non-comparative).
Table 3. Risk of bias of randomised controlled trials.
Microwave therapy
Five studies assessed the effectiveness of microwave therapy, with one RCT (low risk of bias) and non-RCT (quality score (QS): 22/24) comparing microwave therapy against placebo [Citation41] and hot water therapy [Citation40], respectively, and three before-and-after studies (QS: 2, 12, 13/16) [Citation32,Citation34,Citation37]. The one RCT found a statistically significant difference in the proportion of patients, who reported an improvement in feeling of swelling and restricted mobility after the intervention period compared to those who received placebo [Citation41]. There was also a nonsignificant difference in the proportion of patients who reported an improvement in burning pain or feeling heavy, compared to the placebo group. In the non-RCT study, there was a greater improvement in circumference in microwave therapy compared to hot water therapy; however, this was reversed for limb volume [Citation40] (See for full results).
Table 4. Results for effect of heat/cold therapy from baseline to end of study in intervention group.
All five studies that used microwave therapy demonstrated a positive improvement in the intervention group from baseline to end of study for limb circumference [Citation32,Citation34,Citation37,Citation40,Citation41] of which three were statistically significant [Citation32,Citation34,Citation40]. Similarly, all five studies demonstrated a positive improvement in limb volume comparing baseline to end of study [Citation32,Citation34,Citation37,Citation40,Citation41] of which four were statistically significant [Citation32,Citation34,Citation40,Citation41]. Two studies found a statistically significant improvement for both tonometry [Citation32,Citation41] and the number of erysipelas attacks [Citation32,Citation37]. One study found an improvement in subjective reporting of burning pain, feeling of heaviness and a statistically significant improvement in restricted mobility of the affected limb from baseline to end of study [Citation41].
Microwave therapy plus
Four studies assessed the effectiveness of microwave therapy plus additional treatment, with one RCT (high risk of bias) [Citation42] and three before-and-after studies (QS: 11, 10, 12/16) [Citation30,Citation35,Citation45]. One RCT found a statistically significant difference in limb volume between microwave and bandages compared to bandages or microwave therapy alone [Citation42].
When microwave therapy was combined with bandaging, there was a positive improvement in the intervention group in two studies for limb circumference [Citation30] and limb volume [Citation42] from baseline to end of study, of which limb circumference was statistically significantly improved in one study [Citation30]. There was also a statistically significant improvement in tissue tonicity and ABC immunohistochemistry [Citation30].
The two before-and-after studies which used microwave therapy plus vibration plus bandages found a positive improvement for limb circumference [Citation35] and a statistical significant improvement in calcitonin gene-related peptide levels from baseline to end of study [Citation45].
Hot water
Out of the three studies that reported using hot water therapy [Citation19,Citation29,Citation40], only non-RCT (QS: 22/24) and one before and after study (QS: 14/16) reported outcomes [Citation19,Citation40]. Of these two studies, one found a positive improvement [Citation19] and the other study found a statistically significant improvement in limb volume from baseline to end of study in the intervention group [Citation40]. One study also found that at one year, there was no difference between primary, secondary, and acute lymphoedema recovery rates [Citation19].
Light therapy and infrared
The effects of light (heat) therapy were assessed in a non-RCT (QS: 18/24) [Citation39], which compared light therapy with combinations of either light therapy and interferential therapy or light therapy and interferential therapy plus compression, and two before-and-after studies of light therapy (QS: 7/16) [Citation18] or infra-red therapy, respectively (QS: 6/16) [Citation36]. Of these studies, the non-RCT found positive improvement in limb circumference for light therapy plus interferential therapy and combination (light therapy + interferential therapy + compression). When the interventions were compared, there was a statistically significant improvement in total limb circumference reduction at end of study in the compression group (bandage compression) compared to the light therapy group and the combination group [Citation39]. There was a greater improvement in total limb circumference in the light therapy group compared to combination and light therapy plus interferential therapy group [Citation39].
Two out of two studies found a positive improvement in limb circumference for light therapy alone from baseline to end of study in the intervention group [Citation18,Citation39]. Only the before-and-after study used infrared therapy and found a statistically significant improvement in patient subjective reporting of tightness, heaviness, solidity, pain, discomfort, numbness, quality of life, and frequency of lymphedema accompanied with dermatolymphangioadenitis from baseline to end of study [Citation36].
Electromagnetic resonance, electric blanket, and ultrasound
One out of one randomised crossover study found a positive improvement for electric blanket heat treatment plus mechanical lymph drainage for limb volume comparing baseline to end of study (high risk of bias) [Citation44]. However, there was no statistically significant improvement in limb volume when treated with electric blanket heat treatment plus mechanical lymph drainage compared to mechanical lymph drainage alone [Citation44].
One out of one non-randomised control study found an improvement in limb volume and subjective assessment of firmness comparing baseline to end of study (QS: 16/24) [Citation38]. There was a statistically significant improvement in limb volume from baseline to 12 months when comparing the ultrasound therapy group to the mechanical pressure therapy group [Citation38]. There was also a greater reduction in subjective pain at 12 months in the ultrasound group compared to the mechanical pressure therapy group [Citation38].
One out of one study found when using electromagnetic resonance therapy, there was an improvement in limb circumference and number of lymphangitis attacks (attacks per year) (QS: 8/16) [Citation31].
Ethanol–water (cold therapy)
One RCT and one before and after study examined the effect of cold therapy using cold air [Citation43] or ethanol–water [Citation33]. The RCT found a statistically significant difference between the cold air therapy group compared to usual care, for thickness of epidermis and dermis and circumference of the wrist, above and below the elbow at 6 weeks and 12 weeks of treatment (high risk of bias) [Citation43]. The before and after study using ethanol–water found a statistically significant reduction in skin tissue hardness at levels 1.3 mm and 4 mm when comparing baseline to end of study (QS: 16/16) [Citation33]. Tissue dielectric constant (TDC) used to measure skin water level, also had a positive improvement from baseline to end of study [Citation33].
Attrition rate of intervention group
A random effects meta-analysis found the completion rate to be 99% (95% confidence interval 97–100%) of studies, which reported both data points. Only one study had a less than a hundred percent completion rate. There was moderate statistically significant heterogeneity (I2 = –62%). See , for the forest plot.
Adverse events
Only four studies out of the 18 included studies reported adverse events [Citation35,Citation38,Citation42,Citation45]. One study using electromagnetic resonance treatment noted that a “few patients” out of 16 participants reported having a slight headache after treatment [Citation45]. A study using microwave therapy plus bandages reported that two patients out of 30 reported lymph node swelling and cellulitis [Citation42]. In one study using microwave plus vibration and bandaging, one patient out of nine developed transient skin erythema [Citation35]. Another study reported reasons for noncompletion across all interventions studied (i.e., 150 participants received either ultrasound, ultrasound plus elastic sleeve, mechanical pressure, or mechanical pressure plus elastic sleeve), with 12 patients having recurrence of cancer and 42 reporting non-compliance of treatment [Citation38].
Discussion
With no cure for lymphoedema, attention has focused on conservative management approaches to managing its symptoms, specifically through improving the flow of fluid through the lymphatic system and preventing its build-up [Citation46]. Guidance has recommended the wearing of active or passive compression garments, multilayer bandaging, exercise, and specialised massage techniques (e.g., manual lymphatic drainage) to help promote the drainage of fluid and prevent it accumulating in the body [Citation47]. The importance of good skin care to prevent infection is emphasised [Citation47]. Surgery to debulk tissue through liposuction and lymphatic reconstruction through bypass provide other options [Citation47]. Although temperature-based treatments have been used with the intention of improving drainage of lymphatic fluid [Citation48], current clinical advice given to people with lymphoedema indicates that extremes of temperature should be avoided [Citation16,Citation49–51]. It is thought that such extremes may increase blood flow and increase lymphatic load [Citation15]. Uncertainties around possible treatment options cause concern for people with lymphoedema and for those providing treatment who want to receive and provide the most effective care [Citation52].
In systematically reviewing the evidence on the use of “hot” and “cold” therapies for managing lymphoedema, it was evident that there were some benefits, particularly in reducing limb volume. Approaches that applied heat, whether through microwave therapy alone [Citation32,Citation34,Citation37,Citation40,Citation41] or in combination with bandages [Citation30] and bandages plus vibration [Citation35] through hot water treatment [Citation19,Citation29,Citation40], electromagnetic resonance [Citation31], and light therapy alone [Citation18], and in combination with interferential therapy and/or compression [Citation39] showed benefit in reducing limb circumference. Similar benefits from heating were found on limb volume following the use of microwave [Citation32,Citation34,Citation37,Citation38,Citation41], microwave with bandaging [Citation42], hot water [Citation19], ultrasound [Citation38], and electric blankets combined with mechanical lymph node drainage [Citation44]. Measures of indentation force (i.e., skin firmness) improved following the use of heat through the use of microwave [Citation32,Citation41] and microwave plus bandaging [Citation30]. Benefits following heat therapy were found on other symptoms of lymphoedema and subjective measures of quality of life [Citation19,Citation30–33,Citation36,Citation37,Citation41,Citation45]. Cold therapy, through the use of ethanol–water or colder air, had beneficial effects on indentation force (i.e., skin firmness), TDC (measure of tissue water), skin thickness, and limb circumference [Citation33,Citation43]. Adverse events were rarely reported in studies, with those identified including headache [Citation45], lymph node swelling [Citation42], cellulitis [Citation42], and transient skin erythema [Citation35]. Although non-compliance with ultrasound, ultrasound plus elastic sleeve, mechanical pressure, or mechanical pressure plus elastic sleeve were reported [Citation38], attrition from studies was low.
The benefits of “hot” and “cold” therapies were predominantly shown in studies conducted under specific treatment conditions, including controlled temperature ranges (39–42 °C) [Citation19,Citation29,Citation31,Citation34,Citation36,Citation37], prolonged treatment periods (1200–3600 min) [Citation19,Citation34–36,Citation40–42] and within clinical environments, laboratory [Citation19,Citation29,Citation44]; hospital [Citation32,Citation34–37,Citation39–42]; outpatient [Citation30,Citation31,Citation38,Citation45]. It is unclear whether the suggested benefits would be maintained outside these specific conditions, an important consideration given the long-term goal of self-management at home [Citation47]. Importantly, the findings should be interpreted with caution given the varied study designs, their methodological quality and the risk of bias. Only two before-and-after studies [Citation33,Citation37], three non-RCTs [Citation38,Citation39] and one RCT [Citation41] were at low risk of bias, which may have influenced the outcomes reported. With limited comparative studies, it is unclear if the “hot” and “cold” therapies are more or less effective than other treatment options.
In other areas of rehabilitation, the evidence on the benefits of controlled heat and cold exposure for chronic swelling is inconsistent [Citation53–57]. Subsequently making it difficult to make a global recommendation for heat and cold to reduce swelling in all clinical scenarios. The reviews in this area are either very specific to a treatment type [Citation57] or to a specific condition [Citation58,Citation59]. But overall, there is a notable lack of systematic reviews exploring the effect of heat and cold therapy on swelling. When using ultrasound as a heat source, a previous Cochrane review found no evidence that ultrasound reduced swelling compared to sham ultrasound [Citation57]. This was based upon only three low quality random controlled trials.
The findings of this review are consistent with this previous Cochrane review in that there was limited evidence to support the benefit effects of ultrasound on swelling [Citation38]. Regarding other chronic conditions where swelling is common, cryotherapy has shown to help with reducing localised swelling when applied in both patients with rheumatoid arthritis and osteoarthritis [Citation58,Citation59]. However, like this review the evidence is limited and methodologically weak. It is important to note that all three reviews are now at least two decades old and the evidence underpinning this effect may have substantially changed.
Strengths and limitations
This systematic review has certain strengths and limitations that should be taken into consideration when interpreting the findings of this review. The strengths of this systematic review are: that it was registered on Prospero prior to commencing the review; no post hoc amendments were made; a multi-database search was used [Citation60] with citation screening (no additional papers were identified) [Citation61] and a pre-piloted form was used for data extraction [Citation62].
The main limitations of this review were that abstract and title screening was undertaken by a single reviewer with a piloting process [Citation63]. However, substantial agreement was able to be achieved within the 10% piloting process (Cohen kappa score). Similarly, full paper screening, data extraction, and assessment of bias were undertaken by a single reviewer and verified by a second reviewer but not independently. A further limitation was that three papers were unable to be retrieved for full paper screening. An email was sent to the corresponding authors but there was no response. A further request was made to the British library for these articles; however, they were unable to locate them. Nevertheless, these three studies were not identified to be included studies, rather that they were studies which needed additional information to make a more informed decision at full paper screening. Due to the wide heterogeneity of studies and outcome measures, a “vote counting” method was employed. This method does not produce an estimate of effect and does not take into account the individual weighting of studies [Citation26]. This method also does not consider if the difference was statistically significant [Citation26]. Within this review, the number of statistically significant studies was reported but this was not interpreted as an indication of direction, rather that it was reported as an indication of the probability of the improvement occurring by chance within these individual studies. Furthermore, when using vote counting other such principles, such as imprecision, inconsistency, and publication bias are difficult to assess, which is typically needed to establish a certainty in a directional effect. It is important to note that due to the before and after data collection utilised in the majority of studies within this review, that a meta-analysis would not be typically advised due to the before and after data not being independent from each other [Citation64]. Furthermore, due to this association, it is difficult without a control group to establish that the improvements identified in the studies are associated with the intervention rather than other factors [Citation64]. Over time, this review will become less relevant and may possibly become out of date. Thus, it is important to take note of the date of the search strategy when interpreting the findings from this review. However, within the last decade from the point of the search strategy, only three papers have been published.
Recommendations to future research
The current evidence-base is at a point where no specific clinical recommendations can be made. However, there is enough evidence to suggest that further research is warranted.
Initial studies in this area have been mainly undertaken within hospital and laboratory settings and little is known regarding the use of heat or cold therapy outside these environments. Therefore, until a greater safety profile within these more controlled environments is established, home-based studies are not recommended. Most studies included in the review explored the use of heat therapy for lower limb lymphoedema and there is less certainty regarding its effect on upper limb lymphoedema. Subsequently, there is a requirement for future research to explore the effect of heat on this area. Due to the poor reporting standards within the included studies, it was difficult to establish standardisation of approaches used. Future studies should report greater detail regarding the exact intervention delivered indicating the modalities used, frequency, intensity, and duration of treatment [Citation65,Citation66]. Similarly, greater consistency and transparency is required within outcome reporting. Wherever possible, relevant core outcome sets should be utilized [Citation67]. Future studies exploring the use of cryotherapy with lymphoedema should take a cautionary approach as there is currently little evidence to support the efficacy and safety of this intervention.
Conclusions
The current evidence-base suggests that heat therapy may help in reducing limb circumference and limb volume when provided over a long period of time (1200–3600 min) at a specific skin temperature (39–42 °C) in a controlled environment (laboratory/hospital/outpatients) in lower limb lymphoedema. When applied within these parameters, there was no evidence that heat therapy was unsafe for patients with lymphoedema. For the use of cold therapy for lymphoedema, there was limited evidence in both effectiveness and safety. Due to the lack of high-quality evidence, no recommendations to practice can be made at this time for the use of both hot and cold therapy for patients with lymphoedema. Further high-quality RCTs are required to explore the effects of heat therapy on people with lymphoedema, with a particular focus on upper limb lymphoedema, adverse events, effects of different heat modalities, intensities, and duration.
Supplemental Material
Download MS Word (23.3 KB)Supplemental Material
Download MS Word (14.2 KB)Acknowledgements
This research was funded by the National Institute for Health and Care Research Applied Research Collaboration North West Coast (NIHR ARC NWC). Additional funding was received from the University MedTech Solutions Group. Registration number: CRD42022309475.
Disclosure statement
The authors declare that there is no conflict of interest.
Data availability statement
The data that support the findings of this study are available on request from [email protected].
Additional information
Funding
References
- Sleigh BC, Manna BL. Lymphedema. Treasure Island (FL): StatPearls; 2022.
- Lopez M, Roberson ML, Strassle PD, et al. Epidemiology of lymphedema-related admissions in the United States: 2012–2017. Surg Oncol. 2020;35:249–253. doi: 10.1016/j.suronc.2020.09.005.
- Douglass J, Kelly-Hope L. Comparison of staging systems to assess lymphedema caused by cancer therapies, lymphatic filariasis, and podoconiosis. Lymphat Res Biol. 2019;17(5):550–556. doi: 10.1089/lrb.2018.0063.
- Rockson SG. Advances in lymphedema. Circ Res. 2021;128(12):2003–2016. doi: 10.1161/CIRCRESAHA.121.318307.
- Rajan S, Venkatramani H. Recent advances in management of lymphedema. J Skin Sex Transmit Dis. 2021;3:26–32. doi: 10.25259/JSSTD_15_2021.
- Brix B, Sery O, Onorato A, et al. Biology of lymphedema. Biology. 2021;10(4):261. doi: 10.3390/biology10040261.
- Venkatramani H, Shanmugakrishnan RR, Kumaran MS, et al. Surgical debulking, lymphatico venous anastomosis, vascularised lymph node transfer in lower limb lymphoedema. Plast Aesthet Res. 2020;2020:19. doi: 10.20517/2347-9264.2019.70.
- Tzani I, Tsichlaki M, Zerva E, et al. Physiotherapeutic rehabilitation of lymphedema: state-of-the-art. Lymphology. 2018;51(1):1–12.
- Hopkins A. A community nursing guide: multilayer lymphoedema bandaging. Br J Community Nurs. 2008;13(4):S18, S20–S24. doi: 10.12968/bjcn.2008.13.Sup2.29396.
- Tran K, Argáez C. CADTH rapid response reports. Intermittent pneumatic compression devices for the management of lymphedema: a review of clinical effectiveness and guidelines. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2017.
- Moffatt CJ, Burian E, Karlsmark T, et al. Factors predicting limb volume reduction using compression bandaging within decongestive lymphatic therapy in lymphedema: a multicountry prospective study. Lymphat Res Biol. 2021;19(5):412–422. doi: 10.1089/lrb.2021.0060.
- Petrek JA, Pressman PI, Smith RA. Lymphedema: current issues in research and management. CA Cancer J Clin. 2000;50(5):292–307. doi: 10.3322/canjclin.50.5.292.
- Stuiver MM, ten Tusscher MR, Agasi-Idenburg CS, et al. Conservative interventions for preventing clinically detectable upper-limb lymphoedema in patients who are at risk of developing lymphoedema after breast cancer therapy. Cochrane Database Syst Rev. 2015;(2):CD009765. doi: 10.1002/14651858.CD009765.pub2.
- Moffatt CD, D. Morgan P. Best practice for the management of lymphoedema. International consensus. London: Framework, L. Ltd M.; 2006.
- Cemal Y, Pusic A, Mehrara BJ. Preventative measures for lymphedema: separating fact from fiction. J Am Coll Surg. 2011;213(4):543–551. doi: 10.1016/j.jamcollsurg.2011.07.001.
- National Lymphedema Network. Risk reduction practices. New York: National Lymphedema Network; 2022. Available from: https://lymphnet.org/risk-reduction-practices
- Li K, Zhang Z, Liu NF, et al. Far-infrared radiation thermotherapy improves tissue fibrosis in chronic extremity lymphedema. Lymphat Res Biol. 2018;16(3):248–257. doi: 10.1089/lrb.2016.0057.
- Kulkarni AA, Abhyankar SV, Chaudhari GS, et al. Simple and effective method of heat therapy in lymphoedema. Indian J Surg. 2010;72(1):64–65. doi: 10.1007/s12262-010-0013-8.
- Campisi C, Boccardo F, Tacchella M. Use of thermotherapy in management of lymphedema: clinical observations. Int J Angiol. 1999;8(1):73–75. doi: 10.1007/BF01616849.
- Dell DD, Doll C. Caring for a patient with lymphedema. Nursing. 2006;36(6):49–51. doi: 10.1097/00152193-200606000-00041.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71.
- Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4.
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x.
- Higgins JPT, Thomas J, Chandler J, et al, editors. Cochrane handbook for systematic reviews of interventions version 6.3 [updated 2022 Feb]. Cochrane; 2022. Available from: www.training.cochrane.org/handbook
- Hartling L, Hamm M, Milne A, et al. AHRQ methods for effective health care. Validity and inter-rater reliability testing of quality assessment instruments. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012.
- Borenstein MH, Higgins JPT. Meta-analysis methods based on direction and p-values. In: Michael B, Larry VH, Julian PTH, Hannah RR, editors. Introduction to meta‐analysis; 2009. John Wiley & Sons, Ltd. p. 325–330. doi: 10.1002/9780470743386
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2.
- OpenMeta. Completely open-source, cross-platform software for advanced meta-analysis; 2014. Available from: http://www.cebm.brown.edu/openmeta/
- Campisi C, Boccardo F, Tacchella M. Thermotherapy and microsurgery: new trends in the management of lymphedema. Eur J Lymphol Relat Prob. 1995;5(17–18):23–25.
- Cao W, Chang T, Gan J. Effects of microwave heating on systemic and local infiltrating lymphocytes in patients with chronic limb lymphedema. Chin Med J. 1999;112(9):822–827.
- Fox U, Lucani B, Ribaldone G. Hyperthermia in the treatment of chronic limb lymphedemas: 6 years of experience. Eur J Lymphol Relat Prob. 1993;3(11):73–78.
- Gan JL, Li SL, Cai RX, et al. Microwave heating in the management of postmastectomy upper limb lymphedema. Ann Plast Surg. 1996;36(6):576–580, discussion 80–81. doi: 10.1097/00000637-199606000-00003.
- Mayrovitz HN, Yzer JA. Local skin cooling as an aid to the management of patients with breast cancer related lymphedema and fibrosis of the arm or breast. Lymphology. 2017;50(2):56–66.
- Chang TS, Gan JL, Huang WY, et al. A modified microwave oven in the treatment of chronic lymphedema of the extremities. Eur J Plast Surg. 1992;15(5):242–246. doi: 10.1007/BF00193663.
- Ohkuma M. Treatment of peripheral lymphedema by concomitant application of magnetic fields, vibration and hyperthermia: a preliminary report. Lymphology. 2002;35(2):87–90.
- Li K, Liu NF, Zhang YX. Therapeutic effects of far-infrared ray in treating chronic lower extremity lymphedema with dermatolymphangioadenitis. J Shanghai Jiaotong Univ (Med Sci). 2018;38(9):1059–1065.
- Zhang DS, Han LY, Gan JL, et al. Micro-wave: an alternative to electric heating in the treatment of chronic lymphedema of extremities. Chin Med J. 1986;99(11):866–870.
- Balzarini A, Pirovano C, Diazzi G, et al. Ultrasound therapy of chronic arm lymphedema after surgical treatment of breast cancer. Lymphology. 1993;26(3):128–134.
- Gogia SB, Appavoo NC, Mohan A, et al. Comparative results of non-operative multi-modal therapy for filarial lymphoedema. Indian J Plast Surg. 2009;42(1):22–30.
- Liu NF, Olszewski W. The influence of local hyperthermia on lymphedema and lymphedematous skin of the human leg. Lymphology. 1993;26(1):28–37.
- Chang TS, Gan JL, Fu KD, et al. The use of 5,6 benzo-[alpha]-pyrone (coumarin) and heating by microwaves in the treatment of chronic lymphedema of the legs. Lymphology. 1996;29(3):106–111.
- Ohkuma M. Lymphedema treated by microwave and elastic dressing. Int J Dermatol. 1992;31(9):660–663. doi: 10.1111/j.1365-4362.1992.tb03991.x.
- Askary ZM, Elshazly M. Addition of local cryotherapy for treatment of post-mastectomy lymphedema. Lymphology. 2022;55(2):70–76. doi: 10.2458/lymph.5269.
- Mariana VF, de Fatima GGM, Maria PdGJ. The effect of mechanical lymph drainage accompanied with heat on lymphedema. J Res Med Sci. 2011;16(11):1448–1451.
- Hasegawa H, Ohkuma M. Calcitonin gene-related peptide in lymphedema and its change after physiotherapy by pulse magnetic fields, vibration and hyperthermia. Eur J Lymphol Relat Prob. 2010;21(59):1–2.
- National Health Service. Overview: lymphoedema; 2022 [cited 2022 Oct 21]. Available from: https://www.nhs.uk/conditions/lymphoedema/
- O’Donnell TFJr., Allison GM, Iafrati MD. A systematic review of guidelines for lymphedema and the need for contemporary intersocietal guidelines for the management of lymphedema. J Vasc Surg Venous Lymphat Disord. 2020;8(4):676–684. doi: 10.1016/j.jvsv.2020.03.006.
- Li K, Liu N, Fu L, et al. Therapeutic effect of heating and bandage treatment for chronic lymphedema of extremities accompanied with erysipelas: a report of 80 cases. Zhonghua Zheng Xing Wai Ke Za Zhi. 2015;31(1):39–42.
- National Cancer Institute. Lymphedema. (PDQ)-Patient version; 2022 [cited 2022 Oct 21]. Available from: https://www.cancer.gov/about-cancer/treatment/side-effects/lymphedema/lymphedema-pdq
- John Hopkins Medicine. Treating lymphedema: John Hopkins Medicine; 2022. Available from: https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/treating-lymphedema
- Cleveland Clinic. Lymphedema: Cleveland Clinic; 2022. Available from: https://my.clevelandclinic.org/health/diseases/8353-lymphedema
- Rawlins M. In pursuit of quality: the National Institute for Clinical Excellence. Lancet. 1999;353(9158):1079–1082. doi: 10.1016/S0140-6736(99)02381-8.
- Wang ZR, Ni GX. Is it time to put traditional cold therapy in rehabilitation of soft-tissue injuries out to pasture? World J Clin Cases. 2021;9(17):4116–4122. doi: 10.12998/wjcc.v9.i17.4116.
- Miranda JP, Silva WT, Silva HJ, et al. Effectiveness of cryotherapy on pain intensity, swelling, range of motion, function and recurrence in acute ankle sprain: a systematic review of randomized controlled trials. Phys Ther Sport. 2021;49:243–249. doi: 10.1016/j.ptsp.2021.03.011.
- Hubbard TJ, Denegar CR. Does cryotherapy improve outcomes with soft tissue injury? J Athl Train. 2004;39(3):278–279.
- Malanga GA, Yan N, Stark J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad Med. 2015;127(1):57–65. doi: 10.1080/00325481.2015.992719.
- van den Bekerom MP, van der Windt DA, Ter Riet G, et al. Therapeutic ultrasound for acute ankle sprains. Cochrane Database Syst Rev. 2011;2011(6):CD001250.
- Robinson V, Brosseau L, Casimiro L, et al. Thermotherapy for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2002;2002(1):CD002826.
- Brosseau L, Yonge KA, Robinson V, et al. Thermotherapy for treatment of osteoarthritis. Cochrane Database Syst Rev. 2003;2003(4):CD004522.
- Bramer WM, de Jonge GB, Rethlefsen ML, et al. A systematic approach to searching: an efficient and complete method to develop literature searches. J Med Libr Assoc. 2018;106(4):531–541.
- Horsley T, Dingwall O, Sampson M. Checking reference lists to find additional studies for systematic reviews. Cochrane Database Syst Rev. 2011;2011(8):MR000026.
- Büchter RB, Weise A, Pieper D. Development, testing and use of data extraction forms in systematic reviews: a review of methodological guidance. BMC Med Res Methodol. 2020;20(1):259. doi: 10.1186/s12874-020-01143-3.
- Buscemi N, Hartling L, Vandermeer B, et al. Single data extraction generated more errors than double data extraction in systematic reviews. J Clin Epidemiol. 2006;59(7):697–703. doi: 10.1016/j.jclinepi.2005.11.010.
- Cuijpers P, Weitz E, Cristea IA, et al. Pre–post effect sizes should be avoided in meta-analyses. Epidemiol Psychiatr Sci. 2017;26(4):364–368. doi: 10.1017/S2045796016000809.
- Duncan E, O’Cathain A, Rousseau N, et al. Guidance for reporting intervention development studies in health research (GUIDED): an evidence-based consensus study. BMJ Open. 2020;10(4):e033516. doi: 10.1136/bmjopen-2019-033516.
- Moher D, Schulz KF, Altman D, et al. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285(15):1987–1991. doi: 10.1001/jama.285.15.1987.
- Chiarotto A, Ostelo RW, Turk DC, et al. Core outcome sets for research and clinical practice. Braz J Phys Ther. 2017;21(2):77–84. doi: 10.1016/j.bjpt.2017.03.001.