Abstract
Purpose
To translate ABILHAND-NMD and ACTIVLIM into Norwegian and assess their psychometric properties in adults with Myotonic Dystrophy type 1(DM1).
Methods
ABILHAND-NMD and ACTIVLIM were translated into Norwegian through a standardized translation process. Psychometric properties of the translated questionnaires were tested. Intraclass correlation coefficient (ICC3.1) was used to assess test-retest reliability and Cronbach’s α for internal consistency. The validity of the questionnaires was also assessed.
Results
A total of 39 adults with DM1 were included. We found excellent test-retest reliability on ABILHAND-NMD (ICC 0.91) and ACTIVLIM (ICC 0.93). We found a good internal consistency of ABILHAND-NMD with Cronbach’s α (95%CI) of 0.80 (0.69–0.88) and ACTIVLIM with Cronbach’s α (95%CI) of 0.88 (0.82–0.93) An expert group of healthcare professionals and a pilot group reported good face and content validity. We found a high correlation between ABILHAND-NMD and ACTIVLIM (r = 0.75), p < 0.001 implying good convergent validity. ABILHAND-NMD and ACTIVLIM showed no floor effect, but a potential for ceiling effect.
Conclusion
The Norwegian versions of ABILHAND-NMD and ACTIVLIM are reliable and valid patient reported outcome measures for Myotonic Dystrophy type 1. The questionnaires are easy to administer as they take a short time to answer, and the participants reported no problems understanding the questions.
IMPLICATIONS FOR REHABILITATION
Myotonic Dystrophy type 1 cause myopathy and altered muscle function.
Impaired arm- and hand function increases patients’ need for assistance and reduces independence.
The use of patient reported outcome measures (PROMs) to uncover impairments and activity limitations is important in clinical practice and research.
The Norwegian versions of ABILHAND-NMD and ACTIVLIM are reliable and valid measures of manual ability and activity limitations for adults with Myotonic Dystrophy type 1.
Introduction
Myotonic Dystrophy type 1 (DM1) is the most prevalent hereditary neuromuscular disorder among adults [Citation1]. It is a rare disorder, and the prevalence varies between countries, however, an incidence of 13 cases per 100,000 is frequently cited [Citation2]. The disorder is characterized by great variability in age of onset, disease severity, and most prominent symptoms. DM1 is a multisystemic disorder that can present with symptoms in many organs, including the cardiovascular, respiratory, gastrointestinal, and nervous systems [Citation3]. Early cataracts as well as behavioural and cognitive changes are other possible characteristics of affected individuals. The disease causes altered muscle function [Citation3]. Motor signs include myotonia and muscle weakness, and eventually contractures [Citation4]. Muscle weakness most frequently starts distally with gradual proximal progression, but also neck and trunk muscles become affected early [Citation4]. The patient’s muscle severity is commonly categorized by the Muscular Impairment Rating Scale (MIRS) [Citation5]. Muscle weakness and myotonia often lead to reduced arm and hand function that might influence activities related to personal hygiene, and daily activities in work and leisure [Citation6]. A well-functioning arm and hand are key components in maintaining independence and the ability to live at home. The combination of myopathy and myotonia is distinctive for DM1 and gives specific challenges that are important to investigate and monitor.
The aim of the study was to translate and evaluate the psychometric properties of the Norwegian versions of the ABILHAND-NMD and the ACTIVLIM, two patient-reported outcome measures (PROMs) designed for people with neuromuscular disorders. These questionnaires assess manual ability and activity limitations in people with neuromuscular disorders [Citation7,Citation8]. The questions in the ABILHAND-NMD concentrate on fine motor dexterity and hand function while the questions in the ACTIVLIM concentrate on gross motor functions and activity limitations. The questionnaires have the same structure and are developed for use in the same patient population, therefore we wanted to study both in our study. There is a need for reliable and valid outcome measures for research and for clinical assessment of patients. Ideally, one should use a variety of outcome measures including functional tests able to detect challenges on impairment and activity level as well as PROMs. There is no gold standard to assess arm- and hand function for people with DM1 [Citation9]. We used the quick-DASH questionnaire as a reference for comparison, an instrument evaluating arm- and hand function [Citation10]. It already exists Norwegian [Citation11], however not validated for neuromuscular diseases. PROMs are developed to explore patients’ experiences, functional deficits and life situations. This is in line with the demand for a more patient-centred approach to healthcare services [Citation12]. The PROMs focusing on function should ideally be able to identify and capture the limitations relevant to the patient, without exhausting them with countless questions. Cognitive decline, fatigue or pain may shorten the ability for understanding and answering questionnaires. PROMs should therefore be easy to understand and relevant to the target population. The use of an inadequate PROM on functional deficits, neither understandable nor relevant for the participants, increase the risk of not capturing important impairments or changes [Citation13]. Reduced arm and hand function are frequent in several neuromuscular diseases [Citation14,Citation15]. None of the PROMs in Norwegian language, focusing on arm and hand function and activity limitations, has been validated for the neuromuscular population.
Method
Translation process
Permission to translate and adapt the questionnaires into Norwegian was obtained from the developers. The translation process of the ABILHAND-NMD and the ACTIVLIM questionnaires was performed according to recommended guidelines [Citation16]. The questionnaires were forward-translated from English to Norwegian by a bilingual translator. Questions concerning the translation were discussed with the researchers before the Norwegian version was translated back into English by another translator. The translators were professionals and were hired from a translation firm. An expert group consisting of three physiotherapists and three occupational therapists, knowledgeable in English language, reviewed the translated version. It was discussed and adjusted to consensus.
Pilot testing
The final version of the questionnaires was pilot tested in a small sample (n = 12) of people with neurodegenerative and neuromuscular diseases. This was a convenient sample of patients present at a rehabilitation institution who had muscle impairment potentially relatable for the questionnaires. These patients (the pilot group) were between 30 and 60 years old and 50% were women. They answered both questionnaires and gave feed-back concerning time use and whether the questions were understandable, meaningful and relevant for their impairment. The purpose was to ensure that the translated versions were relevant for use.
Validity
The face and content validity of the ABILHAND-NMD and the ACTIVLIM was examined by asking the expert group of healthcare workers (3 physiotherapists and 3 occupational therapists) and the pilot group of patients about the relevance of the questions related to the impairments and activity limitations. For convergent validity, we calculated the correlation between the continuous measure of the logit sum score of the ABILHAND-NMD and the logit sum score of the ACTIVLIM. Furthermore, the correlation between the two questionnaires and the participant’s MIRS score and Quick-Dash score was calculated. All the variables were considered normally distributed using normality plots and statistics, and the Pearson correlation coefficient was used in correlation analysis. Ideally, when translating a questionnaire, one should assess the structural validity by factor analysis or Rasch analysis. This requires a higher number of responders than what was possible for the scope of this study and was not performed. As DM1 is a rare disorder, recruiting enough participants to be able to perform Rasch analysis is difficult. We assessed the floor and ceiling effects of the translated questionnaires by examining the percentages that scored at the lower and upper end of the scale, respectively [Citation16].
Test-retest reliability
Inclusion criteria for participants were age above 18 years and a genetically confirmed DM1 diagnosis. Exclusion criteria were inability to understand or answer in Norwegian and severe cognitive impairment. The participants responded to the ABILHAND-NMD, the ACTIVLIM and quick-DASH questionnaires 2 times, 1–2 weeks apart (called baseline and follow-up). The items in the ABILHAND-NMD and the ACTIVLIM were ordered differently on the two occasions. Both questionnaires are accessible in a set of 10 versions, with the items in a random order to reduce the risk of survey bias. The questionnaires were administered in a set order, first the ABILHAND-NMD, then the ACTIVLIM, and finally Quick-DASH. The questions were read to the participants and the researcher marked the answers. Demographic and disease data, including MIRS scores, were collected at baseline. A sample size of between 30 and 50 participants was considered adequate for test-retest reliability as this is in line with previous studies [Citation17] and has shown to be a meaningful estimate of sample size for reliability studies [Citation18].
The ABILHAND-NMD and the ACTIVLIM
Both the ABILHAND-NMD and the ACTIVLIM were developed in Belgium and each questionnaire consist of 22 questions of either manual ability (ABILHAND-NMD) or activity limitations (ACTIVLIM) [Citation7,Citation8]. Of the 22 items, four are specifically intended for children, four are specially intended for adults and the remaining 14 are for all responders [Citation7,Citation8]. Answer alternatives are easy, difficult or impossible and are scored respectively with a 2, 1 or 0. The 18 answers are summed to give a total score between 0 and 36. Items that the respondents have not tried to perform in the last three months are scored as not applicable, but if they have not performed the activities because they are too difficult the item is scored as impossible [Citation19]. The questionnaires are able to handle missing responses, although they will reduce the precision of the measure [Citation19].
A higher sum score indicates better hand function (the ABILHAND-NMD) or higher capacity for activity (The ACTIVLIM). Both are Rasch-built questionnaires (linearly weighted scales) which allow the conversion of ordinal scores into linear measures [Citation7,Citation8]. This can be performed online using the developers’ website and give a logit score according to the calibration of the scale established for neuromuscular disorders [Citation19]. A higher logit score indicates better function [Citation19]. Both questionnaires exist in French and Dutch, and have been translated to English [Citation19]. The ACTIVLIM has been translated into Spanish, and other versions of the ABILHAND questionnaire have been translated to other languages [Citation7,Citation19,Citation20].
The ACTIVLIM has shown good psychometric properties, with an ICC test-retest reliability of 0.93 and construct validity with high correlation (r = 0.85) with the Functional Measure Motor Score [Citation8]. A previous study has found a high correlation between the ACTIVLIM and gait speed in patients with DM1 [Citation21]. The questionnaire is seen as a valid method for assessing activity limitations in patients with neuromuscular disorders and has shown good agreement between self-reported activity limitations (by the participant) and observed measures of activity limitations (by physiotherapist) (ICC2,1 = 0.87) [Citation22]. It is sensitive to change over time, also for subjects with DM1 [Citation23,Citation24]. The Spanish version showed excellent test-retest reliability (r = 0.96), good internal consistency and construct validity compared with Functional Independence Measure (r = 0.87). Other versions of the ACTIVLIM have been developed for people with stroke and cerebral palsy [Citation25,Citation26]. These versions contain different numbers of questions and slightly different items.
The ABILHAND-NMD has shown a good reliability index (r = 0.95), indicating the ability to statistically distinguish between different levels of manual ability within a patient sample, and high convergent validity when compared with the ACTIVLIM in a previous study (r = 0.76) [Citation7]. There are several versions of the ABILHAND questionnaire, developed for other patient populations including chronic stroke, rheumatoid arthritis, systemic sclerosis, cerebral palsy (CP), and for use after hand surgery [Citation19]. A version has also been validated for multiple sclerosis [Citation27]. The versions for chronic stroke and children with CP are the most translated and used. These versions contain different numbers of questions and slightly different items than the one for the neuromuscular population There is a need for examining the test-retest reliability of the ABILHAND-NMD for neuromuscular patients [Citation7]. Other versions of the ABILHAND have shown high test-retest reliability tested by ICC (0.85≤ r ≤ 0.97) [Citation17,Citation28,Citation29].
Other variables
Information about the participant’s age, working and marital status and their living situation were collected. The use of physiotherapy and rehabilitation services as well as the time since they were diagnosed, and symptom duration were collected. The Muscular Impairment Rating Scale (MIRS) was used to categorize the patient’s muscle weakness severity [Citation5]. Grade 1 implies no muscle impairment, grade 2–3 implies minimal to mild distal muscle impairment and grade 4–5 implies moderate to severe distal and proximal muscle impairment [Citation5]. The -quick- DASH (The shortened Disabilities of the Arm, Shoulder and Hand) questionnaire has previously been used in similar patient populations [Citation15] and a Norwegian version is already available [Citation30]. Quick- DASH was compared with the ABILHAND-NMD and the ACTIVLIM, as there is no gold standard for this population. Quick- DASH is a generic questionnaire for people with physical impairment and/or pain in the arm and hand. The quick-DASH questionnaire has 11 questions and similar psychometric qualities as the original instrument with 30 questions [Citation10]. Each of the 11 questions has answer alternatives from 0- no symptoms to 4-worst symptoms [Citation31]. The scores are added (sum score 0–44), divided by the number of answers, and multiplied by 25. A lower score indicates better function and/or fewer symptoms.
Statistical analyses
Mean and standard deviations (SD) are used to present the continuous variables that were normally distributed. Categorical variables are described with frequencies and percentages. Test-retest reliability was calculated with intraclass correlation coefficient (ICC3,1) with 95% confidence intervals (CI’s). The ICC3,1 is the single-measurement, absolute agreement, 2-way mixed-effects model [Citation32]. An ICC value between 0.5 and 0.75 indicate moderate reliability, values between 0.75 and 0.9 indicate good reliability and values above 0.9 indicate excellent reliability [Citation32]. Internal consistency was measured by Chronbach’s α, and values above 0.7 were considered acceptable. Pearson correlation coefficients (r) were used to examine associations between ABILHAND-NMD and quick-DASH as well as between ACTIVLIM and quick-DASH as measures of validity, and r between 0.10–0.29 was considered as small, between 0.30–0.49 as medium and between 0.50–1.0 as high [Citation33]. The 95% CIs for the correlation coefficients were calculated using bootstrapping [Citation34]. Floor or ceiling effects were presented as the frequency of the lowest or the highest possible score achieved respectively by respondents. If more than 15% of the participants scored the lowest or highest value, a floor or ceiling effect was considered [Citation35]. Statistical analysis was performed using SPSS Statistics 26. P-value less than 0.05 was considered significant for all analyses. The study was approved by the Protection Official Agent at the Oslo University Hospital. All participants received written and oral information about the study and gave written consent to participate.
Results
Translation process
The translation process of the questionnaires revealed no major problems. There was little need for transcultural adaptation as Belgium and Norway are quite similar from a cultural perspective. The expert group of healthcare professionals and the pilot testing group examined the relevance of the questions. This resulted in some minor changes from the verbatim translation, for instance, the item of opening a breadbox was altered to opening a lunch box as this is more in line with everyday routines and use for Norwegians. This is also in accordance with the Danish version [Citation29] and more in line with the original French and Dutch versions [Citation19].
Participants
A sample of 39 adults (16 men and 23 women) with DM1 were recruited from the Norwegian Registry for Hereditary and Congenital Neuromuscular Diseases and Oslo University Hospital. Data were collected between 2018 and 2021. The participant’s characteristics like age and time since diagnosis are described in .
Table 1. Participant characteristics, 39 participants with Myotonic Dystrophy type 1.
Test-Retest reliability and internal consistency
We found that both questionnaires had excellent test-retest reliability. The ABILHAND-NMD had an ICC3,1 = 0.91 (95% CI) of (CI: 0.84–0,95) with p < 0.001 and the ACTIVLIM had an ICC3,1 = 0,93 (95% CI) (CI: 0.87–0.96) with p < 0.001. Internal consistency of the ABILHAND-NMD and the ACTIVLIM was good with Cronbach’s α (95%CI) of 0.80 (0.69–0.88) and 88 (0,82-0,93) respectively. and show the differences in responses between the two test occasions.
Table 2. Discrepancy in answers between test occasions 1 and 2 ABILHAND-NMD.
Table 3. The discrepancy in answers between test occasions 1 and 2 ACTIVLIM.
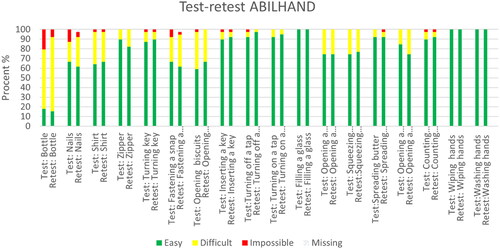
and Citation2 show the percentages of the different answer alternatives in the test and retest for the ABILHAND-NMD and the ACTIVLIM. These figures show that the most difficult items (on the left in the table) have a higher response rate of the answer alternatives difficult and impossible than the easier items (on the right).
Figure 1. Test-retest ABILHAND-NMD.
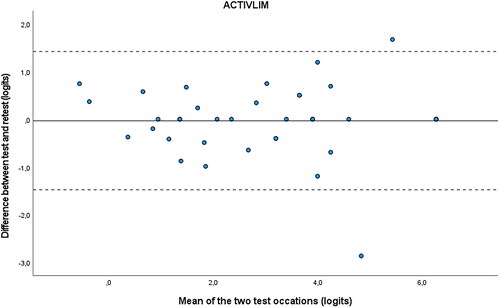
and Citation4 are the Bland-Altman plots, showing the difference (logits) between the measurements plotted against the mean (logits) of the 2 test occasions for the ABILHAND-NMD and the ACTIVLIM. The dotted lines represent the 95% CI for limits of agreement and are drawn at d ± 1.96 × SD difference. The mean systematic difference is small on both occasions, and most of the dots fall between the dotted lines.
Validity and floor and ceiling effect
Participants in the pilot group and the healthcare professionals reported that the ABILHAND-NMD and the ACTIVLIM included relevant questions for people with neuromuscular disorders regarding both impairments and activity limitations. The correlation between the ABILHAND-NMD and the ACTIVLIM was high (r = 0.71. (CI:0.60–0.85)). p < 0.001. The correlations between Quick DASH and both the ABILHAND–NMD and the ACTIVLIM were high and negative (r= −0.81. (CI: −0.81– −0.68)) and (r=–0.83 (CI:–0.90– −0.76)) respectively (p < 0.001). The ACTIVLIM was highly negatively correlated with MIRS (r= −0.72 (CI: −0.84– −0.52)) p < 0.001. There was a medium (r=–0.50 (CI: −0.77 – −0.17)) negative correlation between the ABILHAND–NMD and MIRS p = 0.001. There was no floor effect as all the participants were able to perform with ease at least some of the functions in the ABILHAND-NMD and the ACTIVLIM. There was a potential ceiling effect as six participants (15%) scored all items being easy in the ACTIVLIM, and 5 participants (13%) scored all items being easy in the ABILHAND-NMD.
Discussion
Translation process
The ABILHNAD-NMD and the ACTIVLIM were successfully translated into Norwegian. The participants found both questionnaires easy to understand and answer, and the time to answer was short. None of the participants reported problems understanding the items to the helping researchers. Concerning the item about counting banknotes, it was commented by participants (during the data collection) that Norwegians rarely carry banknotes anymore, as most Norwegians use debit or credit cards for their money transactions. Although this was mentioned by several participants, most had a clear idea of how they could handle real money if they had to. Some items had higher variability in the responses, indicating that the participants understood the questions differently from test to retest. Participants commented on the difficulty of buttoning a shirt depended on the shirt. Moreover, when it came to opening a toothpaste, they wondered if it meant opening the cap (easy) or removing the aluminium foil sealing that is under the cap on a new toothpaste (difficult). Most of the participants found it easy to turn on and off a tap. A few decades ago, most faucets in Norway had knobs where you would turn the water on and off with a twisting motion. Today most faucets require lifting up and pushing down to turn the water on and off. This is arguably easier than the twisting motion and might be an explanation for the high number of participants who reported that this task was easy to perform. In contrast, opening a jar of jam (twisting motion) from the quick-DASH questionnaire was one of the most difficult items to perform. As seen in there are two items on the ACTIVLIM that had a lower response rate (taking a bath and getting out of a bathtub) indicating that these were activities not performed by the participants in the last few months. In Norway, there is a tendency to remove bathtubs in favour of showers, especially when the house owner has muscle impairments or balance issues.
Figure 2. Test-retest ACTIVLIM.
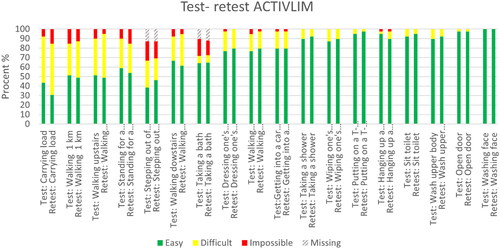
Figure 3. Bland-Altman plot ABILHAND.
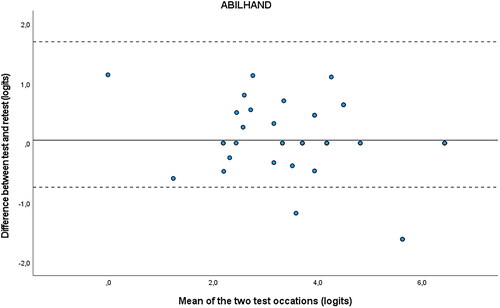
Figure 4. Bland-Altman plot ACTIVLIM.
Participants
The questionnaires were developed for children and adults with all types of neuromuscular disorders. We have tested the translated versions only in adults with DM1. The participants in the study were in all stages of the disorder, with most of them in the earlier stages of the disease (stage 2 and 3 as measured by MIRS), indicating only limited muscle impairment. This is also confirmed by the tendency of ceiling effects. A higher number of participants in more severe stages of the disease would likely have reduced the percentages of participants responding to the items easy to perform and this could also have resulted in floor effects.
Reliability and validity
The test-retest reliability and the content and concurrent validity of the translated versions of the questionnaires are high. We found that the ABILHAND-NMD had an excellent test-retest reliability and internal consistency with an ICC3,1 value of 0.91 and a Cronbach’s alpha of 0.95. The ICC values are in agreement with earlier studies of the ABILHAND reported for other diseases, like stroke (ICC = 0.85–0.91) [Citation17], systemic sclerosis (ICC= 0.96) [Citation36] and rheumatoid Arthritis (ICC= 0.86) [Citation28]. The ACTIVLIM had an excellent test-retest reliability and internal consistency with an ICC value of 0.95 and a Cronbach’s alpha of 0.97. This is in accordance with earlier studies on the ACTIVLIM with ICC values of 0.93 (8) and 0.96 (20). The high correlation and good convergent validity between the ABILHAND-NMD and the ACTIVLIM have also been demonstrated in a previous study [Citation7]. The negative correlation between MIRS and both the ACTIVLIM and the ABILHAND-NMD was as predicted since the instruments are inversely scored. A higher ACTIVLIM and ABILHAND-NMD score indicates a higher activity level and a better hand function while a higher MIRS score indicates more severe muscle impairment.
As reported in this study, we also expected to find a higher negative correlation between MIRS and the ACTIVLIM compared with MIRS and the ABILHAND-NMD. MIRS is an ordinal scale where the lower scores (2 and 3) imply distal weakness while the higher scores (4 and 5) imply proximal weakness [Citation5]. More severe proximal muscle impairment has higher implications for activity limitations like stair climbing and going for walks than it does for hand function. Both the ACTIVLIM and the ABILHAND-NMD were negatively correlated with quick-DASH. We found that quick-DASH had a high test-retest reliability (ICC= 0.93), but we will argue that it has lower validity for the population of patients with neuromuscular diagnoses than the ABILHAND-NMD and the ACTIVLIM. Some of the items in the quick-DASH were difficult for our participants to perform, but not necessarily because of their impaired arm- and hand function which is what the quick-DASH is meant to uncover. Several patients with DM1 might have sleeping difficulties (as is one of the questions asked in quick-Dash) [Citation37], but this is not necessarily related to their impaired arm- and hand function.
We expected the correlation between ABILHAND and quick-DASH to be higher than between quick-DASH and ACTIVLIM. This is because both ABILHAND and quick-Dash are developed to test the level of impairment in the arm and hand [Citation7,Citation10]. But in fact, the correlations were similar, and actually higher between quick-DASH and ACTIVLIM. This might be because some of the questions in the quick-DASH can be related more to activity limitations than arm impairment [Citation10] like doing household chores or carrying a shopping bag.
For people with DM1 performing heavy household chores may be difficult due to reduced balance and motor impairments in the lower extremities [Citation38]. There was a high correlation between the ABILHAND-NMD and the ACTIVLIM. This implies a good convergent validity, as has been shown earlier [Citation7].
We found no floor effect as none of the participants was unable to perform at least some of the items on the ABILHAND-NMD and the ACTIVLIM. Our study showed a potential, but not definite ceiling effect of both instruments when used on our participants. On the ABILHAND-NMD 13% of the participants and on the ACTIVLIM 15% of the participants were able to perform all tasks with ease. A limit of more than 15% of the cases was defined as a ceiling effect [Citation35]. A possible ceiling effect of the ABILHAND-NMD [Citation7] and the ACTIVLIM [Citation20] has been reported earlier and should be taken into consideration when used in future studies. This highlights the need to use several outcome measures (clinical and PROMS) in both research and clinical practice, to ensure that we get the full picture of the participants.
Implications for future use and research
DM1 is a disorder that affects arm and hand function and leads to activity limitations. Therefore, it is important to have tools that identify and assess these issues. To translate and evaluate PROMS targeting these functions is important. Although a disease-specific PROM might have some advantages, there are challenges with translating and testing questionnaires for specific rare disorders. One challenge is to get enough participants according to recommendations [Citation39]. This can be overcome by multicentre collaborations [Citation39], often internationally. But when we want to assess if the translated Norwegian version of the questionnaires is adequate for academic purposes, we need to test them on a Norwegian-speaking population. We have tested the Norwegian versions of the ABILHAND-NMD and the ACTIVLIM with an adult DM1 population, but the questionnaires are designed for use in all neuromuscular disorders and ages [Citation7,Citation8]. To have PROMs that can be used across different types of neuromuscular diseases is useful because that is often the reality for clinicians. It is unrealistic to expect health- care professionals to use patient-specific PROMs in everyday clinics. But we should encourage them to start using PROMs to get a better understanding of their patients and the challenges they face. In research, different types of neuromuscular disorders are often included together in studies on the effect of exercise and physiotherapy. This improves the statistical power of the studies since the number of participants will increase. However, it might introduce more difficulties when interpreting the results, since the neuromuscular disorders are heterogeneous. Even within the DM1 population, there are large variations when it comes to symptoms, age of onset and disease severity [Citation1]. The patient population heterogeneity in addition to the rareness of the disease complicates the designing, performing and analysing clinical trials.
Limitations of the study
The relatively low number of participants is a limitation of the study. The results of the validity of the questionnaires should therefore be interpreted with caution. Structural validity was not assessed using factor analysis or Rasch analysis on the Norwegian version of the questionnaires due to the limited number of participants. However, DM1 is a rare diagnosis, and the number of available participants is limited. Although we have tried to recruit people in all stages of the disease, we acknowledge that there is probably an underrepresentation of severely affected individuals in this study.
Conclusion
We found that the Norwegian versions of the ABILHAND-NMD and the ACTIVLIM are reliable with high test-retest reliability and internal consistency. Both instruments showed high content and concurrent validity, with no floor effect, but a potential ceiling effect. Further studies on the validity should be considered, due to the relatively small sample size in this study. The questionnaires are easily understood by participants, easy to administer and take a short time to answer. The Norwegian versions of the ABILHAND-NMD and the ACTIVLIM can be used in clinical settings as well as potential outcome measures in future clinical trials in DM1.
Acknowledgments
We wish to thank the developers for allowing us to translate and utilize the original questionnaires, and especially Massimo Penta for the collaboration. The National Advisory Unit on Rare Disorders in Norway for partial funding of the study. We wish to thank the Neuromuscular Association of Norway (FFM) for supporting the study and all the patients who participated in the study. The authors from EMAN are members of the European Reference Network for Neuromuscular Diseases (ERN EURO-NMD)
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Johnson NE. Myotonic muscular dystrophies. Continuum. 2019;25(6):1682–1695. doi: 10.1212/CON.0000000000000793.
- Lindberg C, Bjerkne F. Prevalence of myotonic dystrophy type 1 in adults in Western Sweden. Neuromuscul Disord. 2017;27(2):159–162. doi: 10.1016/j.nmd.2016.12.005.
- Ashizawa T, Gagnon C, Groh WJ, et al. Consensus-based care recommendations for adults with myotonic dystrophy type 1. Neurol Clin Pract. 2018;8(6):507–520. doi: 10.1212/CPJ.0000000000000531.
- Solbakken G, Ørstavik K, Hagen T, et al. Major involvement of trunk muscles in myotonic dystrophy type 1. Acta Neurol Scand. 2016;134(6):467–473. doi: 10.1111/ane.12565.
- Mathieu J, Boivin H, Meunier D, et al. Assessment of a disease-specific muscular impairment rating scale in myotonic dystrophy. Neurology. 2001;56(3):336–340. doi: 10.1212/wnl.56.3.336.
- Raymond K, Auger LP, Cormier MF, et al. Assessing upper extremity capacity as a potential indicator of needs related to household activities for rehabilitation services in people with myotonic dystrophy type 1. Neuromuscul Disord. 2015;25(6):522–529. doi: 10.1016/j.nmd.2015.03.015.
- Vandervelde L, Van den Bergh PY, Penta M, et al. Validation of the ABILHAND questionnaire to measure manual ability in children and adults with neuromuscular disorders. J Neurol Neurosurg Psychiatry. 2010;81(5):506–512. doi: 10.1136/jnnp.2009.177055.
- Vandervelde L, Van den Bergh PY, Goemans N, et al. ACTIVLIM: a Rasch-built measure of activity limitations in children and adults with neuromuscular disorders. Neuromuscul Disord. 2007;17(6):459–469. doi: 10.1016/j.nmd.2007.02.013.
- Symonds T, Randall JA, Campbell P. Review of patient-reported outcome measures for use in myotonic dystrophy type 1 patients. Muscle Nerve. 2017;56(1):86–92. doi: 10.1002/mus.25469.
- Wiitavaara B, Florin J. Content and psychometric evaluations of questionnaires for assessing physical function in people with arm-shoulder-hand disorders. A systematic review of the literature. Disabil Rehabil. 2022;44(24):7575–7586. doi: 10.1080/09638288.2021.1979109.
- Mintken PE, Glynn P, Cleland JA. Psychometric properties of the shortened disabilities of the arm, shoulder, and hand questionnaire (QuickDASH) and numeric pain rating scale in patients with shoulder pain. J Shoulder Elbow Surg. 2009;18(6):920–926. doi: 10.1016/j.jse.2008.12.015.
- Kyte DG, Calvert M, van der Wees PJ, et al. An introduction to patient-reported outcome measures (PROMs) in physiotherapy. Physiotherapy. 2015;101(2):119–125. doi: 10.1016/j.physio.2014.11.003.
- Krogsgaard MR, Brodersen J, Christensen KB, et al. What is a PROM and why do we need it? Scand J Med Sci Sports. 2021;31(5):967–971. doi: 10.1111/sms.13892.
- Bergsma A, Cup EH, Geurts AC, et al. Upper extremity function and activity in facioscapulohumeral dystrophy and limb-girdle muscular dystrophies: a systematic review. Disabil Rehabil. 2015;37(12):1017–1032. doi: 10.3109/09638288.2014.948138.
- Eklund E, Svensson E, Häger-Ross C. Hand function and disability of the arm, shoulder and hand in Charcot-marie-tooth disease. Disabil Rehabil. 2009;31(23):1955–1962. doi: 10.1080/09638280902874170.
- De Vet Hc T, Mokkink K. Measurement in medicine. 8th ed. Cambridge: Cambridge University Press; 2017.
- Ekstrand E, Lindgren I, Lexell J, et al. Test-retest reliability of the ABILHAND questionnaire in persons with chronic stroke. Pm R. 2014;6(4):324–331. doi: 10.1016/j.pmrj.2013.09.015.
- Hobart JC, Cano SJ, Warner TT, et al. What sample sizes for reliability and validity studies in neurology? J Neurol. 2012;259(12):2681–2694. doi: 10.1007/s00415-012-6570-y.
- Ucd L. Rehab-Scales.org 2007 [cited 2022 June 09]. Available from: http://rssandbox.iescagilly.be/.
- Pagola I, Torné L, Jericó I, et al. Transcultural adaptation and validation of the Spanish-language version of ACTIVLIM in adults with inherited myopathies using the Rasch model. Neurologia (Engl Ed). 2021;36(7):514–524. doi: 10.1016/j.nrl.2018.03.024.
- Vandervelde L, Van den Bergh PY, Renders A, et al. Relationships between motor impairments and activity limitations in patients with neuromuscular disorders. J Neurol Neurosurg Psychiatry. 2009;80(3):326–332. doi: 10.1136/jnnp.2008.150060.
- Vandervelde L, Dispa D, Van den Bergh PY, et al. A comparison between self-reported and observed activity limitations in adults with neuromuscular disorders. Arch Phys Med Rehabil. 2008;89(9):1720–1723. doi: 10.1016/j.apmr.2008.01.024.
- Vandervelde L, Van den Bergh PY, Goemans N, et al. Activity limitations in patients with neuromuscular disorders: a responsiveness study of the ACTIVLIM questionnaire. Neuromuscul Disord. 2009;19(2):99–103. doi: 10.1016/j.nmd.2008.11.004.
- Batcho CS, Van den Bergh PY, Van Damme P, et al. How robust is ACTIVLIM for the follow-up of activity limitations in patients with neuromuscular diseases? Neuromuscul Disord. 2016;26(3):211–220. doi: 10.1016/j.nmd.2015.12.004.
- Batcho CS, Tennant A, Thonnard JL. ACTIVLIM-Stroke: a crosscultural Rasch-built scale of activity limitations in patients with stroke. Stroke. 2012;43(3):815–823. doi: 10.1161/STROKEAHA.111.638965.
- Bleyenheuft Y, Paradis J, Renders A, et al. ACTIVLIM-CP a new Rrasch-built measure of global activity performance for children with cerebral palsy. Res Dev Disabil. 2017;60:285–294. doi: 10.1016/j.ridd.2016.10.005.
- Grange E, Marengo D, Di Giovanni R, et al. Italian translation and psychometric validation of the ABILHAND-26 and its correlation with upper limb objective and subjective measures in multiple sclerosis subjects. Mult Scler Relat Disord. 2021;55:103160. doi: 10.1016/j.msard.2021.103160.
- Batcho CS, Durez P, Thonnard JL. Responsiveness of the ABILHAND questionnaire in measuring changes in rheumatoid arthritis patients. Arthritis Care Res. 2011;63(1):135–141. doi: 10.1002/acr.20346.
- Hansen A, Poulsen HS, Kristensen HK, et al. Danish translation, adaptation and validation of the ABILHAND-Kids questionnaire for children with cerebral palsy. Disabil Rehabil. 2020;44(5):807–816. doi: 10.1080/09638288.2020.1780482.
- Finsen V. [Norwegian version of the DASH questionnaire for examination of the arm shoulders and hand]. Tidsskr Nor Laegeforen. 2008;128(9):1070.
- Franchignoni F, Vercelli S, Giordano A, et al. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (QuickDASH). J Orthop Sports Phys Ther. 2014;44(1):30–39. doi: 10.2519/jospt.2014.4893.
- Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. doi: 10.1016/j.jcm.2016.02.012.
- Cohen. The effect size. Statistical power analysis for the behavioral sciences. Abingdon: Routledge; 1988.
- Kirkwood B, Sterne J. Essential medical statisticts. 2nd ed: Blackwell Science 2003.
- Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34–42. doi: 10.1016/j.jclinepi.2006.03.012.
- Vanthuyne M, Smith V, Arat S, et al. Validation of a manual ability questionnaire in patients with systemic sclerosis. Arthritis Rheum. 2009;61(5):695–703. doi: 10.1002/art.24426.
- Sansone VA, Proserpio P, Mauro L, et al. Assessment of self-reported and objective daytime sleepiness in adult-onset myotonic dystrophy type 1. J Clin Sleep Med. 2021;17(12):2383–2391. doi: 10.5664/jcsm.9438.
- Landfeldt E, Nikolenko N, Jimenez-Moreno C, et al. Activities of daily living in myotonic dystrophy type 1. Acta Neurol Scand. 2020;141(5):380–387. doi: 10.1111/ane.13215.
- Stoller JK. The challenge of rare diseases. Chest. 2018;153(6):1309–1314. doi: 10.1016/j.chest.2017.12.018.
