Abstract
Purpose
The Freezing of Gait Severity Tool (FOG Tool) was developed because of limitations in existing assessments. This cross-sectional study investigated its validity and reliability.
Methods
People with Parkinson’s disease (PD) were recruited consecutively from clinics. Those who could not walk eight-metres independently (with or without an assistive device), comprehend instructions, or with co-morbidities affecting walking were excluded. Participants completed a set of assessments including the FOG Tool, Timed Up and Go (TUG), and Freezing of Gait Questionnaire. The FOG Tool was repeated and those reporting no medication state change evaluated for test-retest reliability. Validity and reliability were investigated through Spearman’s correlations and ICC (two-way, random). McNemar’s test was applied to compare the FOG Tool and TUG on the proportion of people with freezing.
Results
Thirty-nine participants were recruited [79.5%(n = 31) male; Median(IQR): age–73.0(9.0) years; disease duration–4.0(5.8) years]. Fifteen (38.5%) contributed to test-retest reliability analyses. The FOG Tool demonstrated strongest associations with the Freezing of Gait Questionnaire (ρ = 0.67, 95%CI 0.43–0.83). Test-retest reliability was excellent (ICC = 0.96, 95%CI 0.88–0.99). The FOG Tool had 6.2 times the odds (95%CI 2.4–20.4, p < 0.001) of triggering freezing compared to the TUG.
Conclusions
The FOG Tool appeared adequately valid and reliable in this small sample of people with PD. It was more successful in triggering freezing than the TUG.
IMPLICATIONS FOR REHABILITATION
The Freezing of Gait Severity Tool’s assessment course is more effective than the commonly-used Timed Up and Go’s assessment course for eliciting freezing of gait for clinical evaluation in people with Parkinson’s disease.
The Freezing of Gait Severity Tool can be considered for scoring freezing of gait severity in people with Parkinson’s disease in the clinical setting.
Introduction
Parkinson’s disease (PD) is one of the most rapidly growing neurological disorders worldwide, with prevalence presently estimated at 10 million people globally [Citation1,Citation2]. It is a progressive neurological disorder affecting motor and non-motor systems, producing both motor impairments, such as tremor, rigidity, and bradykinesia, and non-motor symptoms, such as sleep disorders, neuropsychiatric symptoms, and sensory symptoms [Citation3].
Freezing of gait is a motor impairment experienced by approximately half of people with PD [Citation4,Citation5]. It is defined as an episodic and transient inability to generate effective forward translation of the feet, and can manifest as shuffling, festination, trembling-in-place, or a complete arrest of walking [Citation6]. People with PD and freezing of gait have a greater risk of falls, worse disability, and poorer quality of life compared to people with PD without freezing of gait [Citation7–9]. This underscores the importance of adequate management for freezing of gait, which relies on adequate clinical assessment of this episodic impairment.
To date, there is no standardised objective assessment for freezing of gait severity in the clinical setting [Citation10]. Although the use of patient-reported outcome measures, like the Freezing of Gait Questionnaire or New Freezing of Gait Questionnaire, are the most common methods of assessing freezing of gait severity at present [Citation11,Citation12], recent evidence has raised concerns regarding reliability and sensitivity to change [Citation13]. More specifically, the New Freezing of Gait Questionnaire – largely based on the original Freezing of Gait Questionnaire – was found to have only moderate relative reliability (ICC: 0.60–0.68) over a 6-week test-retest period [Citation13]. Absolute reliability (percentage minimal detectable change, %MDC: 28.5–35.5%) was comparable with that previously reported for the Timed Up and Go test over a 2-week test-retest period (%MDC = 29.8%) [Citation13,Citation14]. However, unlike an objective assessment, both the Freezing of Gait Questionnaire and New Freezing of Gait Questionnaire are unsuitable for evaluating same-day treatment effects (e.g., levodopa challenge tests [Citation15], changes immediately after a physiotherapy intervention etc.) due to their required recall period. Experts have recommended complementary use of both patient-reported and objective assessments for freezing of gait severity due to concerns about inaccuracies with patient-reported outcomes [Citation10,Citation16]. Thus, there remains a need for a suitable objective clinical assessment for freezing of gait severity.
A method that has been used for quantifying freezing of gait severity is by measuring percentage of time spent with freezing (i.e., derived by dividing the total duration of freezing episodes by the total time taken to complete the task) in a Timed Up and Go task through manual annotations of video-recordings [Citation11]. Although possible for research use, this is less feasible in the clinical setting where time is often a limiting factor. Furthermore, there is limited evidence for validity and reliability of percentage of time spent with freezing [Citation17].
Two clinician-rated tools for quantifying freezing of gait severity are presently available – the Freezing of Gait score and Dynamic Parkinson Gait Scale [Citation18,Citation19]. The Freezing of Gait score, although easy to score and taking only 15-min to administer, has unclear content validity. For example, it omits frequency and duration of freezing episodes, which are regarded as important markers of freezing of gait severity, and open-space freezing, which has been suggested as the most severe form of freezing [Citation10,Citation17]. Since the Freezing of Gait score assesses opening a door, passing through the doorway, and turning outside before returning, it could be hard to apply in busy clinics where such a specific space may be unattainable. Furthermore, its motor dual-task involves carrying a tray with a plastic cup full of water. This excludes people who use walking aids from completing the assessment. The Dynamic Parkinson Gait Scale presents varied rank descriptors specific to the item being scored and considers duration of freezing episodes [Citation18]. However, it has been infrequently used and rarely reported in literature since its original publication in 2012, which could reflect its uncertain measurement properties or clinical feasibility.
To fill the current lack of a suitable clinician-rated tool for quantifying freezing of gait severity, a new tool (i.e., Freezing of Gait Severity Tool) was recently developed based on the consensus opinion of 28 experts on the items important for inclusion in a clinical assessment of this impairment [Citation12]. In contrast to the Freezing of Gait score and Dynamic Parkinson Gait Scale [Citation18,Citation19], the Freezing of Gait Severity Tool examined tasks specifically intended to differentiate between degrees of freezing severity (i.e., freezing on initiation, open-space freezing, freezing on turning, and freezing on passing through narrow spaces under single- and dual-task conditions; where single-task open-space freezing would allow for differentiation between moderate and severe freezing) and accounted for both frequency and duration of freezing in its scoring [Citation12]. In addition, the Freezing of Gait Severity Tool allowed for walking aid use and did not require a doorway, enabling wider application across people who used walking aids and clinical settings that had no suitable space with a doorway. For feasibility in the clinical setting, the number of items within the Freezing of Gait Severity Tool was also decided based on the average time reportedly spent on clinically assessing freezing of gait [Citation12]. Since the measurement properties of the Freezing of Gait Severity Tool had not been evaluated, this study aimed to investigate its validity and reliability.
The Freezing of Gait Severity Tool was hypothesized to be a valid measure of freezing of gait severity, with a strong correlation expected when compared to the Freezing of Gait Questionnaire (i.e., ρ ≥ 0.70) [Citation20]. Test-retest reliability was hypothesized to be at least good (i.e., ICC ≥ 0.70) [Citation20], with percentage minimal detectable change expected at under 30%.
Methods
Participants
People with PD, as diagnosed by specialised neurologists, were consecutively recruited from outpatient physiotherapy clinics of a tertiary hospital between 23 August 2021 and 20 June 2022. Interested and eligible people with PD attending the outpatient neurology clinics and Movement Disorder clinics were also enrolled. The inclusion criteria were: age of at least 30 years and ability to ambulate 8-metres independently (with or without an assistive device). Those who were unable to follow instructions for study procedures and those who had other neurological or orthopaedic conditions that severely affected walking were excluded. Freezing of gait experience was not a selection criterion to prevent exclusion of people with freezing of gait who failed to recognise the impairment [Citation10]. Study team members provided information on the study for participants’ consideration and consenting participants completed written informed consent prior to commencing study procedures. This study received ethics approval from the tertiary hospital (CIRB 2019/2650).
Study procedures
Due to restrictions on research related to the coronavirus (COVID-19) pandemic, study consent and assessment procedures could only be conducted on the day of participants’ scheduled hospital appointments. While most consenting participants were assessed immediately after their physiotherapy or neurology appointment, a few who had appointment timeslots in the late morning or late afternoon chose to be assessed immediately before instead.
All assessments were carried out by a trained senior physiotherapist who specialised in neurological rehabilitation and the same protocol was applied for all participants. Throughout the procedures, rest breaks were provided as required. The assessments began with the collection of demographic information – including age, gender, social history, past medical history, PD duration, current mobility status, and current medication regime. Thereafter, participants were requested to rate their present medication state on an 11-point numerical rating scale, with “0” representing their worst state where medication effects had completely worn off (i.e., “Off” medication state) and “10” representing their best state where medication effects were at their best (i.e., “On” medication state). Participants were then assessed with the Freezing of Gait Severity Tool and Timed Up and Go test, which is a measure of balance and gait in people with PD involving standing from a chair, walking 3-metres forward, turning 180-degrees and returning to sit on the chair [Citation21]. The Timed Up and Go test is highly recommended by the PD Evidence Database to Guide Effectiveness (EDGE) task force and has demonstrated adequate validity and excellent reliability [Citation22]. Performance on these tests were recorded for post-hoc video analysis. Following this, the Freezing of Gait Questionnaire, the best available clinical assessment of freezing of gait severity with sufficient validity and reliability [Citation17,Citation23]; Parkinson Anxiety Scale, a measure of anxiety in people with PD with adequate validity and excellent reliability [Citation24]; Montreal Cognitive Assessment, a cognitive screening test with adequate validity and good reliability in people with PD [Citation25,Citation26]; and the Movement Disorder Society’s revised Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Parts II and III, both valid and reliable measures of PD-related disability and motor impairments [Citation27], were administered.
The Freezing of Gait Severity Tool was repeated and scores from participants who reported no change in medication state and perceived freezing of gait severity after completion of the MDS-UPDRS, based on the numerical rating scale and a 7-point global impression of change scale respectively [Citation23,Citation24], were included in the evaluation of its test-retest reliability. Since medication state can affect freezing of gait severity and considering the symptom’s varied response to levodopa [Citation28], including only those with no change in both medication state and perceived freezing of gait severity was necessary to ensure no true change was present. The time interval between test and retest was approximately 30-min to an hour (i.e., the time required for the participants to complete the Freezing of Gait Questionnaire, Parkinson Anxiety Scale, Montreal Cognitive Assessment, and MDS-UPDRS Parts II and III). In contrast to having test and retest occasions on separate days, this same-day design was beneficial for ensuring consistent participant-related conditions (e.g., sleep quality, mood etc.) which could have affected between-day performance [Citation29]. It also allowed for specific investigation of the Freezing of Gait Severity Tool for evaluating same-day treatment effects and a more consistent time interval between testing occasions since COVID-19 research restrictions caused unpredictability in the follow-up schedules for between-day designs.
Freezing of Gait Severity Tool
The Freezing of Gait Severity Tool examined freezing on initiation (“start hesitation”), open-space freezing, freezing on turning, and freezing on passing through a narrow space under conditions of single-tasking and cognitive dual-tasking. Start hesitation was evaluated through the subject’s ability to start walking on cue (i.e., a verbal command to “start”). Open-space freezing was evaluated through a 6-metre forward-walk at a comfortable pace in a quiet, uncluttered corridor. Freezing on turning was evaluated by having subjects turn 720° on the spot at the fastest pace they could manage safely, once clockwise and once anti-clockwise. Freezing on passing through a narrow space was evaluated through the subject’s ability to walk through a space created by positioning a standard geriatric chair (120-cm maximum height, 62-cm maximum width) 50-cm away from the wall in the same corridor, similar to the narrow-space protocols of other studies [Citation30–32]. Finally, cognitive dual-tasking was achieved through simultaneous performance of a rule-reversal task, where subjects were required to respond either “big” or “small” to a series of numbers ranging between 1 and 10. These items were always performed in the same order but as separate tasks, with task instructions (see Supplementary Material 1) and a demonstration provided prior to the start of each task – for example, before assessing freezing on turning in a single-task condition, the assessor read the instructions for turning and showed a demonstration. illustrates the course for the Freezing of Gait Severity Tool.
Figure 1. Illustration of the freezing of Gait Severity tool’s assessment course performed under single-task and dual-task conditions.
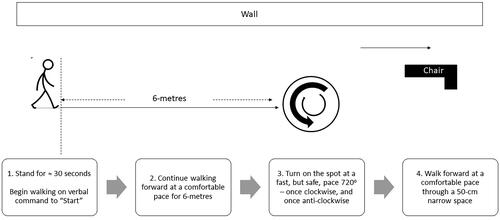
All participants completed one set of the Freezing of Gait Severity Tool. No familiarisation trials were given. Where the assessor observed an incorrect performance, the trial was immediately stopped and the error corrected. The task was then repeated and the score for the correct performance was recorded.
To score freezing of gait severity, the assessor observed the performance and rated each item based on three aspects – “type of freezing,” “number of freezes,” and “average duration of freezes” observed. These were each rated on a 4-point ordinal scale, with a higher score indicating greater freezing severity. “Type of freezing” followed the Freezing of Gait score’s 4-point scale. “Number of freezes” was scored with “0” indicating no freezing of gait, “1” indicating 1 to 3 freezing episodes, “2” indicating 4 to 6 freezing episodes, and “3” indicating at least 7 freezing episodes observed. “Average duration of freezes” was rated “0” for no freezing, “1” for average freeze durations under 3 s, “2” for average freeze durations between 3 to 10 s, and “3” for average freeze durations beyond 10 s. The score for each item was the sum of the three aspects and the Freezing of Gait Severity Tool score was the sum of each item’s score, producing a maximum possible total score of 72 (see Supplementary Material 2). An episode of freezing of gait was defined to start from the point when forward progression of the feet was unintentionally reduced and end at the point where their normal step length was resumed [Citation33,Citation34]. Number of freezing episodes and their individual durations were measured with a lap stopwatch and confirmed by having two reviewers (A.S. and D.T.) revisit the video-recordings.
Statistical analysis
Descriptive data analyses and statistical tests were performed with R, version 4.2.0 (R Foundation, Vienna, Austria). McNemar’s test was applied to compare the Freezing of Gait Severity Tool and the Timed Up and Go test on the proportion of people with freezing behaviour. For criterion-related validity of the Freezing of Gait Severity Tool’s score, correlation with the Freezing of Gait Questionnaire was computed using Spearman’s correlation coefficient with bootstrapped 95% confidence interval. Sufficient criterion-related validity was defined by a correlation of at least 0.70 [Citation20]. The Freezing of Gait Questionnaire was selected as the criterion because it was found to be the best available assessment in a recent systematic review and the existing clinician-rated tools had similarly evaluated validity through correlations with the questionnaire [Citation17–19]. The following hypotheses were tested for construct validity – the Freezing of Gait Severity Tool’s score should have a stronger association with the Freezing of Gait Questionnaire (i.e., an outcome measuring the construct of freezing of gait severity) compared to the MDS-UPDRS Part II and III (i.e., outcomes measuring disability and motor impairment respectively), Montreal Cognitive Assessment (i.e., an outcome measuring cognition), and Parkinson Anxiety Scale (i.e., an outcome measuring anxiety). Similar to criterion-related validity, Spearman’s correlation coefficient was calculated. Intraclass correlation coefficients (ICC, two-way random effects model of absolute agreement) were used for estimation of test-retest reliability, with log-transformation applied. Sufficient reliability was defined by an ICC of at least 0.70 [Citation20]. For absolute reliability, standard error of measurement (SEM) was derived by taking the square root of the ICC’s error variance and minimal detectable change (MDC95) was calculated from the SEM based on the 95% confidence interval (i.e., MDC95 = 1.96 x √2 x SEM) [Citation35,Citation36]. Statistical significance was set at p < 0.05.
Target sample size
This initial validation study sought a sample size comparable to the initial validation studies of existing clinician-rated tools (n = 33 – 35) [Citation18,Citation19] and other recent freezing of gait outcomes (n = 41) [Citation37] to facilitate study feasibility in a period of COVID-19 related research restrictions. While the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) recommends at least 30 participants for an “adequate” study quality for determination of criterion-related validity and a sample size between 50 to 99 participants for an “adequate” study quality for measurement error and reliability, these relate specifically to patient-reported outcome measures [Citation38]. There are no specific recommendations for clinician-rated tools – although, for criterion-related validity, for a correlation of 0.70 with pre-specified confidence interval half-width of 0.10, a sample size of 105 has been recommended [Citation39]; and, for reliability, for an ICC of 0.70 with expected precision of 0.10, a sample size of 101 would be required [Citation40].
Results
Participants
The flow of participants is illustrated in . presents the participant demographics and descriptive results. Of the 39 participants enrolled, 30 participants (76.9%) self-reported to have experienced freezing of gait, based on a score of at least 1 on the Freezing of Gait Questionnaire’s item 3 [Citation41]. Based on previously-published cut-off scores for the Montreal Cognitive Assessment, sixteen (41.0%) participants had mild cognitive impairments (i.e., cut-off score = 25) and six (15.4%) had dementia (i.e., cut-off score = 20) [Citation42]. The majority of participants (82.1%, n = 32) were assessed in the “on” medication state.
Figure 2. Flow of participants.
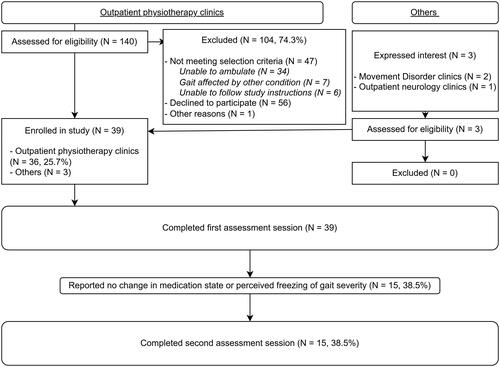
Table 1. Participants (N = 39) demographics and descriptive results.
Two participants (5.1%) could not complete the dual-task condition due to severe freezing of gait in the single-task condition which required the assessor’s assistance – both participants also scored below 20-points on the Montreal Cognitive Assessment. One participant (2.6%) could not perform the walks through the narrow space due to a preference of having their caregiver beside them for confidence. All participants were able to follow task instructions with demonstration and understood the rule-reversal task. Overall, the time taken to complete one set of the Freezing of Gait Severity Tool ranged between 3.5 to 16.0 min [Median (IQR) = 6.5 (4.5) minutes], inclusive of the time provided for rests and set-up (i.e., placement and removal of a standard geriatric chair for creation of the narrow space). The Freezing of Gait Severity Tool triggered freezing behaviour in 31 participants (79.5%). In contrast, freezing of gait was observed in only five participants (12.8%) in the Timed Up and Go test. The Freezing of Gait Severity Tool had 6.2 times the odds (95% CI 2.4–20.4, McNemar’s p < 0.001) of triggering freezing behaviours compared to the Timed Up and Go test.
Validity and reliability
Results of the Spearman’s correlation are summarised in . The Freezing of Gait Severity Tool appeared to have adequate criterion-related validity in this sample of people with PD, with similar correlations demonstrated when all were considered (ρ = 0.67, 95% CI 0.43–0.83) and when only people in the “on” medication state (ρ = 0.70, 95% CI 0.45–0.86; n = 32) were considered. Compared to percentage of time spent with freezing in the Timed Up and Go test, the Freezing of Gait Severity Tool correlated more closely with the Freezing of Gait Questionnaire (ρFOG-Tool = 0.67, 95% CI 0.43–0.83, ρPercentage = 0.34, 95% CI 0.08–0.56; Difference in ρ = 0.34, 95% CI 0.02–0.63 – see ). Adequate construct validity was shown through hypotheses testing. As expected, the Freezing of Gait Severity Tool correlated more strongly with the Freezing of Gait Questionnaire (ρ = 0.67, 95% CI 0.43–0.83) compared to the MDS-UPDRS Parts II (ρ = 0.50, 95% CI 0.24–0.72) and III (ρ = 0.53, 95% CI 0.24–0.77), Montreal Cognitive Assessment (ρ = −0.14, 95% CI −0.47–0.20), and Parkinson Anxiety Scale (ρ = 0.25, 95% CI −0.08–0.55). Frequency histograms are presented in Supplementary Material 3 for visualisation of score distributions. Variations in scores for each participant between tasks are also illustrated in Supplementary Material 3 through interaction plots.
Table 2. Spearman’s correlation coefficients.
Fifteen participants (38.5%) performed a repeat assessment at the same medication state. All were in the “on” state at test and retest. Test-retest reliability was excellent for type-only (ICC = 0.92, 95% CI 0.79–0.97) and total Freezing of Gait Severity Tool scores (ICC = 0.96, 95% CI 0.88–0.99) between two testing occasions approximately 30-min apart. For type-only, SEM and MDC95 were 1.31 and 3.63 respectively (%MDC95 = 15.1%). For total Freezing of Gait Severity Tool score, SEM and MDC95 were 2.19 and 6.08 respectively (%MDC95 = 8.4%).
Discussion
This study found the Freezing of Gait Severity Tool may be valid and reliable as a measure of freezing of gait severity in this sample of people with PD. Taking an average of six minutes to complete, the Freezing of Gait Severity Tool appears feasible to assess freezing of gait severity in the clinical setting. Its test-retest reliability suggests use for evaluating same-day treatment effects may be possible – overcoming this limitation of existing patient-reported outcomes like the Freezing of Gait Questionnaire. With a score based on clinicians’ ratings of observed performance, the Freezing of Gait Severity Tool may also improve on the inaccuracies associated with patient-reported outcomes should freezing of gait not be appropriately recognised [Citation10,Citation16].
Compared to the percentage of time spent freezing in a Timed Up and Go test, the Freezing of Gait Severity Tool showed a stronger relationship with the Freezing of Gait Questionnaire. However, considering only the type of freezing in the Freezing of Gait Severity Tool also produced a comparable correlation with the Freezing of Gait Questionnaire. This suggests computing only the score for type of freezing in the Freezing of Gait Severity Tool may be an equally valid clinical measure of freezing of gait severity. Although criterion-related validity may have been adequate, the content validity of the type-only score of the Freezing of Gait Severity Tool remains uncertain. Missing concepts in these outcomes could compromise their responsiveness [Citation43]. For instance, relying only on the score for type of freezing would mean a person with PD and akinetic freezing would have to demonstrate a complete absence of akinetic freezing or trembling-in-place before the score detects an improvement.
While the task of walking through a narrow space – both under single-task and dual-task conditions – performed best among the tasks included in the Freezing of Gait Severity Tool, completing all tasks of the tool remains important for comprehensiveness. Three distinct subtypes of gait freezing were previously identified using a data-driven approach in a sample of 41 people with PD and freezing of gait [Citation37]. Each subtype demonstrated a unique propensity for specific freezing triggers – the first group experienced freezing most often in motor-related situations, like initiating walking, turning, and walking through narrow spaces; the second group experienced freezing most often in anxiety-related situations, like when rushed, feeling anxious, or distracted; and the third group experienced freezing most often in attention-shifting circumstances, like walking in cluttered spaces [Citation37]. In this study, all tasks demonstrated adequate associations with the Freezing of Gait Questionnaire’s total score, except start hesitation under the single-task condition. Freezing on initiation of walking could have been less observed among the participants as the verbal command to “start” may have had a cueing effect similar to the use of the verbal command “go” [Citation44]. Since the effectiveness of cueing has been known to reduce in dual-task conditions, this could have provided for the stronger association of the Freezing of Gait Severity Tool’s dual-task start hesitation and the Freezing of Gait Questionnaire [Citation45]. Furthermore, visualisation of the data suggested some participants presented a similar unique propensity for specific freezing triggers and the frequency histograms supported that the turning tasks had better distributional properties than other tasks (see Supplementary Material 3), supporting the necessity of a comprehensive assessment with the Freezing of Gait Severity Tool.
Limitations
This study has limitations. First, there is currently no standardised criterion for evaluating criterion-related validity of freezing of gait severity outcomes. On the one hand, although the Freezing of Gait Questionnaire has been used as a comparison by previous studies and was found to be the best available assessment [Citation17–19], reliability may be a concern. On the other hand, percentage of time spent with freezing has limited evidence for validity and reliability [Citation17]. The Freezing of Gait Severity Tool and percentage of time spent with freezing were also both derived from frequency and duration of freezing episodes. This overlap was a concern when considering the criterion to be used [Citation46]. Thus, the selected criterion for criterion-related validity was the Freezing of Gait Questionnaire. Hypotheses testing for construct validity supported overall validity of the Freezing of Gait Severity Tool. In line with earlier studies validating other freezing of gait severity outcomes [Citation41], the MDS-UPDRS Parts II and III were expected to show better correlations than the Parkinson Anxiety Scale and Montreal Cognitive Assessment. Anxiety and cognition may be related to freezing of gait, with a recent study proposing both as contributors to two distinct subtypes of gait freezing – though the presence of these subtypes remain to be confirmed [Citation37].
Second, the Freezing of Gait Severity Tool’s total score did not perform better than the score based on type of freezing alone. This could have been because of the more-common presentation of shuffling or festination and the lack of differentiation of its severity – number and duration of freezing episodes were not suitable quantifications of severity of shuffling or festination and were recorded only for trembling-in-place or akinetic freezing. Future studies could explore quantifying severity of shuffling or festination through step count. The Freezing of Gait Severity Tool adopts the assumption that festination is less severe than trembling-in-place or akinetic freezing. This was a recognised limitation of the Freezing of Gait score and is not a universally-accepted assumption [16]. Future studies could investigate the value of separating these freezing manifestations in scoring.
Third, due to COVID-19-related restrictions on research, assessments could not be deliberately scheduled around medication timings so the effects of medication state on the Freezing of Gait Severity Tool score could not be determined. Further investigation of its ecological validity is warranted since performance is known to fluctuate based on medication state [Citation28]. Additionally, since most participants were assessed after a physiotherapy appointment, their performance could have been impacted if they had just practiced strategies to reduce freezing of gait prior to study testing. Despite this and the majority of study participants being in the “on” medication state, the Freezing of Gait Severity Tool’s assessment course successfully triggered freezing behaviour in 26 more participants than the Timed Up and Go test.
Fourth, the short window of time between test and retest occasions could have influenced the second performance due to familiarity with the first performance of the test. While the benefits of this design were earlier described, further evaluation of the test-retest reliability of the Freezing of Gait Severity Tool is warranted with a longer time interval between testing sessions.
Finally, the inclusion of people with dementia and higher proportion of males relative to typical demographics associated with PD may have influenced findings. While including people with dementia could have improved generalisability, the comprehensibility of the tool’s instructions for people with PD and severe cognitive impairments remains unclear since most of the study’s participants had mild cognitive impairments at worst (84.6%, n = 33). Although validity and reliability appeared to be adequate in this small sample of people with PD, further evaluation of the Freezing of Gait Severity Tool’s validity and reliability in a larger sample is necessary for firm conclusions to be drawn. Furthermore, a Rasch analysis with a larger sample could facilitate identification of redundant items, items with poorer distributional properties, and those that represent a different construct than freezing of gait. Evaluation in a larger sample could also allow for determination of appropriate cut-off scores for each rank.
Conclusion
This study found the Freezing of Gait Severity Tool may be a valid and reliable measure of freezing of gait severity in this small sample of people with PD. Taking an average of six minutes to complete, the Freezing of Gait Severity Tool can be easily administered in the clinical setting with minimal equipment (i.e., a lap stopwatch and a geriatric chair). For observation of freezing behaviour, the Freezing of Gait Severity Tool’s assessment course triggered freezing of gait in more participants than the Timed Up and Go test.
Ethical approval
This study was approved by the ethics committees of the relevant institutions (SingHealth: CIRB 2019/2650, Curtin University: HRE2020-0094).
Supplemental Material
Download MS Word (794.9 KB)Acknowledgements
The authors acknowledge Singapore General Hospital’s Department of Physiotherapy, Department of Neurology, and research assistants Ms Sherry Leow and Ms Abigail Koh for their support during data acquisition. The authors also thank undergraduate physiotherapy students Benjamin Lam and Lee Boon Yee for contributing their honours research budget towards this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting the results of this study is not publicly available, but can be obtained from the corresponding author upon reasonable request.
Additional information
Funding
References
- Global Burden of Disease Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(5):459–480.
- Parkinson’s Foundation. Understanding Parkinson’s - Statistics. 2021. https://www.parkinson.org/Understanding-Parkinsons/Statistics
- Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for parkinson’s disease. Mov Disord. 2015;30(12):1591–1601. doi:10.1002/mds.26424.
- Ge H-L, Chen X-Y, Lin Y-X, et al. The prevalence of freezing of gait in parkinson’s disease and in patients with different disease durations and severities. Chin Neurosurg J. 2020;6:17. doi:10.1186/s41016-020-00197-y.
- Zhang W-S, Gao C, Tan Y-Y, et al. Prevalence of freezing of gait in parkinson’s disease: a systematic review and meta-analysis. J Neurol. 2021;268(11):4138–4150. doi:10.1007/s00415-021-10685-5.
- Nutt JG, Bloem BR, Giladi N, et al. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10(8):734–744. doi:10.1016/S1474-4422(11)70143-0.
- Hiorth YH, Larsen JP, Lode K, et al. Natural history of falls in a population-based cohort of patients with parkinson’s disease: an 8-year prospective study. Parkinsonism Relat Disord. 2014;20(10):1059–1064. doi:10.1016/j.parkreldis.2014.06.023.
- Perez-Lloret S, Negre-Pages L, Damier P, et al. Prevalence, determinants, and effect on quality of life of freezing of gait in parkinson disease. JAMA Neurol. 2014;71(7):884–890. doi:10.1001/jamaneurol.2014.753.
- Tan DM, McGinley JL, Danoudis ME, et al. Freezing of gait and activity limitations in people with parkinson’s disease. Arch Phys Med Rehabil. 2011;92(7):1159–1165. doi:10.1016/j.apmr.2011.02.003.
- Mancini M, Bloem BR, Horak FB, et al. Clinical and methodological challenges for assessing freezing of gait: future perspectives. Mov Disord. 2019;34(6):783–790. doi:10.1002/mds.27709.
- D’Cruz N, Seuthe J, De Somer C, et al. Dual task turning in place: a reliable, valid, and responsive outcome measure of freezing of gait. Mov Disord. 2022;37(2):269–278. doi:10.1002/mds.28887.
- Scully AE, de Oliveira BI, Hill KD, et al. Developing the freezing of gait severity tool: a delphi consensus study to determine the content of a clinician-rated assessment for freezing of gait severity. Clin Rehabil. 2022;36(12):1679–1693. doi:10.1177/02692155221121180.
- Hulzinga F, Nieuwboer A, Dijkstra BW, et al. The new freezing of gait questionnaire: unsuitable as an outcome in clinical trials? Mov Disord Clin Pract. 2020;7(2):199–205. doi:10.1002/mdc3.12893.
- Huang S-L, Hsieh C-L, Wu R-M, et al. Minimal detectable change of the timed “up & go” test and the dynamic gait index in people with parkinson disease. Phys Ther. 2011;91(1):114–121. doi:10.2522/ptj.20090126.
- Saranza G, Lang AE. Levodopa challenge test: indications, protocol, and guide. J Neurol. 2021;268(9):3135–3143. doi:10.1007/s00415-020-09810-7.
- Barthel C, Mallia E, Debû B, et al. The practicalities of assessing freezing of gait. J Parkinsons Dis. 2016;6(4):667–674. doi:10.3233/JPD-160927.
- Scully AE, et al. Measurement properties of assessments of freezing of gait severity in people with parkinson disease: a COSMIN review. Phys Therapy. 2021;101(4): p):1–12. doi:10.1093/ptj/pzab009.
- Crémers J, Phan Ba R, Delvaux V, et al. Construction and validation of the dynamic parkinson gait scale (DYPAGS). Parkinson Related Disord. 2012;18(6):759–764. doi:10.1016/j.parkreldis.2012.03.012.
- Ziegler K, Schroeteler F, Ceballos-Baumann AO, et al. A new rating instrument to assess festination and freezing gait in parkinsonian patients. Mov Disord. 2010;25(8):1012–1018. doi:10.1002/mds.22993.
- Mokkink LB, Terwee CB, Stratford PW, et al. Evaluation of the methodological quality of systematic reviews of health status measurement instruments. Qual Life Res. 2009;18(3):313–333. doi:10.1007/s11136-009-9451-9.
- Podsiadlo D, Richardson S. The timed "up & go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi:10.1111/j.1532-5415.1991.tb01616.x.
- Kegelmeyer D, et al. Recommendations for patients with Parkinson disease. Parkinson Evidence Database to Guide Effectiveness. 2014. https://www.neuropt.org/docs/default-source/parkinson-edge/recommendations-for-patients.pdf?sfvrsn=890d5543_0
- Giladi N, Shabtai H, Simon ES, et al. Construction of freezing of gait questionnaire for patients with parkinsonism. Parkinsonism Relat Disord. 2000;6(3):165–170. doi:10.1016/s1353-8020(99)00062-0.
- Leentjens AFG, Dujardin K, Pontone GM, et al. The parkinson anxiety scale (PAS): development and validation of a new anxiety scale. Mov Disord. 2014;29(8):1035–1043. doi:10.1002/mds.25919.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x.
- Gill DJ, Freshman A, Blender JA, et al. The montreal cognitive assessment as a screening tool for cognitive impairment in parkinson’s disease. Mov Disord. 2008;23(7):1043–1046. doi:10.1002/mds.22017.
- Goetz CG, Tilley BC, Shaftman SR, et al. Movement disorder society-sponsored revision of the unified parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. doi:10.1002/mds.22340.
- McKay L, et al. Freezing of gait can persist after an acute levodopa challenge in parkinson’s disease. NPJ Parkinsons Dis. 2019;5:25.
- Richard IH, Justus AW, Kurlan R. Relationship between mood and motor fluctuations in parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2001;13(1):35–41. doi:10.1176/jnp.13.1.35.
- Janssen S, Heijs JJA, van der Meijs W, et al. Validation of the auditory stroop task to increase cognitive load in walking tasks in healthy elderly and persons with parkinson’s disease. PLoS One. 2019;14(8):e0220735. doi:10.1371/journal.pone.0220735.
- Lord SR, Bindels H, Ketheeswaran M, et al. Freezing of gait in people with parkinson’s disease: nature, occurrence, and risk factors. J Parkinsons Dis. 2020;10(2):631–640. doi:10.3233/JPD-191813.
- Sawada M, Wada-Isoe K, Hanajima R, et al. Clinical features of freezing of gait in parkinson’s disease patients. Brain Behav. 2019;9(4):e01244. doi:10.1002/brb3.1244.
- Mitchell T, Conradsson D, Paquette C. Gait and trunk kinematics during prolonged turning in parkinson’s disease with freezing of gait. Parkinsonism Relat Disord. 2019;64:188–193. doi:10.1016/j.parkreldis.2019.04.011.
- O’Day JJ, et al. The turning and barrier course reveals gait parameters for detecting freezing of gait and measuring the efficacy of deep brain stimulation. PLoS One. 2020;15(4):e0231984.
- Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res. 2005;19(1):231–240. doi:10.1519/15184.1.
- de Vet HCW, et al. Measurement in medicine: a practical guide. Cambridge: Cambridge University Press; 2011.
- Ehgoetz Martens KA, Shine JM, Walton CC, et al. Evidence for subtypes of freezing of gait in parkinson’s disease. Mov Disord. 2018;33(7):1174–1178. doi:10.1002/mds.27417.
- Mokkink LB, et al. COSMIN study design checklist for patient-reported outcome measurement instruments. Amsterdam: COSMIN; 2019. Available from: https://www.cosmin.nl/wp-content/uploads/COSMIN-study-designing-checklist_final.pdf
- Moinester M, Gottfried R. Sample size estimation for correlations with pre-specified confidence interval. TQMP. 2014;10(2):124–130. doi:10.20982/tqmp.10.2.p0124.
- Bonett DG. Sample size requirements for estimating intraclass correlations with desired precision. Stat Med. 2002;21(9):1331–1335. doi:10.1002/sim.1108.
- Giladi N, Tal J, Azulay T, et al. Validation of the freezing of gait questionnaire in patients with parkinson’s disease. Mov Disord. 2009;24(5):655–661. doi:10.1002/mds.21745.
- Skorvanek M, Goldman JG, Jahanshahi M, et al. Global scales for cognitive screening in parkinson’s disease: critique and recommendations. Mov Disord. 2018;33(2):208–218. doi:10.1002/mds.27233.
- Terwee CB, Prinsen CAC, Chiarotto A, et al. COSMIN methodology for evaluating the content validity of patient-reported outcome measures: a delphi study. Qual Life Res. 2018;27(5):1159–1170. doi:10.1007/s11136-018-1829-0.
- Morris S, Morris ME, Iansek R. Reliability of measurements obtained with the timed “up & go” test in people with parkinson disease. Phys Ther. 2001;81(2):810–818. doi:10.1093/ptj/81.2.810.
- Ginis P, Nackaerts E, Nieuwboer A, et al. Cueing for people with parkinson’s disease with freezing of gait: a narrative review of the state-of-the-art and novel perspectives. Ann Phys Rehabil Med. 2018;61(6):407–413. doi:10.1016/j.rehab.2017.08.002.
- Nieuwboer A, Rochester L, Herman T, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with parkinson’s disease and their carers. Gait Posture. 2009;30(4):459–463. doi:10.1016/j.gaitpost.2009.07.108.
