Abstract
Purpose
To assess if a high-level mobility programme (HLMP) can promote sustained participation in physical activity by adolescents with cerebral palsy.
Methods
Eight adolescents with cerebral palsy, Gross Motor Function Classification System levels I-II, 11–16 years, participated in 24 community-based group HLMP sessions across 12 weeks. Participants set attendance, involvement, and physical performance goals, completed activity diaries over 58 weeks and undertook physical capacity tests. Measures of activity frequency and diversity (attendance) and involvement level were collected weekly across baseline (4–6 weeks), intervention (12 weeks), and nine months follow-up (including Covid lockdown).
Results
Median attendance was 23 of 24 HLMP sessions. Attendance goal/s attainment was highest during COVID lockdown. Involvement goals were consistently attained throughout all phases. Physical performance goal/s attainment was highest during intervention phase but reduced during nine months follow-up. Frequency of participation in physical activities varied greatly across study phases (range 0–33 episodes/week) with stable variety of activities and generally high ‘involvement.’ During the intervention, seven participants improved physical capacity and six maintained, or increased, the gains six months later.
Conclusion
Most participants improved physical capacity post-intervention but only some had sustained attendance and involvement in physical activity, highlighting the complexity of physical activity participation.
IMPLICATIONS FOR REHABILITATION
Health professionals’ promotion of sustained participation in physical activity needs to consider individual preferences for frequency, diversity and duration.
Supporting and measuring involvement in physical activity should be prioritised as a key outcome of an intervention.
Physical activity interventions should be followed up for longer than six months to determine sustained changes in participation outcomes
Measuring physical capacity and performance gains alone is insufficient to determine sustained, meaningful participation.
Regular participation in physical activity is important for physical fitness and well-being for everyone, including those with disabilities. Lack of participation in physical activity in the general population has been associated with preventable morbidity [Citation1,Citation2]. Conversely, regular participation in physical activity can improve physical fitness, cardiometabolic, bone and mental health, cognitive outcomes and reduced adiposity in children, youth and adults [Citation1–3]. Despite strong evidence for the benefits of physical activity participation, insufficient evidence is available to determine the dose-response relationships between being active and sedentary, the most effective type of physical activity, and the outcomes for people with disability.
To achieve the greatest benefits, the premise is that physical activity should be sustained. Physical activity that continues over an extended period is considered to be sustained. It is different to infrequent or one-off physical activities or habitual activities of daily living. In the literature, sustained participation may be described as activity extending from 6 weeks to 28 years [Citation4,Citation5], with no definition consensus. Strategies to sustain participation in physical activity in the general population include promoting use of outdoor and green spaces [Citation6], developing online and in-person social networks [Citation7], targeting health behaviour change [Citation8], and increasing skills competence [Citation9].
For children and adolescents with cerebral palsy, physical activity is equally important [Citation10]. Yet, limited research has been conducted to determine the health and wellbeing benefits, particularly benefits that are long term and result in increased participation for this population [Citation11]. Effective interventions to promote physical activity in children with cerebral palsy are not well described, and how they might translate into improvements in being active in everyday life have rarely been measured beyond six months [Citation11,Citation12]. Current 24-hour activity guidelines provide recommended daily and weekly doses of physical activity; ideas to reduce sedentary behaviour; and consideration of a whole-day approach to being active for ambulant and non-ambulant children and adolescents with cerebral palsy [Citation13]. The guidelines do not include how to sustain being active or a time frame required to optimise the likelihood that an activity will be sustained in the future. To advance knowledge in this area, there has been a call for a shift in focus to participation in physical activity as the primary outcome, rather than secondary, in studies of interventions to promote children with cerebral palsy to be more active [Citation14].
Using the Family of Participation-Related Constructs (fPRC) framework [Citation15] the essential participation constructs of attendance and involvement, can form primary outcomes and be linked to relationships with the person (activity competence, sense of self and preferences), the context, and the environment. The fPRC framework promotes understanding of the impact of constructs related to a person’s experience of participating, in this instance, in physical activity. Attendance is defined as ‘being there’ [Citation15] and can be measured as the frequency of attendance and/or the range (diversity) of different activities that the child takes part in. Attendance is commonly used as a participation outcome measure and is widely understood [Citation15,Citation16]. Involvement is the ‘experience of participation while attending’ [Citation15], is less often measured and poorly understood. Essential elements of involvement may include motivation, persistence, affect, engagement, and perhaps social connection, which link the person to all levels of the construct. The fPRC enables clinicians and researchers to choose components of participation and the pathways to be investigated as a means e.g., starting point, or ends e.g., a secondary outcome [Citation15].
To achieve sustained participation in physical activity across the life course, interventions and approaches need to target the constructs of participation attendance and involvement and move beyond therapy provided in clinical settings [Citation14]. Running is one important community physical activity in which all ambulant children can participate. Despite the importance of running as a high-level mobility skill, interventions addressing the skill of running and relationship to community participation in cerebral palsy are rare. In one randomised control trial, ambulant children with cerebral palsy achieved goal-based running and school participation outcomes at the end of the 12-week programme. Sustained changes beyond the programme are unknown as no follow-up was conducted and participation was measured retrospectively using 4-month recall at the end of the intervention [Citation17].
To address the gap in knowledge about real time and sustained changes in attendance and involvement during and beyond interventions, single subject research designs (SSRD) have been recommended [Citation18]. The fPRC was the conceptual framework that underpinned the research design that aimed to investigate the relationship between attendance and involvement and its effect on sustained participation in community-based physical activities as a means. A high-level mobility programme (HLMP) for children with cerebral palsy was developed, using a SSRD, with the primary outcome of participation and secondary outcomes including physical performance and capacity. The aim was to evaluate the effect of a community-based, goal-focused HLMP on physical activity participation outcomes of ambulant children living with cerebral palsy.
Research Questions:
Does a community-based HLMP change attendance and involvement in physical activity of children with cerebral palsy?
To what extent do these children sustain their attendance and involvement in physical activity 9 months after completion of a community-based HLMP?
Does a community-based HLMP improve physical performance and capacity for physical activity in children with cerebral palsy? Are these gains sustained at 6-month follow-up?
Methods
Study design
A non-randomised, concurrent, SSRD study design was used with a multiple-baseline component. The SSRD involved establishing participant goals at baseline and collection of outcomes over five phases: baseline (4–6 weeks in length dependent on recruitment date), intervention (12 weeks) and three follow-up phases (totalling 40 weeks post-intervention). The intended follow-up phase had been 26 weeks; however, because the COVID-19 pandemic interrupted participants’ lives and the study, the protocol was amended to increase the length of follow-up. Follow up phases were: 12 weeks post HLMP, COVID lockdown (12 weeks of home-based physical activity), and 16 weeks post lockdown.
Ethical approval was granted by the Health and Disability Ethics Committee of New Zealand (19/STH/22) and Australian Catholic University Human Research Ethics Committee (2019-49 R). Written informed consent was obtained from each participant and their parents. The study was registered with the Australian New Zealand Clinical Trials Registry (trial identification number ACTRN 12619000126112; universal trial number U1111-1226-8425).
Participant Recruitment
Included participants were children/adolescents with cerebral palsy aged 7–18 years and functioning at Gross Motor Function Classification System (GMFCS) [Citation19] levels I-II. Children needed to be able to follow instructions, understand and speak English, and set goals. Children were excluded if they had received multi-level orthopaedic surgery in the past year or any other surgical intervention in the past six months that may have contraindicated their participation in a running programme. Eligibility was confirmed by each participant’s nominated health professional (e.g., physiotherapist or general practitioner). Recruitment was conducted from healthcare and community physical activity settings using posters, advertising on the website and email posts, and therapists purposefully informing suitable families of the research. Children, assisted by their parents, were asked to rate their gross motor functional levels using the GMFCS [Citation19], upper limb ability using the Manual Ability Classification System (MACS) [Citation20], functional mobility (Functional Mobility Scale – FMS) [Citation21], and communication skills using the Communication Function Classification System (CFCS) respectively [Citation22]. Identification of functional levels helped target physical performance goals, drills and skills for each adolescent e.g., asymmetric propulsion and arm swing in hemiplegia, and for consideration during goal setting and communication throughout the study.
High-level-mobility programme
The HLMP provided task-specific training of running skills for one hour, twice each week, for 12 weeks. The HLMP design was based on motor training principles [Citation23,Citation24] and delivered at school sports fields, a local playground or, in poor weather, indoor therapy rooms. The HLMP was delivered by a qualified physiotherapist with extensive physical activity prescription and coaching experience and assistance from an athletics trainer for half of the sessions; both with experience in physical activity for people with disability. An individualised home exercise programme (HEP) was provided and updated fortnightly; with 2–3 sessions of self-practice recommended each week to enhance goal attainment. Participants had no restrictions on therapy provision during the study period. The design of the HLMP intervention is summarised in Supplementary Table 1 using the Template for Intervention Description and Replication (TIDieR) checklist [Citation25].
Outcome measures
Goal Attainment Scale: Individual attendance, involvement and physical performance goals (that did not need to be running based) were identified using semi-structured interviews with each child and parents. Participants could choose more than one goal in each area. Examples of chosen goals included: “I play tennis with my family every week” (attendance), “I sort of like exercising” (involvement), “I can run 500m without stopping” (physical performance). Goals were scaled in discussion with participants during the baseline period using Goal Attainment Scale (GAS) methods [Citation26]. GAS ratings were collected fortnightly for up to 26 weeks from the start of the HLMP. In total, 16 physical performance goals, 8 attendance and 9 involvement goals were set. Participants retained the same goals throughout the study, progressively resetting the levels of attainment for physical performance when goals were achieved.
Attendance Diary: frequency, diversity and duration of activity: A study-specific diary was designed and piloted prior to use with 12 children, aged 7–16 years. Attendance frequency (how often), diversity (range of activities) and duration of activity greater than 15 minutes were recorded weekly for up to 58 weeks. Participation in school-based physical activity was excluded from diary records.
Involvement Diary: Participants rated their level of involvement each week for each activity in their diary as ‘not at all,’ ‘somewhat’ or ‘very involved.’ Involvement was operationalised for participants as “think about how you felt, how much you were thinking about the activity and how hard you tried during each activity.”
Physical capacity: Three cerebral palsy specific, valid and reliable running tests, and the Revised High-Level Mobility Assessment Tool (HiMAT) [Citation27], assessed physical capacity. All physical testing occurred using a standardised protocol, in the same order and location, with a 3-minute rest between each test [Citation28–33]. Testing took one hour for each participant and was conducted as follows: (i) 2-dimensional gait and running sagittal and frontal video recording to capture strides/20m, time/20m, time/40m and to use for analysis of running form [Citation17]; (ii) Muscle power sprint test (MPST) [Citation33]; (iii) Revised HiMAT [Citation27]; (iv) 10x5m agility test [Citation33]; and (v) 10m modified shuttle run test (10m SRT-I and SRT-II for children classified GMFCS I-II respectively) [Citation32].
Analyses
Descriptive statistics were calculated and tabulated for each phase for each participant from their diary data including: (i) the number of times goal attainment was achieved (GAS score of 0, +1 or +2); (ii) total (min/max) frequency of attendance in physical activity; (iii) mean weekly frequency (total, min/max); (iv) diversity of activities—total (number of specific activities) and median (interquartile range) weekly diversity; (v) duration in minutes (total and range per activity, plus mean (and range) weekly duration and mean minutes per day. The minimum recommended weekly duration of physical activity of 420 min for children and adolescents with cerebral palsy was used as the duration comparator [Citation13]. Weekly level of involvement, and weekly level of involvement for time spent active, during each phase for three ratings of involvement were also calculated (not at all = 1 point, somewhat = 2 points, very involved = 3 points).
Data were displayed graphically for each participant, for each study phase, for all outcome measures. Visual and graphic analysis using SSRD guidelines for change in trend (slope), shift in level, and latency of change were employed [Citation34]. Differences in the standard deviation were calculated when a shift in level was observed visually. Frequency of attendance in the HLMP and HEP (the intervention) were summarised separately to GAS and diary data which reported community-based participation. In addition, data for all participants were pooled, tabulated, and analysed for each phase, enabling comparison of mean frequency and duration, and median diversity of participation in physical activity.
Physical capacity data obtained at the four testing sessions for participants were pooled, tabulated, and expressed as line graphs for graphic, and descriptive analyses. Minimal clinically importance differences (MCID) for each test—MPST, Agility test, and 10 m-SRT I or SRT II—for each participant were calculated based on norm reference values for cerebral palsy [Citation32,Citation33]. No norm referenced values have been developed for the HiMAT for children with cerebral palsy and recommended Minimal Detectable Change (MDC) values for children and adolescents with traumatic brain injury were used [Citation35].
Results
Participant demographics
Eight adolescents were recruited (mean age 13 years 11 months, range 11–16 years, SD 1.5 years). There were seven males and one female, five functioning at GMFCS Level I and three at Level II. The adolescents’ mobilised independently without aids at home and in the community (FMS) [Citation21], had upper limb skills classified in MACS I-III [Citation20] and communication skills using the CFCS at levels I-II [Citation22]. Self- or parent-reported ethnicity was New Zealander or New Zealand European. No participant received therapy or other treatment during the study period except for orthotic management. Participants are referred to as A1-8.
Participation in the HLMP and completeness of data collection
Attendance at the 24 HLMP sessions ranged from 2 to 23 (median = 23). Except for one participant (A4, the one female participant), non-attendance was primarily related to social and school events.
Adverse events
There were no adverse events that limited participation in the 12-week HLMP. A2 and A7 tripped while attempting drills over low hurdles resulting in low level Grade 1 inversion ankle sprains. A2 sprain was on his non-hemiplegic ankle, occurred at the end of a session, and following RICE treatment at home, could participate fully in all remaining sessions thereafter. A7 had a history of bilateral ankle instability and was reviewed by an independent physiotherapist following his Grade 1 sprains on his right side. Conservative management was recommended, and no concerns were raised regarding continued participation in the HLMP. Based on A7 history, drills and skill-based activities were modified to enable full participation and to mitigate risks (e.g., drills over the low hurdles were changed to next to the hurdles). No sessions were missed due to injury.
Full data sets (all GAS and weekly activity charts) were obtained for six participants over 56–58 weeks. The female participant A4 had full data collection until week 21. She returned only 12 (46%) of the 26 activity charts during the COVID and follow-up phases. Due to low completion rate and significant social strain on this family during COVID, the decision was taken to stop data collection at 6-months follow-up. Participant A8 completed 100% of his charts until 6 weeks into the post-lockdown phase (providing 30 weeks of post-HLMP data); he left New Zealand at this time and no further data were collected. Diary data enabled variations in participation (actual activities and attendance/involvement) to be identified e.g., due to injury, illness, being on holiday, which provided important details when collecting a longitudinal record of sustained participation.
Completion of the weekly, personalised home exercise programme (HEP) was low during the HLMP (total range 1–28 of sessions completed, with session duration ranging 15–24 min). One participant (A7) completed the HEP the recommended 2–3 times per week during the intervention phase. Whilst not a study requirement, six participants intermittently completed their HEP during follow-up phases, with 59 sessions completed in total.
Goal attainment
displays the summary of outcomes for all GAS goals across the study phases. Involvement goal attainment was the most consistently attained throughout all phases, with goals achieved at least once by 7 participants. Attainment of attendance goals was highest during the COVID lockdown despite venue restrictions (5/7 goals achieved for 5/7 participants). Physical performance goal attainment was highest during the intervention phase (11/15 goals attained at GAS scores of 0 or above for 7 participants), with reduced attainment during the 9 months of follow-up. Six physical goals were incrementally increased up to four times following attainment of GAS scores of +2 – greater than expected improvements (see Supplementary Tables 2–4 for all GAS goals set and scaled (attendance, involvement, physical performance) and Figures 1–3 for graphed results for each participant for each GAS goal).
Table 1. Participant demographics and summary of study participation.
Table 2. Overall goal attainment for all participants in all study phases.
Attendance outcomes
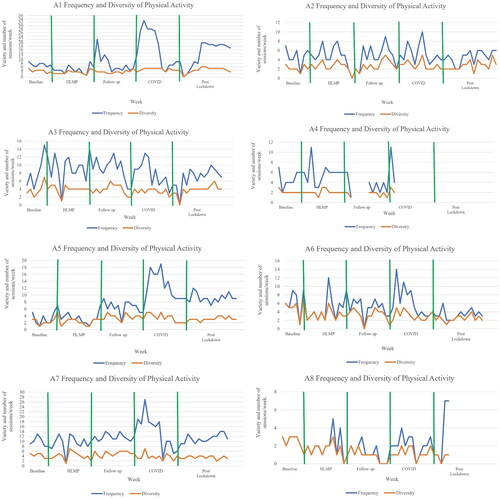
shows variable frequency of attendance in physical activity (mean 1.7–16.7 occasions/week; range 0–33 occasions/week) between participants but stable number of physical activities (median range 1–5.5 activities) over time. Participants with low frequency and diversity at baseline (A4 and A8) remained low through all phases. Participants A1, A5, A6 and A7 increased their frequency of activity over the COVID lockdown phase. Participants reported a range of 8–27 different activities across each study phase, with informal activities most common (total diversity: 14 formal: 36 informal activities). Formal activities included swimming lessons, athletics, personal training, cricket, karate and football, while informal varied from walking, running, biking, trampolining, skiing, go-karting and kayaking. Participants returned to their pre pandemic physical activity choices and frequency following COVID lockdown.
Figure 1. Weekly frequency (times participated) and diversity (number of different activities) of physical activity for each participant across phases.
Note. The multiple baseline period varied from 4 to 6 weeks based on recruitment date. Data were intended to be collected weekly. Y axis variable increments due to large variation in number of sessions of physical activity participated in each week (frequency). Zero scores for frequency and diversity reflect injury, illness, no activity, or need for a week of rest. Gaps in frequency and diversity figures indicate no data returned. Data collection ended at week 42 for A4 and week 47 for A8. HLMP: High level mobility programme; COVID: COVID lockdown period in New Zealand
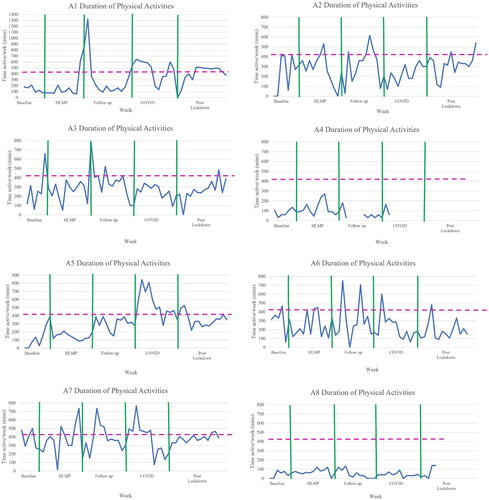
Mean weekly duration of physical activity for all participants over the five study phases ranged from 38 min (A8) to 525 min (A5) (). Six participants achieved at least 420 min of physical activity in some weeks of data collection but only participants A1, A5, and A7 achieved the recommended weekly duration for a whole phase (COVID lockdown phase). The greatest within and between participant variation in mean weekly duration of physical activity occurred during the COVID phase.
Figure 2. Time spent physically active (minutes/week) for each participant across phases.
Note. The multiple baseline period varied from 4 to 6 weeks based on recruitment date. Data were intended to be collected weekly. Y axis displayed in increments of 100 minutes of participating in physical activity/week. Zero scores for duration reflect injury, illness, no activity or need for a week of rest. Gaps in duration figures indicate no data returned. Data collection ended at week 42 for A4 and week 47 A8. Dashed line represents 420 minutes, which is the minimum recommended duration of moderate-vigorous physical activity per week for children with cerebral palsy. HLMP: High level mobility programme; COVID: COVID lockdown period in New Zealand.
Visual analysis showed no consistent changes in slope or shift in levels following the HLMP during the 9 months of follow-up in attendance for any of the participants ( and ). Therefore, planned trend lines and latency of change are not displayed and differences in the standard deviation for a shift in level were not calculated.
Involvement outcomes
Participants consistently rated involvement in their activities of choice as ‘very involved.’ Involvement remained high with increased time spent active for all study phases (Supplementary Figure 4–5). Being ‘not involved’ occurred for a total of seven occasions between four participants over 13 months. Following the HLMP, there was no obvious trend or shift from ‘somewhat involved’ to ‘very involved’ when weekly involvement in activities was measured. Being ‘somewhat involved’ was reported for activities considered chores rather than choice, such as walking the dog (A7), biking for errands (A2), and playing with younger siblings (A1). Being ‘very involved’ was predominantly observed in organised and formal activities, including swimming lessons, sports, karate lessons, and athletics practice and competitions.
Physical capacity outcomes
The seven participants who attended the HLMP showed improvement in at least one measure of physical capacity using MCID values [Citation29,Citation30] and MDC [Citation35]. Six participants maintained or further increased the gains 6 months later (Supplementary Figures 6–10). Gait parameters also improved in participants who made ongoing gains in physical capacity. Participant A4 (non-attendee in HLMP) showed lower scores in MPST, 10 m SRT-I and the Revised HiMAT physical capacity tests over the testing period and had low levels of participation and goal attainment.
Discussion
This study used a SSRD to evaluate whether participation in a 12-week, community-based HLMP delivered using a goal-focused approach could promote individual sustained participation in physical activity over the next 40 weeks. We found that the HLMP was a safe and well attended intervention for seven of eight of the adolescents in this study, consistent with past running interventions for children with cerebral palsy [Citation17]. The HLMP meet recommended intervention guidelines by implementing whole-task running practice within the community, with a focus on self-identified, functional, participation and physical goals, while considering the preferences and ability of each adolescent [Citation36]. In addition, quality physical activity participation experiences were fostered by offering the HLMP as a group based session with their peers with cerebral palsy and providing experienced, knowledgeable instructors in adapted physical activity [Citation37].
At baseline, five adolescents showed stable physical activity frequency, three maintained duration and all had stable diversity. We found highly variable frequency and duration of participation in physical activities between adolescents but less within-participant variability in all phases of the 13 months’ study period. Participation involvement and physical performance goals were more likely to be achieved than attendance goals, with less than 30% attainment during the post lockdown phase. Despite following goal setting guidelines of self-selected, meaningful functional goals in preferred activities [Citation36], adolescents ability to increase their frequency of being active appeared to be challenging.
In all study phases, the diversity of physical activities remained relatively stable. Low diversity but consistently high frequency and duration of physical activity in some adolescents suggested that they had identified their preferences, formed habits, and were able to sustained their participation over the 13 months of the study. The stable within-participant results for frequency and diversity is a positive result, particularly in children aged 10–13 years, when participation in physical activity is known to decline [Citation38].
The HLMP appeared to have a positive influence on five adolescents, who increased their daily duration of physical activity in the follow-up phase. The HLMP participation focused approach promoted “being active” but was not successful in assisting participants to meet or exceed the ‘minimum recommendations’ of 60 min of physical activity accumulated each day. The findings are consistent with past research suggesting even the most able children with cerebral palsy are more sedentary than their peers [Citation39–41]. Focusing on ‘having a go’ and making meaningful changes for low active participants rather than meeting guidelines may be the best starting point for children and adolescents with cerebral palsy, given that ‘doing something is better than nothing’ [Citation42,Citation43].
Consistent ratings of high involvement within participation diary data suggested adolescents had sustained, high levels of effort, focus, motivation and engagement when active. Maintenance of high levels of involvement when adolescents increased their time spent active and high involvement goal attainment over the research period (67–88%), supports the concept that involvement may be of key importance to supporting ongoing participation [Citation15]. Sustaining involvement may be critical to a life course of physical activity, with up to 70% of non-disabled adolescents reportedly withdrawing from youth sports between 7 and 18 years of age [Citation44]. Assessing sustained participation based on involvement appears to be an important inclusion in participation research and exercise prescription. Measuring involvement, however, presents with challenges, given its personal nature, the fluctuations that can exist within a physical activity session, and the proxy reporting required for some children.
The HLMP focused on running as the goal-directed physical activity in view of its importance in recreation, play, and many sporting activities [Citation45]. Learning the skill of running in childhood may improve running proficiency, increase physical output, improve mental health, and lessen the long-term risk of secondary health conditions [Citation46,Citation47]. Running is an important skill for ambulant children with cerebral palsy and was the physical activity most frequently participated in by young New Zealanders with disability and their non-disabled counterparts (47% and 52%, respectively) [Citation48]. Despite the global prevalence of participation in running [Citation49] and the potential health and social benefits, there is a surprising paucity of research on specific interventions to improve running in children and adults with disabilities.
We found high levels of physical goal attainment during the HLMP (73%), which were slightly lower than those reported by Gibson and colleagues of 86% following a similar motor learning based running intervention called Xcelerate [Citation17]. Lower physical goal attainment beyond the HLMP may reflect changing preferences and motivation for the same goals. All seven adolescents who regularly attended the HLMP made clinically meaningful changes in at least one of the four physical capacity measures and six continued to make changes six months beyond the HLMP. Running capacity changes were not shown using the randomised control trial methodology in Xcelerate and sustained changes beyond that programme are unknown as no follow-up was conducted.
The ongoing improvements in physical capacity and physical performance following the HLMP provided evidence that children with cerebral palsy could learn the skill of running within a participation focused approach and translate gains into daily physical activities; which is not always demonstrated [Citation50]. The improvements beyond the HLMP, with minimal uptake of the HEP, indicates the relative low dose of 24 h of intervention across the intervention period was effective. The findings compare favourably to other therapy interventions requiring higher dosing [Citation51], participation focused interventions [Citation43,Citation52,Citation53] and school based interventions in typically developing peers where sustained impacts beyond six months have been limited to improvement in duration of physical activity of 3–14 min/day and not fitness or skills [Citation54].
Participant A4, the only female, attended 2 of the 24 programme sessions. Participant A4 showed reduction in some physical capacity outcomes, had habitually low levels of attendance in physical activity and poor goal attainment throughout. Low levels of leisure time physical activity diversity and a lack of participation in organised sport in adolescence may translate to reduced participation in the adult years [Citation55,Citation56] and risk of secondary morbidities [Citation10] which raises further reasons for concern in an already sedentary adolescent.
COVID
Six adolescents maintained or increased their participation and physical capacity during the New Zealand three-month COVID lockdown period. This may be explained by more family time and more free time to be active. Participants may have developed positive physical activity participation habits and could practice these more in everyday activities at home. Participant involvement and diversity scores were consistent during the COVID period, suggesting that adolescents were able to replace their formal activities (such as swimming lessons due to lockdowns) with informal activities, in which they reported high levels of involvement. Return to the same physical activity levels post COVID was a positive outcome, with international literature finding COVID lockdown were very challenging for many families with children and young adults with disabilities [Citation57].
The complexity of sustaining physical activity participation
Morris et al. [Citation58] developed the Framework of Sustained Participation to provide a structure that may assist clinicians and facilitators to promote sustained participation in young people with cerebral palsy. The authors defined sustained participation as attending the same activity for one year or two seasons; however, this may not meet the preferences or needs of the adolescent and family or address the components of involvement. Determining what needs to be supported and for how long to achieve sustained physical activity participation remains a challenge. Kalhert [Citation59] describes a lapse–recovery relationship for participation in physical activity and an expected volatility, consistent with the adolescent diary data in this study. Fluctuating frequency and high levels of enjoyment (one component of involvement) have also been found in the early years of children with cerebral palsy; with context, motor function and parental empowerment influencing participation [Citation60]. The importance of opportunities and support to participate for children with cerebral palsy from their parents, their community, and stakeholders from an early age to ensure sustained physical activity participation cannot be underestimated [Citation61].
In the present study, some participant’s results demonstrated a disconnect between outcomes and highlight the complexity of participation. Goal attainment and frequency, diversity, and duration of physical activity were not consistent, irrespective of gains in physical capacity for some participants. This may reflect an intention-behaviour gap regarding physical activity [Citation62], that is, what we say we will do, is not always what we do. For example, Participant 5 attained his involvement goal and had more ratings of being ‘very involved’ as the study progressed. He increased frequency of participation and improved in three physical outcome measures at the end of the HLMP and 6 months follow up. Yet, his goal of increasing his frequency of running was achieved only once and his physical goal of running 800 m without stopping was attained during the HLMP and not attempted beyond the programme despite much greater physical competency.
Strengths and limitations
The SSRD allowed each adolescents response to participating in the HLMP and the effect of the HLMP on sustained attendance and involvement to be investigated for 9 months post HLMP. The SSRD involved repeated, weekly measurements that provided nuanced insight about the results, for example, impacts of illness, injury, being on holiday and COVID. We found that longitudinal measurement of participation in physical activity in ambulant adolescents with cerebral palsy was feasible with almost full data collection achieved. The high level of complete data (including during a COVID lockdown period) may indicate the value placed on the research by these adolescents and the relationships that can be formed during research using an SSRD. Longitudinal measurements of this type have long been called for [Citation63,Citation64] with shorter time frames considered inadequate to detect change [Citation65] or determine retention of gains [Citation66].
Although a valuable design, the SSRD was not without limitations. There was no randomisation element and fewer data points in a relatively short baseline (4–6) meant stability was not reached for some participants for some measures e.g., duration for five adolescents [Citation18]. The variability in the data meant it was not appropriate to apply statistical analysis methods. Gaps in weekly participation and goal attainment data for A4 after the HLMP led to early cessation of data collection and limited the conclusions that could be drawn for that participant. Measuring the same goals repeatedly for the extended 13-month time-period of the SSRD did not allow for changing preferences or personal expectations. This is unlikely to be a problem in clinical practice, where more frequent evaluations and opportunities to reset or replace goals are possible. Missing data for some participation goals were inevitable due to activity and location restrictions during the COVID phase.
Future research
There is growing evidence to support community-based, participation-focused interventions [Citation43,Citation52]. However, changes have been measured for a maximum of six months post interventions [Citation43,Citation52,Citation53], which cannot be considered to represent sustained participation. Whiteneck and Dijkers [Citation67] described participation as the ultimate health outcome and rehabilitation goal. While ‘participation’ might imply an ongoing experience, for physical activity, sustained participation is needed to confer health benefits and thus should be the explicit aim. Understanding sustained participation requires further definitional clarity and longitudinal measurement. Interventions and longitudinal research addressing sustained participation in physical activity are essential to promote increased activity levels among people with cerebral palsy over the life course to support their health and well-being [Citation68–70].
The HLMP was based on contemporary expectations, was pragmatic, and embraced real-world physical activity participation experiences of adolescents with cerebral palsy [Citation15]. Future research should involve trialling the HLMP in younger ambulant children with cerebral palsy. Developing high level mobility skills at a younger age might support establishing positive health habits from a young age, which is an imperative. Running appears to be a good choice due to its popularity as a physical activity and importance as a skill in a range of other physical activities [Citation48,Citation71,Citation72].
Piloting the HLMP in ambulant adults with cerebral palsy using a participation-based approach is also recommended. Adults with cerebral palsy lose mobility, report increased fatigue, pain and mental health issues, which can be minimised by exercise [Citation70,Citation73,Citation74]. There is evidence to support increased participation in physical activity in adults with cerebral palsy, most of whom do not meet the guidelines for physical activity [Citation75]. Participation in a community-based HLMP may have benefits beyond participation and competence in physical activities.
Qualitative exploration of sustained participation in physical activity is also recommended [Citation76]. The voice of people with lived experience may help inform the way interventions are delivered to encourage lifelong participation and translate current clinical practice to meaningful everyday physical activity. Participants’ experiences were explored qualitatively during the SSRD and findings are reported separately [Citation77].
Conclusions
The HLMP was a safe community-based, targeted and goal-directed intervention for ambulant adolescents with cerebral palsy, delivered using a participation-focused approach. Delivery of the HLMP twice weekly for 12 weeks appears to have been adequate to maintain, improve and sustain participation attendance, involvement and physical capacity, skill and goal attainment, nine months beyond the programme for some, but not all, of the eight participants. Variability in results within and between participants over the 13 months highlights the complex, dynamic and perhaps unpredictable nature of participation in physical activity.
Authors contributions
GK performed the high-level mobility intervention, extracted the data for analysis, conducted the analysis, and wrote the paper. GK, CI, SS, BA, MS and AH were involved in the design of the research, drafting, and review of the paper.
Supplemental Material
Download MS Word (4.3 MB)Acknowledgements
The authors would like to acknowledge the eight participants and their families for their commitment to this research over a 13-month period, including a three month period of COVID lockdown in New Zealand. GK would like to acknowledge the contribution of the Australian Commonwealth Government for support through an Australian Government Research Training Program Scholarship during her PhD at the Australian Catholic University and the University of Melbourne.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Gibson‐Moore H. UK chief medical officers’ physical activity guidelines 2019: What’s new and how can we get people more active? Nutr Bull. 2019;44(4):320–328. doi: 10.1111/nbu.12409.
- Physical Activity Guidelines Advisory Committee. Physical activity guidelines advisory committee scientific report. 2018.
- Chaput J-P, Willumsen J, Bull F, et al. 2020 WHO guidelines on physical activity and sedentary behaviour for children and adolescents aged 5–17 years: summary of the evidence. Int J Behav Nutr Phys Act. 2020;17(1):141. doi: 10.1186/s12966-020-01037-z.
- Palomäki S, Hirvensalo M, Smith K, et al. Does organized sport participation during youth predict healthy habits in adulthood? A 28-year longitudinal study. Scand J Med Sci Sports. 2018;28(8):1908–1915. doi: 10.1111/sms.13205.
- Robroek SJ, Van Lenthe FJ, Van Empelen P, et al. Determinants of participation in worksite health promotion programmes: a systematic review. Int J Behav Nutr Phys Act. 2009;6(1):26. doi: 10.1186/1479-5868-6-26.
- Bedimo-Rung AL, Mowen AJ, Cohen DA. The significance of parks to physical activity and public health: a conceptual model. Am J Prev Med. 2005;28(2 Suppl 2):159–168. doi: 10.1016/j.amepre.2004.10.024.
- Rovniak LS, Sallis JF, Kraschnewski JL, et al. Engineering online and in-person social networks to sustain physical activity: application of a conceptual model. BMC Public Health. 2013;13(1):753. doi: 10.1186/1471-2458-13-753.
- Tombor I, Michie S. Methods of health behavior change. Oxford: Oxford University Press; 2017.
- Stodden DF, Goodway JD, Langendorfer SJ, et al. A developmental perspective on the role of motor skill competence in physical activity: an emergent relationship. Quest. 2008;60(2):290–306. doi: 10.1080/00336297.2008.10483582.
- Cribb CF, Keko M, Creveling S, et al. Mental health, physical activity, and sports among children with cerebral palsy. Child Care Health Dev. 2023;19:1–8. doi: 10.1111/cch.13122.
- Ryan JM, Cassidy EE, Noorduyn SG, et al. Exercise interventions for cerebral palsy. Cochrane Database Syst Rev. 2017;6(6):CD011660. doi: 10.1002/14651858.CD011660.pub2.
- Kilgour G, Adair B, Stott NS, et al. Do physical activity interventions influence subsequent attendance and involvement in physical activities for children with cerebral palsy: a systematic review. Disabil Rehabil. 2022;44(9):1682–1698. doi: 10.1080/09638288.2021.1909151.
- Verschuren O, Hulst RY, Voorman J, et al. 24-hour activity for children with cerebral palsy: a clinical practice guide. Dev Med Child Neurol. 2021;63(1):54–59. doi: 10.1111/dmcn.14654.
- Gorter JW. Physical activity interventions for children and young people with cerebral palsy. Dev Med Child Neurol. 2017;59(10):990–991. doi: 10.1111/dmcn.13550.
- Imms C, Granlund M, Wilson PH, et al. Participation, both a means and an end: a conceptual analysis of processes and outcomes in childhood disability. Dev Med Child Neurol. 2017;59(1):16–25. doi: 10.1111/dmcn.13237.
- Adair B, Ullenhag A, Rosenbaum P, et al. Measures used to quantify participation in childhood disability and their alignment with the family of participation‐related constructs: a systematic review. Dev Med Child Neurol. 2018;60(11):1101–1116. doi: 10.1111/dmcn.13959.
- Gibson N, Chappell A, Blackmore AM, et al. The effect of a running intervention on running ability and participation in children with cerebral palsy: a randomized controlled trial. Disabil Rehabil. 2018;40(25):3041–3049. doi: 10.1080/09638288.2017.1367426.
- Tate R, Perdices M. Single-case experimental designs for clinical research and neurorehabilitation settings: planning, conduct, analysis and reporting. Milton Park: Routledge; 2019.
- Palisano RJ, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x.
- Eliasson AC, Krumlinde‐Sundholm L, Rösblad B, et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48(7):549–554. doi: 10.1017/S0012162206001162.
- Graham HK, Harvey A, Rodda J, et al. The functional mobility scale (FMS). J Pediatr Orthop. 2004;24(5):514–520. doi: 10.1097/01241398-200409000-00011.
- Hidecker MJC, Paneth N, Rosenbaum PL, et al. Developing and validating the communication function classification system for individuals with cerebral palsy. Dev Med Child Neurol. 2011;53(8):704–710. doi: 10.1111/j.1469-8749.2011.03996.x.
- Williams G, Morris ME. High-level mobility outcomes following acquired brain injury: a preliminary evaluation. Brain Inj. 2009;23(4):307–312. doi: 10.1080/02699050902774170.
- Williams G, Schache AG. Evaluation of a conceptual framework for retraining high-level mobility following traumatic brain injury: two case reports. J Head Trauma Rehabil. 2010;25(3):164–172. doi: 10.1097/HTR.0b013e3181dc120b.
- Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348(3):g1687. doi: 10.1136/bmj.g1687.
- Kiresuk TJ, Smith A, Cardillo JE. Goal attainment scaling: applications, theory, and measurement. New Jersey: Lawrence Erlbaum Associates; 1994.
- Williams G, Pallant J, Greenwood K. Further development of the high-level mobility assessment tool (HiMAT). Brain Inj. 2010;24(7-8):1027–1031. doi: 10.3109/02699052.2010.490517.
- Ratel S, Williams C, Oliver J, et al. Effects of age and recovery duration on performance during multiple treadmill sprints. Int J Sports Med. 2006;27(1):1–8. doi: 10.1055/s-2005-837501.
- Verschuren O, Bloemen M, Kruitwagen C, et al. Reference values for aerobic fitness in children, adolescents, and young adults who have cerebral palsy and are ambulatory. Phys Ther. 2010;90(8):1148–1156. doi: 10.2522/ptj.20090318.
- Verschuren O, Bloemen M, Kruitwagen C, et al. Reference values for anaerobic performance and agility in ambulatory children and adolescents with cerebral palsy. Dev Med Child Neurol. 2010;52(10):e222-e8. doi: 10.1111/j.1469-8749.2010.03747.x.
- Verschuren O, Bongers BC, Obeid J, et al. Validity of the muscle power sprint test in ambulatory youth with cerebral palsy. Pediatr Phys Ther. 2013;25(1):25–28. doi: 10.1097/PEP.0b013e3182791459.
- Verschuren O, Takken T, Ketelaar M, et al. Reliability and validity of data for 2 newly developed shuttle run tests in children with cerebral palsy. Physical Therapy. 2006;86(8):1107–1117. doi: 10.1093/ptj/86.8.1107.
- Verschuren O, Takken T, Ketelaar M, et al. Reliability for running tests for measuring agility and anaerobic muscle power in children and adolescents with cerebal palsy. Pediatr Phys Ther. 2007;19(2):108–115. doi: 10.1097/pep.0b013e318036bfce.
- Kazdin AE. Single-case experimental designs. Evaluating interventions in research and clinical practice. Behav Res Ther. 2019;117:3–17. doi: 10.1016/j.brat.2018.11.015.
- Kissane AL, Eldridge BJ, Kelly S, et al. High-level mobility skills in children and adolescents with traumatic brain injury. Brain Inj. 2015;29(13–14):1711–1716. doi: 10.3109/02699052.2015.1075174.
- Jackman M, Sakzewski L, Morgan C, et al. Interventions to improve physical function for children and young people with cerebral palsy: international clinical practice guideline. Dev Med Child Neurol. 2022;64(5):536–549. doi: 10.1111/dmcn.15055.
- Shirazipour CH, Evans MB, Leo J, et al. Program conditions that foster quality physical activity participation experiences for people with a physical disability: a systematic review. Disabil Rehabil. 2020;42(2):147–155. doi: 10.1080/09638288.2018.1494215.
- Baksjoberget PE, Nyquist A, Moser T, et al. Having fun and staying active! children with disabilities and participation in physical activity: a follow-up study. Phys Occup Ther Pediatr. 2017;37(4):347–358. doi: 10.1080/01942638.2017.1281369.
- Verschuren O, Peterson MD, Balemans AC, et al. Exercise and physical activity recommendations for people with cerebral palsy. Dev Med Child Neurol. 2016;58(8):798–808. doi: 10.1111/dmcn.13053.
- Gorter JW, Noorduyn SG, Obeid J, et al. Accelerometry: a feasible method to quantify physical activity in ambulatory and nonambulatory adolescents with cerebral palsy. Int J Pediatr. 2012;2012:329284–329286. doi: 10.1155/2012/329284.
- Reedman SE, Johnson E, Sakzewski L, et al. Sedentary behavior in children with cerebral palsy between 1.5 and 12 years: a longitudinal study. Pediatr Phys Ther. 2020;32(4):367–373. doi: 10.1097/PEP.0000000000000740.
- World Health Organisation. WHO Guidelines on Physical Activity and Sedentary Behaviour. 2020.
- Reedman SE, Boyd RN, Trost SG, et al. Efficacy of participation-focused therapy on performance of physical activity participation goals and habitual physical activity in children with cerebral palsy: a randomized controlled trial. Arch Phys Med Rehabil. 2019;100(4):676–686. doi: 10.1016/j.apmr.2018.11.012.
- Petlichkoff LM. The drop-out dilemma in youth sports. Child Adolescent Athlete. 1996;6:418–430.
- Chappell A, Gibson N, Morris S, et al. Running in people with cerebral palsy: a systematic review. Physiother Theory Pract. 2019a;35(1):15–30. doi: 10.1080/09593985.2018.1434846.
- Chappell A, Allison GT, Williams G, et al. The effect of a running training intervention on ankle power generation in children and adolescents with cerebral palsy: a randomized controlled trial. Clin Biomech. 2020;76:105024. doi: 10.1016/j.clinbiomech.2020.105024.
- Pedisic Z, Shrestha N, Kovalchik S, et al. Is running associated with a lower risk of all-cause, cardiovascular and cancer mortality, and is the more the better? A systematic review and meta-analysis. Br J Sports Med. 2020;54(15):898–905. doi: 10.1136/bjsports-2018-100493.
- Sport New Zealand. Active NZ spotlight on disability. Wellington: Sport New Zealand; 2018.
- Hulteen RM, Smith JJ, Morgan PJ, et al. Global participation in sport and leisure-time physical activities: a systematic review and meta-analysis. Prev Med. 2017;95:14–25. doi: 10.1016/j.ypmed.2016.11.027.
- Smits D-W, Gorter JW, van Schie PE, et al. How do changes in motor capacity, motor capability, and motor performance relate in children and adolescents with cerebral palsy? Arch Phys Med Rehabil. 2014;95(8):1577–1584. doi: 10.1016/j.apmr.2014.04.013.
- Jackman M, Lannin N, Galea C, et al. What is the threshold dose of upper limb training for children with cerebral palsy to improve function? A systematic review. Aust Occup Ther J. 2020;67(3):269–280. doi: 10.1111/1440-1630.12666.
- Anaby D, Avery L, Gorter JW, et al. Improving body functions through participation in community activities among young people with physical disabilities. Dev Med Child Neurol. 2020;62(5):640–646. doi: 10.1111/dmcn.14382.
- Anaby DR, Law M, Feldman D, et al. The effectiveness of the pathways and resources for engagement and participation (PREP) intervention: improving participation of adolescents with physical disabilities. Dev Med Child Neurol. 2018;60(5):513–519. doi: 10.1111/dmcn.13682.
- Lai SK, Costigan SA, Morgan PJ, et al. Do school-based interventions focusing on physical activity, fitness, or fundamental movement skill competency produce a sustained impact in these outcomes in children and adolescents? A systematic review of Follow-Up studies. Sports Med. 2014;44(1):67–79. doi: 10.1007/s40279-013-0099-9.
- Mäkelä S, Aaltonen S, Korhonen T, et al. Diversity of leisure-time sport activities in adolescence as a predictor of leisure-time physical activity in adulthood. Scand J Med Sci Sports. 2017;27(12):1902–1912. doi: 10.1111/sms.12837.
- Kjønniksen L, Anderssen N, Wold B. Organized youth sport as a predictor of physical activity in adulthood. Scand J Med Sci Sports. 2009;19(5):646–654. doi: 10.1111/j.1600-0838.2008.00850.x.
- Theis N, Campbell N, De Leeuw J, et al. The effects of COVID-19 restrictions on physical activity and mental health of children and young adults with physical and/or intellectual disabilities. Disabil Health J. 2021;14(3):101064. doi: 10.1016/j.dhjo.2021.101064.
- Morris A, Imms C, Kerr C, et al. Sustained participation in community-based physical activity by adolescents with cerebral palsy: a qualitative study. Disabil Rehabil. 2019;41(25):3043–3051. doi: 10.1080/09638288.2018.1486466.
- Kahlert D. Maintenance of physical activity: Do we know what we are talking about? Prev Med Rep. 2015;2:178–180. doi: 10.1016/j.pmedr.2015.02.013.
- Kalleson R, Jahnsen R, Østensjø S. Exploring participation in family and recreational activities among children with cerebral palsy during early childhood: How does it relate to motor function and parental empowerment? Disabil Rehabil. 2022;44(9):1560–1570. doi: 10.1080/09638288.2021.1894608.
- Martin Ginis KA, Ma JK, Latimer-Cheung AE, et al. A systematic review of review articles addressing factors related to physical activity participation among children and adults with physical disabilities. Health Psychol Rev. 2016;10(4):478–494. doi: 10.1080/17437199.2016.1198240.
- Rhodes R, de Bruijn GJ. How big is the physical activity intention–behaviour gap? A meta‐analysis using the action control framework. Br J Health Psychol. 2013;18(2):296–309. doi: 10.1111/bjhp.12032.
- King G, McDougall J, Dewit D, et al. Predictors of change over time in the activity participation of children and youth with physical disabilities. Child Health Care. 2009;38(4):321–351. doi: 10.1080/02739610903237352.
- Shikako-Thomas K, Shevell M, Schmitz N, et al. Determinants of participation in leisure activities among adolescents with cerebral palsy. Res Dev Disabil. 2013;34(9):2621–2634. doi: 10.1016/j.ridd.2013.05.013.
- Van Wely L, Balemans AC, Becher JG, et al. The effectiveness of a physical activity stimulation programme for children with cerebral palsy on social participation, self-perception and quality of life: a randomized controlled trial. Clin Rehabil. 2014;28(10):972–982. doi: 10.1177/0269215513500971.
- Rimmer JH. Physical fitness levels of persons with cerebral palsy. Dev Med Child Neurol. 2001;43(3):208–212. doi: 10.1111/j.1469-8749.2001.tb00189.x.
- Whiteneck G, Dijkers MP. Difficult to measure constructs: conceptual and methodological issues concerning participation and environmental factors. Arch Phys Med Rehabil. 2009;90(11 Suppl):S22–S35. doi: 10.1016/j.apmr.2009.06.009.
- Jacobson DNO, Lowing K, Tedroff K. Health-related quality of life, pain, and fatigue in young adults with cerebral palsy. Dev Med Child Neurol. 2020;62(3):372–378. doi: 10.1111/dmcn.14413.
- Østergaard CS, Pedersen NSA, Thomasen A, et al. Pain is frequent in children with cerebral palsy and negatively affects physical activity and participation. Acta Paediatr. 2021;110(1):301–306. doi: 10.1111/apa.15341.
- Peterson MD, Ryan JM, Hurvitz EA, et al. Chronic conditions in adults with cerebral palsy. JAMA. 2015;314(21):2303–2305. doi: 10.1001/jama.2015.11025.
- Hirvensalo M, Lintunen T. Life-course perspective for physical activity and sports participation. Eur Rev Aging Phys Act. 2011;8(1):13–22. doi: 10.1007/s11556-010-0076-3.
- Boyer ER, Palmer M, Walt K, et al. Validation of the gait outcomes assessment list questionnaire and caregiver priorities for individuals with cerebral palsy. Dev Med Child Neurol. 2022;64(3):379–386. doi: 10.1111/dmcn.15054.
- Maher CA, Toohey M, Ferguson M. Physical activity predicts quality of life and happiness in children and adolescents with cerebral palsy. Disabil Rehabil. 2016;38(9):865–869. doi: 10.3109/09638288.2015.1066450.
- Ryan JM, Crowley VE, Hensey O, et al. Habitual physical activity and cardiometabolic risk factors in adults with cerebral palsy. Res Dev Disabil. 2014;35(9):1995–2002. doi: 10.1016/j.ridd.2014.03.051.
- Usuba K, Oddson B, Gauthier A, et al. Leisure-time physical activity in adults with cerebral palsy. Disabil Health J. 2015;8(4):611–618. doi: 10.1016/j.dhjo.2015.05.006.
- Fraser-Thomas J, Côté J, Deakin J. Understanding dropout and prolonged engagement in adolescent competitive sport. Psychol Sport Exerc. 2008;9(5):645–662. doi: 10.1016/j.psychsport.2007.08.003.
- Kilgour G, Stott NS, Steele M, et al. More than just having fun! understanding the experience of involement in physical activyt. Disabil Rehabil. 2023. doi: 10.1080/09638288.2023.2251395.
