 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
The aim of this prospective single group, time-series study was to determine the feasibility, adherence, and effectiveness of an individualized physical therapist (PT)-driven hybrid model of the exercise component of cardiac rehabilitation (CR) that uses a novel intensity-recovery progression protocol (IRPP) and cardiac testing template (CTT) to assess achieved heart rate (HR) intensity, HR recovery, and intensity-recovery total to guide treatment.
Methods
Assessment of a questionnaire, treadmill 6-min walk (6MW), 1-min sit-to-stand (1STS), 1-min step, and 1-min agility square tests were assessed on 100 participants. Compared to traditional CR the 36-visit 1:1 hybrid model was individualized using the IRPP program and CTT.
Results
Adherence was 35% (timely) and 44% (overall) completion. The per-protocol analysis (PPA) and intent-to-treat (ITT) analysis suggest significant improvement in objective assessments baseline to visit 15 (PPA = 11 of 14) (ITT = 13 of 14), baseline to visit 30 (PPA = 12 of 14) (ITT = 12 of 14) and visit 15 to visit 30 (PPA = 9 of 14) (ITT = 10 of 14). Improvement beyond the minimal clinically important difference (MCID) was 94.3% in the 6MW and 91.4% in the 1STS.
Conclusions
The PT-driven IRPP program was feasible in terms of adherence and safety, showing significant improvement in a majority of assessments. Analysis of HR using the CTT may help clinical decision making for progression in CR.
Cardiac rehabilitation (CR) is an underutilized means of improving health for people recovering from cardiac surgery.
People recovering from cardiac surgery have complex reasons for why they choose to enroll in, drop out from, or complete a CR program.
Reporting of outcomes in CR and progression in intensity is not often individualized.
An individualized physical therapist driven CR program using both subjective and objective assessments may be successful at improving adherence and effectiveness in this cohort.
Implications for rehabilitation
Introduction and purpose
Cardiovascular disease remains the leading cause of death within the USA [Citation1]. According to the 2022 update from the American Heart Association, the most recent estimate of yearly performed inpatient cardiovascular procedures included over: 1 000 000 cardiac catheterizations, 480 000 percutaneous coronary interventions, 434 000 stents, 371 000 coronary artery bypass grafts (CABGs), 351 000 pacemakers, and 156 000 heart valve replacements [Citation2]. To recover from these conditions, cardiac rehabilitation (CR) is the standard of care as a class I level A recommendation [Citation3–5]. A systematic review concluded that the benefit of exercise induced physical activity in CR is correlated with reduced cardiovascular mortality and hospital admissions [Citation6,Citation7]. CR includes safe exercise progression, nutritional education, psychosocial counseling, and education on lifestyle adaptation [Citation8].
Despite growing evidence on the benefits of CR and increases in education and referral rates, attendance and adherence remain poor [Citation9]. Analysis by Keteyian et al. found only 8% of Medicare eligible individuals attended and completed a full set of 36-visits [Citation10]. Reviews in 2020 by Ritchey et al. [Citation11] and 2022 by Keteyian et al. [Citation10] of 366 103 and 412 080 eligible individuals, respectively, found that only 24.4% and 28.6% participated in CR, only 24.3% had timely initiation into the program (<21 days post cardiac event), and only 26.9% and 27.6% of those who enrolled completed the program. The National Cardiovascular Data Registry reported an increase in the rate of referrals for myocardial infarction from 72.9% in 2007 to 80.7% in 2012 [Citation12]. However, when analyzing eligible individuals from 2005 to 2015, <39% self-reported attending CR [Citation13].
Current CR treatment guidelines () have a standardized frequency, time, volume, and exercise type [Citation19]. With these guidelines in place, the reasons behind poor attendance and adherence remains in question. A systematic review by Resurrección et al. reported several factors that lead to poor attendance and adherence included intrapersonal barriers, interpersonal barriers, logistical barriers, CR program design issues, and health system limitations [Citation20]. Limitations in traditional CR program design include the absence of standardized universal reporting criteria of treatment results. CR program design issues related to dropout include a lack of an individualized exercise program, an overall low exercise intensity, inadequate consistent progressions, and group training with a one size fits all nature to treatment [Citation14]. The proposed treatment using a hybrid model, which we have termed intensity-recovery progression protocol (IRPP) is aimed at addressing these limitations.
Table 1. Traditional program vs. hybrid program.
The primary aim of this study (aim 1 – feasibility) was to determine the feasibility using adherence and adverse events of the IRPP, a novel 36 visit, PT-driven 1:1 model of CR program utilizing a HR intensity-recovery algorithm to determine treatment progression. Another primary aim of this study (aim 2 – subjective/objective assessments) was to determine if the IRPP program was associated with better subjective and objective measures using a questionnaire and physical performance testing throughout the 36-visits of the program.
Methods
Participants
All individuals referred to Good Shepherd Rehabilitation, an outpatient phase II cardiopulmonary clinic in Las Vegas, Nevada, that met the inclusion criteria were asked if they would like to participate in the study. Of 192 individuals referred, 20 individuals were excluded and 100 elected to participate. The study began in July 2019 and data collection was completed by July 2022. Prospective participants were considered eligible for the study if they had undergone cardiac surgery (i.e., CABG, stent, or valve replacement) within a year of their evaluation date and could independently ambulate with or without an assistive device into the facility. The following cardiac procedures were excluded: pacemakers, heart transplants, and all other cardiac procedures not listed. Although these types of procedures would not be excluded from using the IRPP program, they were excluded from this specific study due to the presence of heart rate (HR) controlling devices and/or post-transplant intensity progression protocol limitations having an expected decreased achieved HR intensity (AHRI%), an increased HR recovery (HRR%) during treatment. These types of surgical procedures would require an IRPP program design with modifications to lower intensity and increased utilization of subjective measures (e.g., Borg Rating of Perceived Exertion). Other exclusion criteria included persons who had undergone a non-related secondary surgery within the previous 12 months or any individual that had been previously enrolled in a phase II CR program. The sample size needed was estimated using the “one-way repeated measures contrast test” on PASS 19.0.1 (NCSS, LLC, Kaysville, UT, www.ncss.com/software/pass) for aim 2 with seven time points, 80% power, alpha of 0.05, estimated effect size of 0.40 between the first and last measurements, dropout rates of 72.4% [Citation10] 73.1% [Citation11], and correlations of 0.600 between each of the measurements. Based on these parameters, 102 and 105 participants would need to be recruited to have 28 participants who completed all phases of the program.
Design
This study was a prospective single group, time-series design with a 36-visit program length including 30-visits for data collection and 6-visits for discharge planning to phase III CR maintenance. The IRPP program was implemented at Good Shepherd Rehabilitation under the supervision of a licensed physical therapist (PT) with assistance from physical therapy assistants and telemetry monitoring by a registered nurse. A physician (medical director) was available if needed for consultation. All treatment data were collected by a PT and PT assistants. The data were collected during submaximal performance testing and were later extracted from the medical records. Participants were expected to attend at a frequency of twice per week with visits lasting 75–90-min. All participants who started the IRPP program signed a consent form prior to being evaluated. This research was conducted under approval/exemption from the Institutional Review Board on ethical standards on human experimentation at BRANY IRB.
The IRPP program consists of a novel HR intensity-recovery algorithm to determine and inform individualized treatment tolerance and progression. The IRPP program utilizes a cardiac testing template (CTT) which is a tool of communication that helps a clinician analyze AHRI%, HRR%, and intensity recovery total (IR%) during treatment to improve clinical decision making about when a participant should be progressed. We propose that modifications to the traditional guidelines, using the hybrid model, would help bolster attendance, adherence, and outcomes. This IRPP hybrid model highlights some key difference to the exercise component of CR from the traditional model. These differences include a change in the lead licensed personnel carrying out the program to a PT, an individualized program design, improved clinician to participant ratio of 1:1, longer treatment duration, a consistent treatment progression using the CTT to report and analyze results, and the addition of high intensity interval training (HIIT) added to the standard continuous aerobic endurance training (CAET) [Citation15–18] (). HIIT was added to the IRPP model due to its being recommended and deemed safe for CR [Citation18]. HIIT has been shown to improve overall functional capacity, walking speed and endurance, peak oxygen uptake, improved myocardial function, an increase in achieved HR response to activity, and improved quality of life [Citation21–24].
Outcomes
Aim 1: feasibility
Feasibility was assessed using adherence in terms of attendance and completion of the IRPP program. Attendance and completion were determined from a medical chart review assessing how many days of treatment each participant was present and participated. Completion was defined as attending >30 days (). Since the IRPP program was designed to ensure that participants were training and progressing at a specific HR intensity, adverse events during the IRPP program (e.g., injury requiring medical attention, falling, loss of consciousness, cardiac emergency requiring medical attention, and hospitalizations) were recorded.
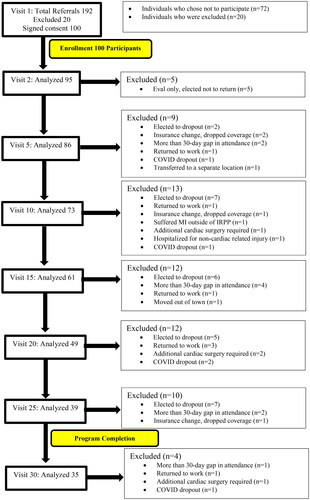
Figure 1. Consort flow diagram of participants throughout the analysis. Patient enrollment at visit 1 and dropout across seven phases in the program, assessed every five visits until program completion at visit 30. A total of 100 participants enrolled out of 192 referred and 35 completed the program. Reasons for dropout included failure to return after evaluation, electing to discontinue, insurance change, prolonged gap in attendance, return to work, COVID, hospitalization, cardiac surgery, and transfer to another clinic.
Aim 2: subjective/objective assessments
Subjective questionnaire
A three-question survey was used to assess the perceived improvement of endurance throughout the program and was given at baseline, visit 15, and visit 30 ().
Table 2. Subjective questionnaire and physical performance test reliability and validity.
Objective measurements
All participants were assessed on the following submaximal physical performance tests every 5th visit: treadmill 6-min walk (6MW), 1-min sit-to-stand (1STS), 1-min step test (1ST), and the 1-min agility square test (1AST) (). The American College of Cardiology has reported that maximal stress testing <2 years after a cardiac procedure is inappropriate and thus these submaximal physical performance tests were carried out to help determine individualized CAET, HIIT, and resistance training program parameters [Citation25,Citation26]. The results of the physical performance tests were input into the CTT to help analyze current endurance levels, program tolerance, and individual treatment progression. These physical performance tests highlight at what intensity a participant is able to perform at so that a similar intensity can be replicated throughout their individual four-part treatment program (). Vital signs were assessed during each of the tests using telemetry and finger pulse oximetry. To control for bias in testing, participants and clinicians were kept blind to previous testing results. Participants were not informed of their previous test results and clinicians were instructed to not look back at previous medical records until testing was completed. While the basic performance-based tests were an important objective assessment, there were additional data collected related to HR intensity and recovery (i.e., AHRI%, HRR%, and IR%) taken during the 6MW and the 1AST.
Table 3. IRPP four-part treatment details.
IRPP program
Upon arrival to the clinic, participants were connected to a (Philips Series C, Amsterdam, Netherlands) telemetry model. The telemetry recording was assessed for a 3-min period allowing for a resting HR (RHR) to be determined. Blood pressure was assessed manually pre- and post-treatment. Based on the current visit number, participants were either guided through their submaximal physical performance tests or taken through their treatment program. Each program was continuously adjusted based on a participant’s present condition including vital signs, telemetry, pain, and physical performance test results. Each participant’s comorbidities and functional limitations were considered in the creation of their individualized four-part program (). Part 1 included a subjective participant health update, vital sign assessment, and telemetry connection. Part 2 was divided into two subcategories, a 30–45-min machine based aerobic/anaerobic component and a 15–30-min strength component. Part 3 was divided into two subcategories, a 10–15-min non-machine based aerobic/anaerobic component and a 10–15-min balance and/or orthopedic impairment-based component. Part 4 included post treatment assessment of vital signs, telemetry, and education on daily performance, physical activity, and lifestyle habits. Each participant went through all four-parts to the program for each visit and received the same education on nutrition, stress, smoking, alcohol, weight loss, and sleep quality. These positive lifestyle practices as discussed by Dean and Lomi were introduced at a participant’s evaluation and continuous verbal education occurred at each visit [Citation27]. These non-exercise components of CR were not tracked in this study and were unchanged from traditional CR guidelines.
CTT
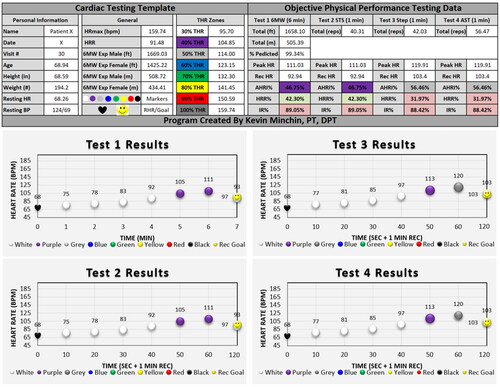
The IRPP program was designed to meet individualized HR intensity and recovery goals determined by each participant’s specific physical performance test results using the CTT (). The CTT provided a recommended universal language template used to communicate results to participants and physicians. The CTT included the total 6MW distance achieved and the target normative distance for each participant, determined based on formulas created by Enright and Sherrill [Citation28] It also contained each participant’s descriptive information including name, testing date, visit number, age, body mass characteristics, RHR, and blood pressure. This information was used to determine the individual target HR (THR) zones for each participant. Test 1–4 submaximal physical performance tests were recorded using the test 1–4 columns where peak HR, recovered HR, AHRI%, HRR%, and IR% were listed. The AHRI% was color coordinated based on what intensity zone the participant performed in.
Figure 2. Cardiac testing template (CTT) of physical performance tests 1–4. Graphs are coordinated with the above numeric data, using the average data for visit 30 from the present study. Recommended universal language template for reporting treatment data to physicians and insurance providers. Peak HR: peak heart rate; Rec HR: 1-minute recovered heart rate; AHRI%: achieved heart rate intensity; HRR%: heart rate recovery; IR%: total intensity recovery; 6MW: 6-minute walk; STS: 1-minute sit-to-stand; ST: 1-minute step; AST: 1-minute agility square test. Heart rate graph during the objective tests of 6-minute walk, sit to stand, step, and agility square tests. Left columns show name, date, age, height, weight, vitals, and normative six-minute walk score. Center columns show colored target heart rate zones including 30% (white), 40% (purple), 50% (grey), 60% (blue), 70% (green), 80% (yellow), 90% (red), and 100% (black). Right columns show numeric test results along with peak heart rate, recovered heart rate, achieved heart rate intensity %, heart rate recovery %, and total intensity-recovery %. Results colored green and red to show if recovery goals were successfully met.
To establish the THR, the HR max was determined using the Seals equation (EquationEquation (1)(1)
(1) ) [Citation29]. HR reserve (HRR) was determined using EquationEquation (2)
(2)
(2) [Citation30]. THR zones were determined based on a participant’s RHR using EquationEquation (3)
(3)
(3) [Citation28, Citation30]. The maximum HR achieved as determined by telemetry during each of the objective assessments was recorded. THR zones of intensity were divided into color coordinated sections (). The color coordinated zones of intensity, AHRI%, HRR%, and subjective participant report were used to assist in decision-making regarding the rate of progression of treatment. The colored zones were used to provide visual feedback to the participant. The maximum HR achieved and the HR after 1-min post exercise as determined by telemetry during each of the physical performance tests were recorded. AHRI% after completion of each of the objective assessments was analyzed using EquationEquation (4)
(4)
(4) [Citation28,Citation29]. HRR% was analyzed by percentage of recovery determined using EquationEquation (5)
(5)
(5) . IR% was determined using EquationEquation (6)
(6)
(6) .
(1)
(1)
(2)
(2)
(3)
(3)
(4)
(4)
(5)
(5)
(6)
(6)
Using research on HRR% on healthy adults by Facioli et al. as a guideline, a HRR% cutoff was created with an abnormal HRR% as <33% across all intensities of HR performance [Citation31]. The IR% goal was quantified as >100% for light and moderate intensity exercise, >115% for high intensity exercise, and >125% for vigorous intensity exercise. Light intensity exercise was based on an AHRI% between 0 and 55% (zones 0–2.5), moderate intensity exercise between 55 and 75% (zones 2.5–4.5), high intensity exercise between 75 and 85% (zones 4.5–5.5), and vigorous intensity exercise between 85 and 100% (zones 5.5–6) () [Citation32]. The HRR% and IR% were color coordinated green and red based on if the participant hit the target recovery. If a participant achieved a green passing score for the HRR% and IR%, it was determined that they could likely tolerate progression of activity into the next THR zone of intensity within their treatment program.
Table 4. Intensity recovery progression protocol (IRPP) to determine progression/regression. (Achieved Heart Rate Intensity (AHRI%), Heart Rate Recovery (HRR%), Intensity Recovery (IR%))
Data analysis
Data were analyzed using SPSS version 28.0 (IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY) and incompatibility of the data with the null was set at α = 0.05. As there was significant attrition during the IRPP, both a per-protocol analysis (PPA) and an intent-to-treat (ITT) analysis [Citation33] were performed with missing data in the ITT analysis being replaced using the last observation carried forward method. For aim 1 (feasibility), basic descriptive statistics were utilized. For aim 2 (subjective/objective assessments), a repeated measures ANOVA was used for each of the subjective and objective assessments. Violations of sphericity were handled using the Greenhouse–Geisser correction according to ANOVA heuristics. The proportion of participants who improved beyond the minimal clinically important difference (MCID) [Citation34] values for the 6MW (50 m [Citation35]) and the 1STS (three repetitions [Citation36]) were calculated at visit 15 and visit 30.
Results
Participants
The participants analyzed (73 males, 27 females) had an average starting age of 66.1 years (SD = 9.6; range 41–88 years). The admitting diagnoses were 50% stent, 37% CABG, 8% valve replacement, and 5% a combination of the above ().
Table 5. Participant characteristics.
Aim 1: feasibility
Out of 100 participants, 35 completed all phases of the IRPP without any gap in coverage (defined as >30 days) (). Thus, the adherence to the full program was 35%. However, there were 9 participants that had a prolonged gap in coverage and were removed from the analysis but eventually returned and completed all 30 visits. Thus, the adherence is 44% if these participants were to be included.
The mean treatments attended was 18.4 visits over an average length of program of 11.4 weeks. When allowing for a gap in coverage, the mean treatments attended was increased to 22.6 visits. The average duration to complete 36-visits was 22.3 weeks. Details about adherence and reasons for dropout throughout the study are included in . There were no adverse events observed for any participant during the IRPP program. However, one participant had a myocardial infarction outside of the program, one participant was hospitalized for a non-cardiac related illness, and four others required additional cardiac procedures. It is not believed that any of those events were a result of the IRPP program.
Aim 2: subjective/objective assessments
The results for the repeated measures ANOVAs for both the PPA and ITT analyses for all objective assessments are given in . The PPA analyses suggest that 11 of 14 assessments were significantly improved from baseline to visit 15 (ITT = 13 of 14), 12 of 14 from baseline to visit 30 (ITT = 12 of 14), and 9 of 14 from visit 15 to visit 30 (ITT = 10 of 14) (). There were only a few minor discrepancies between the PPA and ITT analysis: 6MW AHRI% baseline to visit 15 pairwise comparison p value, 1AST HRR% overall omnibus p value, Q2 for baseline to visit 30 pairwise comparison p value, and Q3 for visit 15 to visit 30 pairwise comparison p value. All other ITT analyses were consistent with the PPAs. For the PPA, of the 61 participants that completed the IRPP program up to visit 15, 59 (96.7%) improved beyond the MCID for the 6MW and 54 (88.5%) improved beyond the MCID for the 1STS. Of the 35 participants that completed the IRPP program up to 30 visits, 33 (94.3%) improved beyond the MCID for the 6MW and 32 (91.4%) improved beyond the MCID for the 1STS. For the ITT, at visit 15 of the IRPP program, 82 of 100 (82.0%) improved beyond the MCID for the 6MW and 75 (75.0%) improved beyond the MCID for the 1STS. At visit 30, 82 (82.0%) improved beyond the MCID for the 6MW and 75 (75.0%) improved beyond the MCID for the 1STS.
Table 6. Between group and within group analysis using means, standard deviations (SDs), and 95% confidence intervals (CI) for the baseline, visit 15, and visit 30 outcome measures.
Discussion
The results suggest that a PT-driven hybrid model that incorporates the novel IRPP program and CTT to guide treatment intensity is feasible in terms of adherence with 35% completing the study in timely fashion (44% overall completion). The IRPP program was a safe program with no adverse events requiring additional medical attention during treatment and no mortalities. The results suggest an association with improvement in a majority of the subjective and objective assessments. However, these results should be interpreted with caution as the design does not lend itself to causal inference. Additionally, it is unknown if improvements were a result of the entire IRPP model or a part of the hybrid model such as individualized 1:1 treatment alone which has been shown to be beneficial in previous research [Citation37].
The IRPP program had better program adherence compared to previous studies using the traditional model of CR. For instance, Ritchey et al. reported a 26.9% completion rate in 89 300 participants and Keteyian et al. reported a 27.6% completion in 117 700 participants [Citation10,Citation11]. The IRPP program contains a more rigorous treatment design than traditional CR (). Due to this, the IRPP program was designed to be PT-driven using the CTT for ongoing assessments of program tolerance that require continuous individual modifications. PTs have a unique skillset of the musculoskeletal system that allows them to best determine how to create, adapt, and progress a plan of care based on the physiologic response to exercise and/or presence of injury. A requirement of the IRPP program is the skilled assessment of the individual CTT results which is a component not observed in the traditional CR format. The improved adherence is promising, however, results of this study should be interpreted with caution due to the comparatively small sample size () [Citation8, Citation15–19].
A few major factors impacted adherence and program completion. First, the IRPP program was interrupted by the COVID-19 pandemic which caused some participants to withdraw and others to go on prolonged absences which directly impacted overall adherence. There were five participants who verbalized dropping out due to either a COVID infection or fear from being in a public setting during the pandemic in the months of March–December 2020. Additionally, there were nine participants who returned for treatment after prolonged absences during the months of the COVID outbreak (). It is unknown if these prolonged absences were the result of the COVID pandemic or other factors. The study design incorporated an immediate withdrawal upon a 30-day gap in treatment. Prolonged gaps due to poor motivation, illness, health or family related issues, and/or poor program compliance, led to withdrawal regardless of if the participant returned for further treatment. Comparatively, the Ritchey et al. and Keteyian et al. studies did not withdraw participants for a prolonged absence, but rather provided a 36-week window to complete treatment [Citation10,Citation11]. Additionally, this study had a longer time to initiate treatment (surgery to CR) compared to those studies. Current research defines timely initiation as starting CR <21 days after a cardiac event [Citation9]. The average initiation of this program was 76.4 days () compared to 47 ± 38.6 days and 45 ± 52 days in the Ritchey et al. and Keteyian et al. studies, respectively [Citation10,Citation11]. Research by Thomas et al. indicates early initiation into CR improves overall enrollment and may be associated with improved outcomes [Citation9]. It is hypothesized that an earlier initiation would have resulted in an increased adherence of the IRPP program.
The IRPP program was associated with subjective and objective improvements. Participant subjective responses all improved from visit 1 to visit 30. Participant perception of endurance improved from 43.5% to 68.0%, their estimation of their tolerance to stairs improved from 28.0 to 39.3 stairs, and overall report of weekly exercise improved from 4.2 to 8.1 h. These subjective assessments suggest that, on average, participants attending the IRPP program felt an improvement in their abilities that correlated to an increase in their weekly activity. The physical performance tests for the 6MW, 1STS, 1ST, and 1AST all improved significantly from baseline to visit 30. Based on the MCID of 50 m for the 6MW, 96.7% of the participants improved beyond the MCID at visit 15 and 94.3% improved beyond the MCID at visit 30. The 6MW improved 157.2 m in the PPA and 131.6 m in the ITT from baseline to visit 15 and 205.7 m in the PPA and 149.9 m in the ITT from baseline to visit 30. These results are clinically meaningful considering research by Grundtvig et al. concluded that the 6MW distance achieved is a strong predictor of mortality for persons with heart failure [Citation38]. Using the formulas by Enright and Sherrill, it was determined that, on average, participants who completed the program were able to achieve the normative predicted distance in the 6MW [Citation28]. At baseline in the PPA, participants averaged 54.8% compared to 100.3% of the predicted norm distance at visit 30. Based on the MCID of three reps for the 1STS, 88.5% of the participants improved beyond the MCID at visit 15 and 91.4% improved beyond the MCID at visit 30. The 1STS improved 11.4 reps in the PPA and 7.8 reps in the ITT from baseline to visit 15 and 17.8 reps in the PPA and 10.3 reps in the ITT from baseline to visit 30.
It is important to note that in the present study, the Stevens treadmill protocol was utilized [Citation39]. Although the American Thoracic Society recommends testing the 6MW in a flat corridor, treadmills are also used for testing [Citation40,Citation41]. As a result of spacing issues, many clinics are limited in the possibility of carrying out this test in its standardized form and may utilize a treadmill or reduced corridor length as an alternative testing method [Citation40, Citation42,Citation43]. These variations have been shown to significantly reduce overall 6MW distance [Citation44]. In order to compare results, it is recommended the test be performed in a corridor 30-m in length and 3-m in width. Research comparing a 30-m corridor and treadmill during the 6MW on people with pulmonary impairments found a 51- and 102-m difference in favor of the corridor [Citation39, Citation45]. Additionally, research by Laskin et al. found the treadmill 6MW is a reliable tool to measure distance and HR [Citation46]. Based on this research comparing the 6MW variations, it is hypothesized that participants would have achieved an improved 6MW total distance if the American Thoracic Society corridor guidelines were to be used. It is important to note that the difference in testing surfaces should be considered when interpreting results in this study as results are not interchangeable.
While more research is needed, these results provide preliminary evidence to support the utilization of AHRI%, HRR%, and IR% to help determine tolerance to activity for use in progressing treatment intensity. Due to the variance in AHRI% in exercise testing, we proposed that HRR% and IR%, as opposed to HR numeric differential (HRR#) target of >12 bpm or >18 bpm used in previous research, are better indicators to assess tolerance to activity and progression of treatment [Citation47–50]. The use of HRR% and IR% over HRR# is a key addition to the hybrid model over traditional CR. HRR# is defined as the numeric difference in HR from peak activity to 1-min recovery. This cutoff is the same regardless of if an individual is completing a low, moderate, high, or vigorous intensity task. We expect low intensity activities would result in a faster and, thus, higher HRR% and high intensity activities would result in a slower and, thus, lower HRR%. Due to the variance in recovery at different intensities, we propose that HRR% is a better objective assessment than HRR#. In the present study, AHRI% in the aerobic and anaerobic assessments improved by 19.4% and 14.6%, respectively, from visit 1 to visit 30. HRR% in the same aerobic and anaerobic assessments did not show significant improvement. Despite the lack of improvement in HRR%, participants were able to maintain a similar HRR% while achieving a higher overall AHRI% at peak performance. We propose this is a result of AHRI% and HRR% being inversely related. Taking this into consideration, we analyzed the IR% to further explain tolerance to physical exertion across all intensities. The IR% improved by 19.8% and 21.1% in the aerobic 6MW and anaerobic 1AST assessments from visit 1 to visit 30. Analyzing IR% is a novel analysis that can be utilized to explain recovery across all intensities of exertion. Future research should consider adding HRR% and IR% to CR programs to assess intensity and recovery together as a means to appropriately progress individuals. From a clinical perspective, this has the potential to better help clinicians safely progress a person to the next level of treatment intensity. This is done by assessing a person’s tolerance to their current level of treatment intensity while analyzing if an adequate HRR% was met. If a person is unable to recover in a timely manner (e.g., HRR% <33%, IR% <100%), the clinician should consider staying at the current intensity level within treatment. If a person is able to recover adequately (e.g., HRR >33%, IR% >125%), the clinician should consider that an increase in treatment intensity may be appropriate. These objective assessments should be utilized in addition to a person’s subjective response and monitoring of vital signs to determine progression and regression of treatment. This is an improvement over traditional CR progression which is often progressed subjectively.
One variable not analyzed was the effect of cardiac medications on AHRI%, HRR%, and IR%. To help analyze the effect of prescribed medication on participant condition, blood pressure was tracked and assessed pre- and post-treatment for each session. shows the average blood pressures recorded and, qualitatively, both systolic and diastolic blood pressure were stable throughout the study. The use of ace inhibitors, beta blockers, calcium channel blockers, and cardiac glycosides all have an effect on decreasing HR [Citation9, Citation51–54]. Research by Godlasky et al. reviewed stress test echocardiograms for persons with CAD and observed significant differences in HR max, dependent upon if beta blockers were taken, held for 12–24 h, or not prescribed [Citation55]. Research by Sydó et al. determined that there was no significant difference in HRR% with the use of beta blockers [Citation50]. Due to the discrepancy of the effect on beta blockers on RHR and HR max, but not HRR%, further research is needed to quantify the effect of cardiac medications on RHR, AHRI%, and HRR%. It is hypothesized that the individuals who would qualify for and attend CR would have a lower AHRI% and IR% as a result of their prescribed medications. Due to the variability across the cohort in dosage, frequency, time medication was taken, time of treatment session, and total amount of prescribed medications, this factor was not assessed. This is a study limitation and future research should consider an analytic approach to control this confound.
Limitations
The study design consisted of a small sample size and absence of a control group which limits causal inference. Therefore, we are unable to determine how much of the observed improvement was a result of IRPP program directly or due to other things like natural disease history or placebo. While we did not meet our original target of 102–105 recruited participants, we did exceed the sample size estimate of 28 participants needed with 35 participants who completed all phases of the study. Another limitation is that medication type and dosages were unique to each patient and were not tracked or controlled analytically. Additionally, this study was interrupted by COVID-19 pandemic adding to issues with program adherence. COVID-19 had an impact on participant fear to attend, increased inactivity, and drop-out. The objective physical performance test assessments had some limitations. The 6MW used a treadmill as opposed to the standardized 30-m walkway. The 1STS and 1ST were completed for 1 min as opposed to the standardized 30 s and 2 min, respectively. There was a lack of psychometrics for the novel 1AST and the three-question survey. Further limitations include the effect of treatment frequency on results. All participants were placed on a 2× a week schedule and would likely have benefited more from an increased frequency of 3× a week. It is also not certain what element/s (e.g., duration, individualized program, HIIT, intensity progression using CTT, and 1:1 supervision) of this program underlie the adherence and improvements observed. Future research will be needed to tease this out. Additional research may also consider incorporating the surgical diagnoses excluded from this design to determine if the IRPP program can be utilized effectively for other diagnoses. While there were clearly limitations, the strengths of the study were that the PT-driven program design had no adverse events, was individualized to each participant, is promising in terms of adherence and intensity escalation, and provided preliminary evidence that the IRPP program and CTT may be beneficial in CR.
Conclusions
The present study offers evidence in support of utilizing the IRPP program as a new approach to the exercise component of CR. The PT-led hybrid model incorporating individualized treatment programs was associated with improved adherence compared to other CR programs using the traditional model. Additionally, the hybrid model had significant improvement in subjective measures and objective physical performance-based assessments. These results suggest, the proposed IRPP and CTT with analysis of AHRI%, HRR%, and IR% are useful tools to determine individualized progression within CR. Further research to compare this novel CR program to traditional CR is needed as well as research to quantify the effect of prescribed cardiac medications on RHR, AHRI%, HRR%, and IR%. The results of the study warrant future research using more rigorous designs with control groups to help establish causality of the IRPP program.
Ethical approval
BRANY Institutional Review Board.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- National Vital Statistics System. Mortality multiple cause-of-death. Centers for Disease Control and Prevention; 2021 [cited 2023 Jan 9]. Available from: https://www.cdc.gov/nchs/nvss/mortality_public_use_data.htm
- Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–e639. doi: 10.1161/CIR.0000000000001052.
- Hillis LD, Smith PK, Anderson JL, et al. ACCF/AHA guideline for coronary artery bypass graft surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2011;58(24):e123–e210. doi: 10.1016/j.jacc.2011.08.009.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for cardiovascular angiography and interventions. Catheter Cardiovasc Interv. 2012;79(3):453–495. doi: 10.1002/ccd.23438.
- Wenger NK. Current status of cardiac rehabilitation. J Am Coll Cardiol. 2008;51(17):1619–1631. doi: 10.1016/j.jacc.2008.01.030.
- Winzer EB, Woitek F, Linke A. Physical activity in the prevention and treatment of coronary artery disease. J Am Heart Assoc. 2018;7(4):e007725. doi: 10.1161/JAHA.117.007725.
- Origuchi H, Itoh H, Momomura SI, et al. Active participation in outpatient cardiac rehabilitation is associated with better prognosis after coronary artery bypass graft surgery. Circ J. 2020;84(3):427–435. doi: 10.1253/circj.CJ-19-0650.
- Mampuya WM. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther. 2012;2(1):38–49. doi: 10.3978/j.issn.2223-3652.2012.01.02.
- Thomas RJ, Balady G, Banka G, et al. ACC/AHA clinical performance and quality measures for cardiac rehabilitation: a report of the American College of Cardiology/American Heart Association Task Force on performance measures. J Am Coll Cardiol. 2018;71(16):1814–1837. doi: 10.1016/j.jacc.2018.01.004.
- Keteyian SJ, Jackson SL, Chang A, et al. Tracking cardiac rehabilitation utilization in Medicare beneficiaries: 2017 update. J Cardiopulm Rehabil Prev. 2022;42(4):235–245. doi: 10.1097/HCR.0000000000000675.
- Ritchey MD, Maresh S, McNeely J, et al. Tracking cardiac rehabilitation participation and completion among Medicare beneficiaries to inform the efforts of a national initiative. Circ Cardiovasc Qual Outcomes. 2020;13(1):e005902. doi: 10.1161/CIRCOUTCOMES.119.005902.
- Beatty AL, Li S, Thomas L, et al. Trends in referral to cardiac rehabilitation after myocardial infarction: data from the National Cardiovascular Data Registry 2007 to 2012. J Am Coll Cardiol. 2014;63(23):2582–2583. doi: 10.1016/j.jacc.2014.03.030.
- Peters AE, Keeley EC. Trends and predictors of participation in cardiac rehabilitation following acute myocardial infarction: data from the behavioral risk factor surveillance system. J Am Heart Assoc. 2017;7(1):e007664. doi: 10.1161/JAHA.117.007664.
- Lee M, Wood T, Chan S, et al. Cardiac rehabilitation program: an exploration of patient experiences and perspectives on program dropout. Worldviews Evid Based Nurs. 2022;19(1):56–63. doi: 10.1111/wvn.12554.
- Franklin B, Fern A, Fowler A, et al. Exercise physiologist’s role in clinical practice. Br J Sports Med. 2009;43(2):93–98. doi: 10.1136/bjsm.2008.055202.
- Balady GJ, Williams MA, Ades PA, et al. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update. J Cardiopulm Rehabil Prev. 2007;27(3):121–129. doi: 10.1097/01.HCR.0000270696.01635.aa.
- Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update. Circulation. 2007;116(5):572–584. doi: 10.1161/CIRCULATIONAHA.107.185214.
- Price KJ, Gordon BA, Bird SR, et al. A review of guidelines for cardiac rehabilitation exercise programmes: is there an international consensus? Eur J Prev Cardiol. 2016;23(16):1715–1733. doi: 10.1177/2047487316657669.
- Squires RW, Kaminsky LA, Porcari JP, et al. Progression of exercise training in early outpatient cardiac rehabilitation: an official statement from the American Association of Cardiovascular and Pulmonary Rehabilitation. J Cardiopulm Rehabil Prev. 2018;38(3):139–146. doi: 10.1097/HCR.0000000000000337.
- Resurrección DM, Motrico E, Rigabert A, et al. Barriers for nonparticipation and dropout of women in cardiac rehabilitation programs: a systematic review. J Womens Health. 2017;26(8):849–859. doi: 10.1089/jwh.2016.6249.
- Villelabeitia-Jaureguizar K, Vicente-Campos D, Senen AB, et al. Effects of high-intensity interval versus continuous exercise training on post-exercise heart rate recovery in coronary heart-disease patients. Int J Cardiol. 2017;244:17–23. doi: 10.1016/j.ijcard.2017.06.067.
- Wehmeier UF, Schweitzer A, Jansen A, et al. Effects of high-intensity interval training in a three-week cardiovascular rehabilitation: a randomized controlled trial. Clin Rehabil. 2020;34(5):646–655. doi: 10.1177/0269215520912302.
- Papathanasiou JV, Petrov I, Tokmakova MP, et al. Group-based cardiac rehabilitation interventions. A challenge for physical and rehabilitation medicine physicians: a randomized controlled trial. Eur J Phys Rehabil Med. 2020;56(4):479–488. doi: 10.23736/S1973-9087.20.06013-X.
- Dun Y, Smith JR, Liu S, et al. High-intensity interval training in cardiac rehabilitation. Clin Geriatr Med. 2019;35(4):469–487. doi: 10.1016/j.cger.2019.07.011.
- Shah BR, Cowper PA, O’Brien SM, et al. Patterns of cardiac stress testing after revascularization in community practice. J Am Coll Cardiol. 2010;56(16):1328–1334. doi: 10.1016/j.jacc.2010.03.093.
- Noonan V, Dean E. Submaximal exercise testing: clinical application and interpretation. Phys Ther. 2000;80(8):782–807.
- Dean E, Lomi C. A health and lifestyle framework: an evidence-informed basis for contemporary physical therapist clinical practice guidelines with special reference to individuals with heart failure. Physiother Res Int. 2022;27(3):e1950. doi: 10.1002/pri.1950.
- Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1384–1387. doi: 10.1164/ajrccm.158.5.9710086.
- Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37(1):153–156. doi: 10.1016/s0735-1097(00)01054-8.
- Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate: a longitudinal study. Ann Med Exp Biol Fenn. 1957;35(3):307–315.
- Facioli TP, Philbois SV, Gastaldi AC, et al. Study of heart rate recovery and cardiovascular autonomic modulation in healthy participants after submaximal exercise. Sci Rep. 2021;11(1):3620. doi: 10.1038/s41598-021-83071-w.
- Riebe D, Ehrman JK, Liguori G, et al. ACSM’s guidelines for exercise testing and prescription. 10th ed. Philadelphia: Wolters Kluwer; 2018.
- Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res. 2011;2(3):109–112. doi: 10.4103/2229-3485.83221.
- McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA. 2014;312(13):1342–1343. doi: 10.1001/jama.2014.13128.
- Perera S, Mody SH, Woodman RC, et al. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x.
- Crook S, Büsching G, Schultz K, et al. A multicentre validation of the 1-min sit-to-stand test in patients with COPD. Eur Respir J. 2017;49(3):1601871. doi: 10.1183/13993003.01871-2016.
- Christle JW, Schlumberger A, Haller B, et al. Individualized vs. group exercise in improving quality of life and physical activity in patients with cardiac disease and low exercise capacity: results from the Doppelherz trial. Disabil Rehabil. 2017;39(25):2566–2571. doi: 10.1080/09638288.2016.1242174.
- Grundtvig M, Eriksen-Volnes T, Orn S, et al. 6 min walk test is a strong independent predictor of death in outpatients with heart failure. ESC Heart Fail. 2020;7(5):2904–2911. doi: 10.1002/ehf2.12900.
- Stevens D, Elpern E, Sharma K, et al. Comparison of hallway and treadmill six-minute walk tests. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1540–1543. doi: 10.1164/ajrccm.160.5.9808139.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117.
- Lenssen AF, Wijnen LC, Vankan DG, et al. Six-minute walking test done in a hallway or on a treadmill: how close do the two methods agree? Eur J Cardiovasc Prev Rehabil. 2010;17(6):713–717. doi: 10.1097/HJR.0b013e32833a1963.
- Prochaczek F, Winiarska H, Krzyzowska M, et al. Six-minute walk test on a special treadmill: primary results in healthy volunteers. Cardiol J. 2007;14(5):447–452.
- Gochicoa-Rangel L, Ramírez-José MC, Troncoso-Huitrón P, et al. Shorter corridors can be used for the six-minute walk test in subjects with chronic lung diseases. Respir Investig. 2020;58(4):255–261. doi: 10.1016/j.resinv.2019.12.009.
- Scivoletto G, Tamburella F, Laurenza L, et al. Validity and reliability of the 10-m walk test and the 6-min walk test in spinal cord injury patients. Spinal Cord. 2011;49(6):736–740. doi: 10.1038/sc.2010.180.
- de Almeida FG, Victor EG, Rizzo JA. Hallway versus treadmill 6-minute-walk tests in patients with chronic obstructive pulmonary disease. Respir Care. 2009;54(12):1712–1716.
- Laskin JJ, Bundy S, Marron H, et al. Using a treadmill for the 6-minute walk test: reliability and validity. J Cardiopulm Rehabil Prev. 2007;27(6):407–410. doi: 10.1097/01.HCR.0000300270.45881.d0.
- Cole CR, Blackstone EH, Pashkow FJ, et al. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341(18):1351–1357. doi: 10.1056/NEJM199910283411804.
- Ghaffari S, Kazemi B, Aliakbarzadeh P. Abnormal heart rate recovery after exercise predicts coronary artery disease severity. Cardiol J. 2011;18(1):47–54.
- Lipinski MJ, Vetrovec GW, Gorelik D, et al. The importance of heart rate recovery in patients with heart failure or left ventricular systolic dysfunction. J Card Fail. 2005;11(8):624–630. doi: 10.1016/j.cardfail.2005.06.429.
- Sydó N, Sydó T, Gonzalez Carta KA, et al. Prognostic performance of heart rate recovery on an exercise test in a primary prevention population. J Am Heart Assoc. 2018;7(7):e008143. doi: 10.1161/JAHA.117.008143.
- Pierdomenico SD, Bucci A, Lapenna D, et al. Heart rate in hypertensive patients treated with ace inhibitors and long-acting dihydropyridine calcium antagonists. J Cardiovasc Pharmacol. 2002;40(2):288–295. doi: 10.1097/00005344-200208000-00014.
- Paolillo S, Dell’Aversana S, Esposito I, et al. The use of beta-blockers in patients with heart failure and comorbidities: doubts, certainties and unsolved issues. Eur J Intern Med. 2021;88:9–14. doi: 10.1016/j.ejim.2021.03.035.
- White P. Calcium channel blockers. AACN Clin Issues Crit Care Nurs. 1992;3(2):437–446. doi: 10.4037/15597768-1992-2015.
- Erdmann E. Digitalis therapy – relevance of heart rate reduction. Basic Res Cardiol. 1998;93(Suppl. 1):156–159. doi: 10.1007/s003950050244.
- Godlasky E, Hoffman T, Weber-Peters S, et al. Effects of beta-blockers on maximal heart rate prediction equations in a cardiac population. J Cardiopulm Rehabil Prev. 2018;38(2):111–117. doi: 10.1097/HCR.0000000000000328.
- Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80(7):837–841. doi: 10.1016/s0003-9993(99)90236-8.
- Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: six-minute walk test, Berg balance scale, timed up & go test, and gait speeds. Phys Ther. 2002;82(2):128–137. doi: 10.1093/ptj/82.2.128.
- Hamilton DM, Haennel RG. Validity and reliability of the 6-minute walk test in a cardiac rehabilitation population. J Cardiopulm Rehabil. 2000;20(3):156–164. doi: 10.1097/00008483-200005000-00003.
- Demers C, McKelvie RS, Negassa A, et al. Reliability, validity, and responsiveness of the six-minute walk test in patients with heart failure. Am Heart J. 2001;142(4):698–703. doi: 10.1067/mhj.2001.118468.
- Bellet RN, Adams L, Morris NR. The 6-minute walk test in outpatient cardiac rehabilitation: validity, reliability and responsiveness – a systematic review. Physiotherapy. 2012;98(4):277–286. doi: 10.1016/j.physio.2011.11.003.
- Uszko-Lencer N, Mesquita R, Janssen E, et al. Reliability, construct validity and determinants of 6-minute walk test performance in patients with chronic heart failure. Int J Cardiol. 2017;240:285–290. doi: 10.1016/j.ijcard.2017.02.109.
- Lee MC. Validity of the 6-minute walk test and step test for evaluation of cardio respiratory fitness in patients with type 2 diabetes mellitus. J Exerc Nutrition Biochem. 2018;22(1):49–55. doi: 10.20463/jenb.2018.0008.
- Saba MA, Goharpey S, Attarbashi Moghadam B, et al. Correlation between the 6-min walk test and exercise tolerance test in cardiac rehabilitation after coronary artery bypass grafting: a cross-sectional study. Cardiol Ther. 2021;10(1):201–209. doi: 10.1007/s40119-021-00210-0.
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70(2):113–119. doi: 10.1080/02701367.1999.10608028.
- Tremblay Labrecque PF, Harvey J, Nadreau E, et al. Validation and cardiorespiratory response of the 1-min sit-to-stand test in interstitial lung disease. Med Sci Sports Exerc. 2020;52(12):2508–2514. doi: 10.1249/MSS.0000000000002423.
- Bohannon RW, Crouch R. 1-minute sit-to-stand test: systematic review of procedures, performance, and clinimetric properties. J Cardiopulm Rehabil Prev. 2019;39(1):2–8. doi: 10.1097/HCR.0000000000000336.
- Wang Z, Yan J, Meng S, et al. Reliability and validity of Sit-To-Stand Test protocols in patients with coronary artery disease. Front Cardiovasc Med. 2022;9:841453. doi: 10.3389/fcvm.2022.841453.
- Kohlbrenner D, Benden C, Radtke T. The 1-minute sit-to-stand Test in lung transplant candidates: an alternative to the 6-minute walk test. Respir Care. 2020;65(4):437–443. doi: 10.4187/respcare.07124.
- Chhajed BS. Correlation between 6MWT, 2MST and TUG among hypertensive older individuals. Indian J Physiother Occup Ther. 2014;8:139–143. doi: 10.5958/J.0973-5674.8.1.027.
- Nogueira MA, Almeida TDN, Andrade GS, et al. Reliability and accuracy of 2-minute step test in active and sedentary lean adults. J Manipulative Physiol Ther. 2021;44(2):120–127. doi: 10.1016/j.jmpt.2020.07.013.
- Braghieri HA, Kanegusuku H, Corso SD, et al. Validity and reliability of 2-min step test in patients with symptomatic peripheral artery disease. J Vasc Nurs. 2021;39(2):33–38. doi: 10.1016/j.jvn.2021.02.004.
- Bennett H, Parfitt G, Davison K, et al. Validity of submaximal step tests to estimate maximal oxygen uptake in healthy adults. Sports Med. 2016;46(5):737–750. doi: 10.1007/s40279-015-0445-1.
