Abstract
Purpose
Research suggests that rates for autism may be higher in cerebral palsy than in the general population. For those with severe bilateral physical impairment (GMFCS level IV and V) and little or no speech, describing a profile of social communication skills has been difficult because there are currently no assessments for early social communication specifically tailored for these children. Our aim was to explore the assessment of aspects of joint attention and social reciprocity in this group of children with CP.
Methods
We compared the performance of children with bilateral CP on carefully designed assessments of joint attention and social responsiveness with groups of children with Down syndrome and autism. All three groups were matched for chronological age and mental age.
Results
Approximately 30% of the children with bilateral CP had early social communication scores similar to the autistic children. The remaining 70% of children with CP had a range of early social communication scores similar to the children with Down syndrome.
Conclusion
It is possible to assess key early social communication skills in non-speaking children with bilateral motor disability. This could provide insights to help clinicians and caregivers as they discuss abilities and explore potential areas for intervention.
IMPLICATIONS FOR REHABILITATION
With carefully designed activities, which do not rely on motor skills or verbal exchanges, it was possible to assess joint attention and social responsiveness skills in a group of non-speaking children with bilateral motor disability.
We were able to identify a subgroup of non-speaking children with severe motor disability (approximately 30% of our cohort) whose scores on our assessments were similar to a group of autistic children.
The ability to describe key early social communication skills should provide insights to help clinicians and caregivers as they discuss abilities and explore potential areas for intervention.
Introduction
Cerebral palsy (CP) is the most common motor disability in childhood affecting between 1.6 and 3.4 per 1000 births worldwide. The motor impairment of CP is often accompanied by difficulties in language development, cognitive functioning and social communication [Citation1–3]. Recent evidence also indicates higher rates of autism, attention deficit hyperactivity disorder, depression and anxiety in children with CP compared to peers of a similar age [Citation4,Citation5]. However, for children with CP who do not use speech or who use speech minimally/unreliably and have significant motor impairment affecting all four limbs, behavioural assessments can be difficult to engage with because their motor impairments limit the use of gestures such as pointing and showing [Citation6]. In the current study we attempt to assess early social communication skills in a group of non-speaking children with CP, and we compare their performance with a group of autistic children and a group of children with Down syndrome.
Prevalence estimates for autism in children with cerebral palsy (CP) are reported to be higher than in the general population, ranging from 3% to 30% [Citation5,Citation7–10]. Nordin and Gillberg [Citation10], and later Kilincaslan and Mukaddes [Citation9] used the Autism Behaviour Checklist (ABC) [Citation11], and the Childhood Autism Rating Scale (CARS) [Citation12], as screening and classification measures in CP population studies, finding a frequency of co-occurring autism of 11% and 15% respectively. While these prevalence rates are high, a limitation for both of these studies was that items on the ABC and the CARS could not be scored for children with the most significant physical disability. Delobel-Ayoub and colleagues [Citation7] reported slightly lower rates of autism (9%) following a review of the records of 1225 children with CP from Iceland, Sweden, France and England. Factors associated with an autism diagnosis were male sex, presence of epilepsy, intellectual disability, and, notably, severity of motor impairment. The latter association showed that children with less severe levels of motor impairment presented with higher levels of autism. Delobel-Ayoub and colleagues hypothesised that the association between a diagnosis of autism and less severe motor involvement likely reflects difficulties in assessing children with bilateral CP who are non-speaking, rather than a lack of social communication difficulties or other autistic characteristics in this subgroup. Christensen et al. [Citation13] reviewed the records of 451 children with CP in four areas of the USA, reporting a co-occurring frequency of autism of 7%. Lower prevalence rates from records-based studies as opposed to direct screening may reflect the difficulty in assessing social communication skills in the most significantly affected children in the clinical setting.
More recently, in a population-based study of 200 children with CP, Pahlman and colleagues [Citation5] used a range of established instruments as part of a diagnostic assessment for autism: the Diagnostic Interview for Social and Communication Disorders [Citation14], the CARS [Citation12], and when applicable the Autism Diagnostic Observation Schedule, Second Edition [Citation15]. While the authors reported that they were able to carry out an assessment for autism in many children with CP using these instruments, alongside a medical history and clinical observations (finding a 30% prevalence rate for autism), they also pointed out that “the diagnostic procedure with established instruments…was not suitable for the most disabled children.” It is the group of children with CP who are described as “most disabled” that we focus on in the current study.
There have also been attempts to focus specifically on assessing social communication skills, rather than a full autism diagnosis, in children with bilateral CP. While these studies have been important and informative, the findings are difficult to interpret as no control groups were used. Cress and colleagues [Citation16] recorded the communicative engagement patterns (e.g., gaze toward people and gaze shifts between people and objects) in 25 developmentally young children with disabilities, including 12 children with CP in the context of structured play sessions. The structured activities took place in short, naturally occurring interludes during a two-hour assessment protocol. These exchanges typically involved adults scaffolding child interactions by eliciting child responses through toy play with their preferred toys, responding contingently to child engagement behaviours, and repeating activities that elicited such behaviours. The findings were compared to the frequency of engagement behaviours seen in free play in an earlier study involving the same group of children [Citation17]. The authors were interested in whether different contexts for assessment influenced behaviour. The frequency of engagement in social communication was described as low in both contexts (relative to norms for the activities used), although significantly more engagement behaviours were observed in the structured versus the free play context. This at least suggests that structured play-based assessments have potential to reveal the child’s potential for social communication, even if no direct comparison was made with a control group.
Despite the difficulty in providing a full diagnosis for autism in non-speaking children with bilateral CP, there is value in assessing selected early social communication skills. No clinical assessment can realistically measure all the features of social communication. However, it may be possible to assess aspects of early social communication in this group, such as initiation of triadic joint attention, response to triadic joint attention, response to adult emotion, response to social smile, response to name, anticipation of familiar routine, and requesting a turn. This would not only help to identify skill profiles associated with autism, but would also help clinicians, teachers, and parents in planning appropriate support with the aim of optimising their own and children’s skills and enhancing shared participation through changes in adult interaction practices. In the current study we focussed on directly assessing joint attention and social responsiveness, behaviours which have been identified as “pivotal” for young autistic children [Citation18] and as key features in the diagnosis of autism [Citation19].
Joint attention has been described as the “simultaneous engagement of two or more individuals in mental focus on one and the same external thing” [Citation20] and is typically expressed through the triadic co-ordination of gaze between self, other, and some third object, event, or symbol; although it can also be expressed through other senses such as hearing or touch. Infants with typical development begin to share attention with others in the first year of life, initially by responding to the joint attention cues of others to share attention, for example by following gaze, and later by directing the attention of others [Citation21]. There is clear evidence for an association between joint attention skills early in development and later language skills [Citation22], and, it has been argued, it is through these joint attention behaviours that infants first begin to engage with others’ communicative intentions and mental states [Citation23].
By contrast, infants and young children who later receive a diagnosis of autism have been shown to have limitations in the triadic co-ordination of gaze for joint attention in the first two years of life [Citation24–26] and associations have been reported between the degree to which children engage in triadic joint attention and later language and social development in autism [Citation27]. In fact, limitations in joint attention expressed through gaze behaviours, particularly early in development, are now used as markers for autism by established diagnostic instruments (e.g., the DISCO) [Citation14] the CARS [Citation12], and the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) [Citation15].
In addition to triadic joint attention, another aspect of social communication that could potentially be observable in non-speaking children with severe CP is social responsiveness. This can be assessed by observing gaze behaviours and expressions of emotion (e.g., smiles, laughter) in a social context. Social responsiveness refers to a range of behaviours including looks towards another person in response to their expression of emotion, on hearing one’s own name, or in anticipation of a social routine or during a deliberately delayed turn-taking activity. It can also refer to facial expressions and other expressions of emotion, for example in response to a social smile [Citation15]. A lack of social responsiveness has also been identified as being characteristic of autism, particularly early in development. For example, autistic children are reported to look less often at a clinician’s face than do neurotypical children during assessments where the clinician attempts to elicit eye contact (e.g., by playing peek-a-boo or by suddenly displaying an emotional expression such as fear or surprise) [Citation28]. A range of behaviours considered to indicate social responsiveness is used in the ADOS-2 diagnostic assessment for autism [Citation15].
In the current study we identified two subsets of the “early socio-cognitive measures” in the Very Early Processing Skills (VEPS) assessment [Citation29], designed to measure triadic joint attention and social responsiveness, which could be accessible to young children using gaze direction as a response. The VEPS has previously been used to assess early social communication skills in a large cohort of typically developing young children, aged 2;6–3;6 years of age, and has been found to be a good predictor of later language and social communication skills [Citation29]. We also included additional novel tasks designed to assess joint attention and social responsiveness. Task selection was also guided by the characteristic behaviours suggestive of early social communication difficulties, as identified by Wetherby and colleagues [Citation30] and tasks included in the Autism Diagnostic Observation Schedule (ADOS-2) [Citation28].
The triadic joint attention tasks we used were designed to identify whether the child shows evidence of the triadic co-ordination of gaze between self, other and an object. For example, whether the child, after having been prompted to gaze at an object of interest that is suddenly shaken, voluntarily shifts gaze towards the adult thus potentially engaging the adult in an interaction “about” the object. Another task examined whether the appearance of an object that has previously been shared between adult and child, prompts the child to first shift gaze between the object and the adult’s face and then to attempt to direct the adult’s attention to the object. Two further measures focus on another element of triadic joint attention: the ability to follow another person’s attentional signals (e.g., gaze direction and pointing). We observe first whether gaze direction alone is enough to elicit joint attention and then whether, on a second attempt, an additional point and vocalisation elicits a response.
The social responsiveness tasks we used were chosen based on VEPS items and also on items typically failed by young autistic children in the ADOS-2 procedure [Citation28]. This involved a range of tasks assessing response to emotion, reciprocal smile, response to name, anticipation of a social routine and requesting a turn.
The assessments we used in the current study were specially selected and adapted for non-speaking children with CP [Citation29,Citation30]. We also used the same tasks to assess groups of autistic children and children with Down syndrome. Both comparison groups were expected to show different profiles of performance on our selected early social communication tasks with lower scores predicted for the autistic children than for the Down syndrome children. This would suggest that our selected measures of early social communication were sensitive enough to identify difficulties in the autistic children and strengths in the Down syndrome children. Our aim in choosing a Down syndrome children here was to assess a comparison group that was matched with our CP group for chronological age, non-verbal cognition and language comprehension, but who did not have a diagnosis of autism. If this group showed a different profile of performance on our selected early social communication tasks (e.g., with higher than the autism group), this would suggest that task performance is not simply reflecting age, cognitive ability or language comprehension. We could have chosen other potential comparison groups, however we had access to this clinical population and all children had a well-defined clinical diagnosis with no indication of autism.
In addition, based on prevalence rates for autism in the CP population from the most recent population-based study [Citation5], we predicted that a proportion (up to 30%) of our group of non-speaking children with CP would have early social communication scores similar to the autism comparison group. By contrast, since none of our Down syndrome children had a dual diagnosis of autism we did not expect any overlap in scores with the autistic children.
Finally, in the current study we rely on each child’s gaze patterns as an indication of their engagement in the assessments. However, some children with motor impairments may have difficulties with gaze fixation and gaze transfer [Citation31]. There is also an increased incidence of cerebral visual impairment (CVI) in cerebral palsy [Citation31] and a range of visual impairments which could affect responses. Such issues present a particular challenge for these assessments: is a lack of response to be explained by the child’s difficulties in the functional use of vision or by impairment in triadic joint attention and social responsiveness? For the CP group then, we also used a non-social task to assess functional gaze control skills, in particular the ability to fixate and shift gaze—the key gaze behaviours important for our assessments. As with the social communication assessments, the functional gaze control assessment was designed for ease of use by clinicians, who may be non-vision specialists, but who work with non-speaking children with complex motor disorders. Our aim here was to examine whether there was a relationship between scores on our selected early social communication assessments and the level of functional gaze control in our CP group.
To summarise, our research questions were as follows:
Can we assess joint attention and social responsiveness skills in non-speaking children with severe CP?
Are the assessments sensitive enough to identify difficulties in a group of autistic children and strengths in a group of children with Down syndrome?
Are early social communication scores for the CP group associated with more general difficulties in functional gaze control?
Is there an association between early social communication scores and mental age?
Do our early social communication assessments identify a proportion of children with CP with a similar profile to the autistic children?
Methods
Participants
Ethical approval for the study was obtained from NHS Health Research Authority (London Hampstead Committee), reference 12/LO/1243, and University College London (UCL)’s Ethics Committee, reference 1328/005. Informed consent was gained from parents of all children participating in the research.
Sixty-six children with bilateral CP, 19 children with Down syndrome and 10 autistic children were recruited by contacting schools in the southeast of England. School staff were asked to identify children who they thought might meet a set of inclusion criteria and to forward an invitation to families to take part in the study. Inclusion criteria for all three groups were: (i) a chronological age between 3 and 12 years; (ii) little or no speech; (iii) hearing levels (including corrected) adequate for speech recognition; iv) epilepsy, if present, described as controlled; (v) language understanding (Pre-school Language Scales [Citation32] at 12–60 months); (vi) intellectual ability (Mullen Scales of Early Learning [Citation33] at 12–60 months). We made one exception by including a child whose language understanding was 15 months but for whom we had no Mullens assessment data (subsequently that child was tested and had an intellectual ability score of 9 months; we decided to retain this child in the study as they had already taken part and met all other criteria). For the CP group additional criteria were: (i) bilateral cerebral palsy (GMFCS categories IV and V [Citation34]; (ii) school recommends that the child is able to use functional vision for communication—and a score of at least 50% (≥ 7 out of 14) on our functional gaze control assessment. All participants in the autistic children’s group had a clinical record of diagnosis made by an NHS team adhering to the Diagnostic and Statistical Manual of Mental Disorders [Citation19].
Of the 66 children with CP referred by schools, 32 met the inclusion criteria. Primary reasons for exclusion were physical ability above criterion (n = 1), unable to engage in research activities/poor health/uncontrolled epilepsy (n = 8), unable to fixate gaze consistently (n = 10), language understanding above criterion (n = 5), language understanding below criterion (n = 10). The motor, communication, and speech profile of the remaining 32 children with CP (19 dyskinetic; 11 mixed; 2 spastic CP), are presented in , including descriptive level on: Gross Motor Function Classification Scale (GMFCS) [Citation34]; Manual Ability Classification System (MACS) [Citation35]; Communication Function Classification System (CFCS) [Citation36]; Viking Speech Scale [Citation37].
Table 1. Functional ability profile of children with CP.
Of 19 children with Down syndrome, 16 met criteria for inclusion and of 10 autistic children, 9 met the inclusion criteria. We assessed all participants using the Mullen Scale of Early Learning (Visual Reception subscale) [Citation33] for non-verbal ability and the Pre-school Language Scales (PLS-4 Auditory Comprehension subscale) [Citation32] for verbal ability. For the Mullen Visual Reception subscale we made the following adaptations. We enlarged the picture materials from A5 sizing (148.5 × 210 mm), to A4 format (210 mm × 297 mm). 5 of the 33 items were excluded as they were highly dependent on motor manipulation (items: 5, 10, 11, 13, 18). For the Auditory Comprehension subscale of the PLS-4, 13 of the 62 items were excluded as they could not be scored through a response based on use of gaze direction or auditory scanning (items: 9, 15, 17, 18, 19, 22, 24, 26, 28, 32, 33, 52, 58). We used the following formula to take account of the missing items [raw score (items used) ÷ total possible score at ceiling (items used)] multiplied by the total possible score at ceiling in standard administration = adapted raw score. The adapted raw score was then used to derive an age equivalent score from the standardised tables. Given these adaptations, the absolute scores derived should be treated with caution. However, for comparison purposes, the same adapted version of both tests was used for all three groups of children.
All three groups were matched for chronological age, F (2,54) = 0.62, p = 0.54, non-verbal cognition, F (2, 54) = 0.23, p = 0.79, and language comprehension, F (2,54) = 1.67, p = 0.20 (see ).
Table 2. Mean, range and standard deviation (SD) chronological age, non-verbal age, and language age equivalent for each group.
Procedure
All children were assessed in school or in the child language testing facility at the University, with their parents present. All assessments were carried out by a senior Speech and Language Therapist. The activities were video recorded with a single camera directed at the child’s eyes; notes and scoring were also recorded live by KP during the assessment itself.
Functional gaze control (children with CP only)
The materials comprised two targets (either star, square or triangle) of 5 cm diameter, coloured yellow on one side and black on the other, on the end of black stick handles. The targets were held up against a blackboard background (1 m × 0.75 m) 1 m from the child, with either the coloured surface facing the child or the black (camouflaged) surface. The experimenter could move the targets to different locations against the black background and manually flip the target 180 degrees, making it more visible (coloured surface) or less visible (black surface) by twisting the handle. A target was “presented” by flipping the coloured surface to face the child. A small whole (5 mm diameter) in the centre of the board allowed the experimenter (positioned behind the board and out of sight of the child) to observe the child’s gaze direction. The task was designed to be accessible by children with normal to moderate levels of visual acuity (6/6 to 6/60; Snellen scale; [Citation38]), and assessed the ability to fixate gaze and to carry out a combination of fixating and shifting gaze.
Gaze fixation
On each of 10 trials a target was presented in random order in one of 4 positions (centre, top, bottom, left and right) and held for 5 s. A score of 1 was given for each fixation (total score = 10).
Gaze fixation and shift
On each of 4 trials a target was first presented centrally for 5 s. If the child fixated on this first target (fixation time > 1 s), a second target was presented (an equal number of times, in random order) on either the left or the right of the black board, with the central target remaining visible. The experimenter judged whether gaze transferred from central fixation to peripheral fixation on each trial. A score of 1 was awarded for a successful fixation followed by gaze transfer (Total = 4).
Total maximum score for Functional Gaze Control = 14
Selected measures of early social communication
We adapted two subsets of the “early socio-cognitive measures” in the Very Early Processing Skills (VEPS) assessment [Citation29]. For VEPS joint attention measures we enlarged the toy material to increase visual salience and to encourage child engagement, presented materials at a closer distance and used fewer trials. For the VEPS social responsiveness measures, we removed the “response to anger” item as we were concerned this might trigger a startle reflex or general distress.
We also included two novel tasks assessing joint attention and social responsiveness based on behaviours suggestive of social communication difficulties, as identified by Wetherby and colleagues [Citation30] and tasks included in the Autism Diagnostic Observation Schedule (ADOS) [Citation28].
Joint attention tasks
The child and adult (experimenter) were seated opposite each other on either side of a table.
Adapted VEPS measures
Shaken egg/new toy
Three large plastic eggs (40 cm in height) were placed on the table. On each of three trials an egg was picked up in one hand (left or right) by the adult and, without speaking and while looking at the child, held out at arm’s length to the side and shaken. The egg was then opened by the adult to reveal a small object (e.g., a small doll). One point was awarded if the child looked from the egg to the researcher’s face when the egg was selected and/or opened (maximum score = 3).
Matching toy
Larger versions of the objects found in the eggs were on display approximately 1.5 m and 45 degrees to the left or right of the child. The adult looked from the small toy towards the larger matching version and commented on their similarity: for example, saying, I brought my tiger with me today. Two points were awarded if the child spontaneously followed the adult’s gaze direction from the small toy to the large toy. If the child failed to follow the researcher’s line of sight, the researcher repeated the prompt, accompanied by a finger-point towards the larger object. One point was awarded if the child subsequently transferred their gaze. This was repeated for each of the three toys (maximum score = 6).
To support the VEPS assessment we developed a further protocol for which child looking behaviour alone would offer a reliable response modality.
Initiation of joint attention
This assessment focused more directly on probing for initiation of joint attention (IJA). In a warm-up activity the child was shown three toy rabbits and encouraged to choose one to play with for a short time. The researcher then counted the rabbits with the child; “one, two, three rabbits!” and invited the child to look out for more rabbits, prompting the child to initiate joint attention with the researcher. Subsequently, more rabbit items were presented in three different trials during different play contexts: (i) as a picture item in a 5-piece form board puzzle; (ii) as one of a set of five wind-up toys, and (iii) as a surprise pop-up rabbit toy appearing behind the assessor’s head. If no attempt at IJA was observed, the researcher held up the rabbit and said “Is this another rabbit? Wow yes!” to support the child in their engagement in the “noticing and telling” task. A score of 1 was awarded for the child looking from the rabbit to the adult, plus a “knowing” smile and/or vocalisation and/or gesturing (including eye-pointing) to the rabbit item (maximum score = 3).
Response to joint attention
The adult invited the child to help find toy rabbits partially hidden approximately 1.5 m away in the room by asking “Shall we try and find some more rabbits?” The researcher smiled and directed her own gaze to each of two hidden toy rabbits in turn. A score of 2 was given if the child followed the gaze direction of the tester for either of the hidden rabbits. If the child failed to follow the researcher’s direction of gaze spontaneously, the adult added a point and/or a verbal prompt such as “Look! I can see one!” A score of 1 was awarded if the child followed the adult’s gaze direction in either of these prompted trials (maximum score = 2).
Total maximum score for joint attention = 14
Scoring
For the first VEPS task (shaken egg/new toy) and the initiation of joint attention task there were 3 trials each (each scoring 1 point for a response). On the Matching toy and response to joint attention tasks (each with 3 trials) we used a graded scoring system so that a response to a gaze only cue is scored higher (2 points) than a response only on an additional presentation with added cues (gaze + pointing + vocalisation). The additional presentation was only used if the child did not respond to the initial cue. The total maximum score for joint attention was derived by adding together the highest possible scores for each task.
Social responsiveness tasks
The social responsiveness measures examined the child’s (i) response to adult emotion, (ii) response to social smile, (iii) response to name, (iv) anticipation of a familiar routine and v) requesting a turn.
Response to adult emotion
On five trials the adult acted out a play activity accompanied by an emotional facial expression (hurt, surprise, frustration, fear and achievement). Child looks to the adult’s face in response to each emotion were scored 2 points (looks ≥ 1 s) or 1 point for a fleeting look (looks ≤ 1 s) (maximum score =10).
Response to social smile
During one-to-one play activities with the child, the adult would offer a relaxed social smile to the child. A single point was awarded if the child smiled back in response to adult’s smile (maximum score = 1).
Response to name
During one-to-one play activities with the child, the adult attempted to gain the child’s attention by calling out his/her name; this was done three times. One point was awarded if the child looked towards the adult’s face at least once (maximum score = 1).
Anticipation of a familiar routine
On each of 3 trials the adult demonstrated a pop-up toy rabbit to the child, saying “ready, steady, go!” A single point was awarded if the child displayed anticipation by looking at the adult’s face, and showing heightened affect, and/or vocalisation/laughter/body movement, on any one occasion during the three presentations (maximum score = 1).
Requesting a turn
During the same three presentations of the pop-up toy rabbit activity, 1 point was awarded if the child displayed looking behaviours, heightened affect, vocalisation/laughter, body movement, interpretable as a request for the activity to be repeated on any single occasion (maximum score = 1).
Total maximum score for social responsiveness = 14
Scoring
In observing the child’s response to an adult emotion there were five trials with scoring for each trial; we scored higher for a more sustained look (greater than 1 s) (2 points) as we could be more confident of the child’s intention, but a lower score for a “fleeting” look (less than 1 s) (1 point). For response to smile and response to name there was one trial each (each scoring 1 point for a response). For anticipation of familiar routine and for requesting a turn it can sometimes be difficult to elicit a response and often, in our clinical experience, requires more than one prompt—we therefore chose to score 1 point for a response on any of three trials. The total maximum score for social responsiveness was derived by adding together the highest possible scores for each task.
Results
Reliability analysis
For the functional gaze control measures, inter-rater reliability was evaluated from live scoring by two experimenters (both experienced Speech and Language Therapists) on 10 children with CP. Cohen’s Kappa co-efficient indicated that agreement was good for gaze fixation (k = 0.62) and excellent for gaze transfer (k = 0.79).
For the social communication measures combined, inter-rater reliability was also calculated from the scoring of video recordings by four different coders (all Speech and Language Therapists) and compared with a single coder. Intra-class correlation analysis revealed an excellent level of agreement r(ICC = 0.74).
Functional gaze control
The distribution of scores on the functional gaze control measures is shown in . There was no difference in fixation for location of stimuli presentation (centre, top, bottom, left and right) χ2(2) = 0.183, p = 0.996. There was no difference in fixation + gaze transfer performance in relation to the location of the second target (left or the right of the central target) χ2(2) = 0.9, p = 0.343.
Table 3. Distribution of performance on functional gaze control tasks.
Joint attention and social responsiveness tasks
The scores for the joint attention (JA) task and social responsiveness (SR) task, and for the two combined (social communication score) are shown in .
Table 4. The mean, standard deviation (SD) and range of scores on the joint attention and social responsiveness tasks by group.
Joint attention score
First, we compared group performance on our joint attention assessment only. A one-way ANOVA revealed a significant main effect of group, F (2, 54) = 8.87, p < 0.001, ηp2 = 0.247.
Post-hoc analysis using Tukey’s HSD indicated that the joint attention scores for the CP group (M = 6.9, SD = 3.2) were significantly lower than those for the DS group (M = 9.7, SD = 2.8) (p = 0.013). There was no significant difference between scores for the CP group and those for the autism group (M = 4.4, SD = 3.3) (p = 0.97). Scores for the DS group were significantly higher than those for the autism group (p < 0.001).
Social responsiveness score
Next, we compared group performance on the social responsiveness assessment. Kruskal-Wallis ANOVA revealed a significant main effect of group: H (2) = 9.64, p = 0.008. Mann-Whitney tests were used to follow up the finding from the ANOVA. A Bonferroni adjustment was applied and so all effects are reported at a 0.0167 level of significance. There was no significant difference in performance between the children with CP (Mdn = 8.0) and DS (Mdn = 10.0), U = 205.5, p = 0.23, r = 0.16, and no significant difference in performance between the children with CP (Mdn = 8.0) and autism (Mdn = 5.0), U = 74, p = 0.035, r = 0.33. Scores for the DS group were significantly higher than those for the autism group, U = 11.5, p = 0.001, r = 0.69.
Early social communication score (JA and SR scores combined)
Finally, we compared the group scores for social communication (total score—JA and SR combined). shows each child’s score for the social communication assessment, arranged by diagnostic group.
Figure 1. The mean social communication score by group (CP, DS, autism)*.
*A jittered scatter plot is presented to represent overlapping data points.
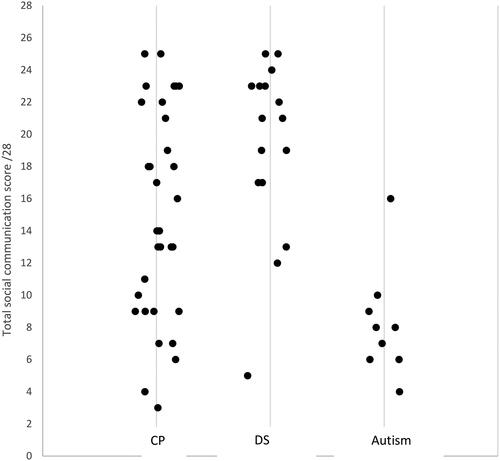
Welch’s ANOVA revealed a significant main effect of group, F (2, 54) = 8.83, p < 0.001. ηp2 = 0.246. Post-hoc analysis using the Games-Howell test indicated that social communication scores for the CP group (M = 14.9, SD = 6.5) were significantly lower than scores for the DS group (M = 19.1, SD = 5.5) (p = 0.04). The CP group scored significantly higher than the autism group (M = 8.8, SD = 4.1) (p < 0.001). The DS group also scored significantly higher than the autism group (p < 0.001).
We also examined the relationship between early social communication scores and background age equivalent scores for each group. There was a significant positive correlation between early social communication scores and non-verbal mental age for all three groups (CP: r (32) = 0.70, p < 0.001; DS: r (16) = 0.53, p<.05; autism: r (9) = 0.79, p<.05). There was also a significant positive correlation between early social communication scores and language understanding age equivalents for the CP group (r(32) = 0.53, p < 0.001) and for the autism group (r(32) = 0.73, p<.05), but not for the DS group (r(16) = 0.47, p > 0.05). For all three groups there was no significant correlation between chronological age and early social communication scores (CP: r(32) = −0.14, p > 0.05; DS: r(16) = 0.34, p > 0.05; autism: r(9) = 0.18. p > 0.05).
For the CP group we also examined whether there was an association between early social communication scores and functional gaze control score. There was no significant correlation between these two measures (r(32) = 0.29, p > 0.05).
We also compared the total functional gaze control scores (maximum = 14) for the subgroup of 10 children with CP with the lowest social communication scores (Low SC) (overlapping those from the autism group—see ), with the 10 children with CP who had the highest social communication scores (High SC). The functional gaze control scores for the Low SC group (M = 11.4; SD = 2.6) and the High SC group (M = 13.0; SD = 1.1) were compared using a Mann-Whitney test as Levene’s test for equality of variances was significant (F = 4.49, p = 0.048). The analysis revealed no significant difference in functional gaze control scores between the Low SC group (Mdn = 12) and the High SC group (Mdn = 13), U = 30, p = 0.14, r = 0.08.
The Low SC group had a lower non-verbal age than the High SC group (M = 17.2 and M = 38.5 months respectively; t(18)= −5.21, p < 0.001), and a lower language comprehension age (M = 19.8 and M = 33.6 months respectively, t(18) = −3.2, p < 0.001).
Discussion
Recent research suggests that autism may be more common in children with CP than the general population, but for those with significant bilateral physical impairment and little or no speech, describing a profile of social communication skills has been difficult because there are currently no assessments for early social communication specifically tailored for these children. In the current study, our aim was to explore the assessment of aspects of joint attention and social reciprocity in this group of children with CP.
Our early social communication assessments worked well with the children we tested. For the group of children with CP we were able to obtain a range of scores even though we relied mainly on eye gaze behaviours or facial expressions as responses. There did not appear to be floor or ceiling effects using these assessments when testing the children with CP, at least in the sense that the assessments were not all failed by all of the children or all passed by all of the children. The results for our two comparison groups were also of interest. The assessments were sensitive enough to identify difficulties in social communication in the autistic children and strengths in the Down syndrome children, who scored significantly higher. There was virtually no overlap in scores between these two comparison groups apart from one child with Down syndrome whose score was in the range of scores obtained for the autistic children, and one child with autism whose score overlapped with the range for Down syndrome (albeit in this case only 2 children with Down syndrome had a lower score). We did not expect an overlap in scores of course as none of the children with Down syndrome had previously been given a dual diagnosis of Down syndrome and autism, but it was useful to see that scores produced by our assessments were consistent with this.
Our next question was whether the early social communication scores for the children with CP were associated with functional gaze control skills. Although a variety of CVI problems can be found in children with CP [Citation31], our concern here was to assess the most basic functional vision skills that might be required for our joint attention and social responsiveness assessments—the ability to fixate on an object and to fixate and shift gaze between objects. We found no significant correlation between early social communication score and functional gaze control score. Furthermore, the 10 children with CP with the lowest early social communication scores (whose scores overlapped with the autistic children) did not differ in functional gaze control skills when compared to the top 10 early social communication scorers from the CP group. Of course, we need to be relatively cautious, accepting that this was a fairly small sample size. With that caveat, our findings suggest that scores on our assessments are unlikely to be accounted for by level of functional gaze control.
We also examined whether there were any associations between early social communication scores in the CP group and measures of language and non-verbal abilities. Here we found that there were positive correlations between early social communication scores and both non-verbal age equivalent and verbal comprehension age equivalent. This was not specific to the group of children with CP. Similar associations were found for both the autistic group and the Down syndrome group. This was a surprising finding as the early social responsiveness skills we assessed, such as requesting repetition, returning a social smile etc. would normally be in place in typically developing children by at least 9 months of age, and joint attention measures by 15 months [Citation39] whereas the verbal and non-verbal ages of our CP group were all above 15 months (with the exception of one child with a non-verbal age of 9 months). Our findings suggest that for the children in all three groups, social responsiveness and joint attention skills are still developing with mental age. Early social communication scores were not correlated with chronological age in any of the three groups. It is worth noting another possibility, that the PLS and the Mullens assessments both require the child to engage socially with the assessor, at least in terms of responding to requests to engage and indicating answers by pointing (either finger pointing or eye pointing). It’s possible that common requirements for these assessments account for the correlations.
Our final research question was whether our early social communication assessments identify some children with CP with a similar profile to the autistic children. Here we found that 10 children from our group of 32 had low early social communication scores overlapping with the scores for the autistic children. We need to be cautious not to overinterpret this finding. This is not an epidemiological study and our CP group is not necessarily a representative sample of non-speaking children with severe motor impairment. For example, excluding more linguistically and intellectually able children may have influenced incidence rates. In addition, our early social communication assessments are quite selective, designed to be accessible for assessing this cohort and scores are not equivalent to a full diagnosis of autism. The finding does, however, appear to reveal that quite a high proportion of our children with CP have joint attention and social responsiveness scores equivalent to the autistic children and it is worth noting that in a recent population study assessing autism in children with CP [Citation5] 30% of children with CP were diagnosed with autism. Although our study was not designed to provide a full diagnosis of autism, assessment of joint attention and social responsiveness are typically part of autism diagnosis (e.g., under the module “reciprocal and social interaction”—ADOS-2 [Citation15]), and limitations in this domain are a strong indication of autism. Our data, at least to the extent that we showed a co-occurrence of early social communication differences in children with CP and autistic children, suggests that the co-occurrence may extend across the clinical continuum and may not only be associated with more verbal children or those with less significant physical impairment.
It is still unclear whether studies showing an increased rate of autism with CP indicate a shared causative mechanism. As Zwaigenbaum [Citation40] has pointed out, both autism and CP are characterised by etiological heterogeneity [Citation41], so that there may be a number of factors underlying the co-occurrence of autism and CP. It is notable also that non-speaking children with significant physical impairments are known to experience different, often restrictive, patterns of social interaction with others which may limit opportunities to establish joint attention as expressed in typical ways, which may have consequences for the development and expression of social communication skills explored in this study [Citation42,Citation43]. These are important topics for future research.
An important clinical outcome from our research is that we have established that it is possible to assess early social communication skills in this group of children with bilateral motor disability and little to no speech. The assessments we have used here, including the functional gaze control measure, can be used by clinicians who do not necessarily specialise in social communication assessment or do not have specialist vision training as an ophthalmologist, and they are relatively short (could be carried out within a two hour clinic session). Our assessments of social communication skills could provide clinicians and caregivers with insights to inform their joint discussions and family-led decision-making.
There are a number of limitations to consider in assessing our findings, and suggestions for future research. Our sample was not fully representative of non-speaking children with CP with bilateral motor impairment. One reason we know this is because a number of children referred to us still had to be excluded from our research sample, including those who were unable to fix gaze and those with very low levels of language understanding. It’s possible that these excluded children had CVI which restricts the visually-based assessments for early social communication. We also don’t have data on potential CVIs in the children we did include; it’s possible that this may have influenced performance on our early social communication assessments, and this would be interesting to know, but data from our functional gaze control assessment at least suggests that the behaviours we were observing were not influenced by an ability to fixate and shift gaze. Finally, future research could also focus on developing therapeutic resources for those children identified with impairments in joint attention and social responsiveness, with the ultimate aim of enhancing participation in ways that are meaningful and enjoyable for families and children.
Acknowledgements
The authors wish to thank the children, families, schools and clinicians who took part in the research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Pennington L, Dave M, Rudd J, et al. Communication disorders in young children with cerebral palsy. Dev Med Child Neurol. 2020;62(10):1161–1169. doi:10.1111/dmcn.14635.
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol. 2007;49(Suppl. 2):8–14. doi:10.1111/j.1469-8749.2007.tb12610.x.
- Soriano JU, Hustad KC. Speech-language profile groups in school aged children with cerebral palsy: nonverbal cognition, receptive language, speech intelligibility, and motor function. Dev Neurorehabil. 2021;24(2):118–129. doi:10.1080/17518423.2020.1858360.
- Bjorgaas HM, Hysing M, Elgen I. Psychiatric disorders among children with cerebral palsy at school starting age. Res Dev Disabil. 2012;33(4):1287–1293. doi:10.1016/j.ridd.2012.02.024.
- Påhlman M, Gillberg C, Himmelmann K. Autism and attention-deficit/hyperactivity disorder in children with cerebral palsy: high prevalence rates in a population-based study. Dev Med Child Neurol. 2021;63(3):320–327. doi:10.1111/dmcn.14736.
- Sargent J, Clarke M, Price K, et al. Use of eye-pointing by children with cerebral palsy: What are we looking at?. Int J Lang Commun Disord. 2013;48(5):477–485. doi:10.1111/1460-6984.12026.
- Delobel-Ayoub M, Klapouszczak D, van Bakel MME, et al. Prevalence and characteristics of autism spectrum disorders in children with cerebral palsy. Dev Med Child Neurol. 2017;59(7):738–742. doi:10.1111/dmcn.13436.
- Craig F, Savino R, Trabacca A. A systematic review of comorbidity between cerebral palsy, autism spectrum disorders and Attention Deficit Hyperactivity Disorder. Eur J Paediatr Neurol. 2019;23(1):31–42. doi:10.1016/j.ejpn.2018.10.005.
- Kilincaslan A, Mukaddes NM. Pervasive developmental disorders in individuals with cerebral palsy. Dev Med Child Neurol. 2009;51(4):289–294. doi:10.1111/j.1469-8749.2008.03171.x.
- Nordin V, Gillberg C. Autism spectrum disorders in children with physical or mental disability or both. I: clinical and epidemiological aspects. Dev Med Child Neurol. 1996;38(4):297–313. doi:10.1111/j.1469-8749.1996.tb12096.x.
- Cassidy A. Autism behavior checklist. Encyclopedia of autism spectrum disorders. New York: Springer; 2013. p. 342–343.
- Schopler E, Reichler RJ, DeVellis RF, et al. Toward objective classification of childhood autism: childhood Autism Rating Scale (CARS). J Autism Dev Disord. 1980;10(1):91–103. doi:10.1007/BF02408436.
- Christensen D, Van Naarden Braun K, Doernberg NS, et al. Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning – Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev Med Child Neurol. 2014;56(1):59–65. Jan doi:10.1111/dmcn.12268.
- Wing L, Leekam SR, Libby SJ, et al. The diagnostic interview for social and communication disorders: background, inter-rater reliability and clinical use. J Child Psychol Psychiatry. 2002;43(3):307–325. doi:10.1111/1469-7610.00023.
- Gotham K, Risi S, Pickles A, et al. The autism diagnostic observation schedule: revised algorithms for improved diagnostic validity. J Autism Dev Disord. 2007;37(4):613–627. Apr doi:10.1007/s10803-006-0280-1.
- Cress CJ, Arens KB, Zajicek AK. Comparison of engagement patterns of young children with developmental disabilities between structured and free play. Educ Train Dev Disabil. 2007;42(2):152–164.
- Arens K, Cress CJ, Marvin CA. Gaze-shift patterns of young children with developmental disabilities who are at risk for being nonspeaking. Educ Train Dev Disab. 2005;40(2):158–170.
- Charman T. Why is joint attention a pivotal skill in autism?. Philos Trans R Soc Lond B Biol Sci. 2003;358(1430):315–324. doi:10.1098/rstb.2002.1199.
- Association AP. Diagnostic and statistical manual of mental disorders: DSM-5™, 5th ed. (DSM-5). Washington, DC: American Psychiatric Publishing, a division of American Psychiatric Association; 2013.
- Mundy P, Sigman M, Kasari C. A longitudinal study of joint attention and language development in autistic children. J Autism Dev Disord. 1990;20(1):115–128. doi:10.1007/BF02206861.
- Mundy P, Block J, Delgado C, et al. Individual differences and the development of joint attention in infancy. Child Dev. 2007;78(3):938–954. doi:10.1111/j.1467-8624.2007.01042.x.
- Morales M, Mundy P, Delgado CEF, et al. Responding to joint attention across the 6-through 24-month age period and early language acquisition. Journal of Applied Developmental Psychology. 2000;21(3):283–298. doi:10.1016/S0193-3973(99)00040-4.
- Tomasello M. Joint attention as social cognition. Joint attention: its origins and role in development. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1995. p.103–130.
- Ibañez LV, Grantz CJ, Messinger DS. The development of referential communication and autism symptomatology in high-risk infants. Infancy. 2013;18(5):687–707. doi:10.1111/j.1532-7078.2012.00142.x.
- Nyström P, Bölte S, Falck-Ytter T. Responding to other people’s direct gaze: alterations in gaze behavior in infants at risk for autism occur on very short timescales. J Autism Dev Disord. 2017;47(11):3498–3509. doi:10.1007/s10803-017-3253-7.
- Nyström P, Thorup E, Bölte S, et al. Joint attention in infancy and the emergence of autism. Biol Psychiatry. 2019;86(8):631–638. doi:10.1016/j.biopsych.2019.05.006.
- Yoder P, Stone WL, Walden T, et al. Predicting social impairment and ASD diagnosis in younger siblings of children with autism spectrum disorder. J Autism Dev Disord. 2009;39(10):1381–1391. doi:10.1007/s10803-009-0753-0.
- Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223.
- Chiat S, Roy P. Early phonological and sociocognitive skills as predictors of later language and social communication outcomes. J Child Psychol Psychiatry. 2008;49(6):635–645. doi:10.1111/j.1469-7610.2008.01881.x.
- Wetherby AM, Woods J, Allen L, et al. Early indicators of autism spectrum disorders in the second year of life. J Autism Dev Disord. 2004;34(5):473–493. doi:10.1007/s10803-004-2544-y.
- Deramore Denver B, Froude E, Rosenbaum P, et al. Measurement of visual ability in children with cerebral palsy: a systematic review [Review. Dev Med Child Neurol. 2016;58(10):1016–1029. doi:10.1111/dmcn.13139.
- Zimmerman I. Preschool language scale, Fourth Edition (PLS-4 UK). London: Pearson; 2009.
- Mullen E. Mullen scales of early learning. MN: AGS Circle Pines; 1995.
- Palisano RJ, Avery L, Gorter JW, et al. Stability of the gross motor function classification system, manual ability classification system, and communication function classification system. Dev Med Child Neurol. 2018;60(10):1026–1032. doi:10.1111/dmcn.13903.
- Eliasson AC, Krumlinde-Sundholm L, Rösblad B, et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability [Review. Dev Med Child Neurol. 2006;48(7):549–554. doi:10.1017/S0012162206001162.
- Hidecker MJC, Cunningham BJ, Thomas-Stonell N, et al. Validity of the communication function classification system for use with preschool children with communication disorders. Dev Med Child Neurol. 2017;59(5):526–530. doi:10.1111/dmcn.13373.
- Pennington L, Virella D, Mjøen T, et al. Development of The Viking Speech Scale to classify the speech of children with cerebral palsy. Res Dev Disabil. 2013;34(10):3202–3210. doi:10.1016/j.ridd.2013.06.035.
- Hetherington R. The Shellen chart as a test of visual acuity. Psychol Forsch. 1954;24(4):349–357. doi:10.1007/BF00422033.
- Tomasello M, Carpenter M, Call J, et al. Understanding and sharing intentions: the origins of cultural cognition. Behav Brain Sci. 2005;28(5):675–691. doi:10.1017/S0140525X05000129.
- Zwaigenbaum L. The intriguing relationship between cerebral palsy and autism. Dev Med Child Neurol. 2014;56(1):7–8. doi:10.1111/dmcn.12274.
- Pakula AT, Van Naarden Braun K, Yeargin-Allsopp M. Cerebral Palsy: classification and epidemiology. Phys Med Rehabil Clin N Am. 2009;20(3):425–452. doi:10.1016/j.pmr.2009.06.001.
- Clarke M, Kirton A. Patterns of interaction between children with physical disabilities using augmentative and alternative communication systems and their peers. Child Lang Teach Ther. 2003;19(2):135–151. doi:10.1191/0265659003ct248oa.
- Pennington L, McConachie H. Mother-child interaction revisited: communication with non-speaking physically disabled children. Int J Lang Commun Disord. 1999;34(4):391–416. doi:10.1080/136828299247351.
