ABSTRACT
Three experiments investigated younger (18–25 yrs) and older (70–88 yrs) adults’ temporary memory for colour–shape combinations (binding). We focused upon estimating the magnitude of the binding cost for each age group across encoding time (Experiment 1; 900/1500 ms), presentation format (Experiment 2; simultaneous/sequential), and interference (Experiment 3; control/suffix) conditions. In Experiment 1, encoding time did not differentially influence binding in the two age groups. In Experiment 2, younger adults exhibited poorer binding performance with sequential relative to simultaneous presentation, and serial position analyses highlighted a particular age-related difficulty remembering the middle item of a series (for all memory conditions). Experiments 1–3 demonstrated small to medium binding effect sizes in older adults across all encoding conditions, with binding less accurate than shape memory. However, younger adults also displayed negative effects of binding (small to large) in two of the experiments. Even when older adults exhibited a greater suffix interference effect in Experiment 3, this was for all memory types, not just binding. We therefore conclude that there is no consistent evidence for a visual binding deficit in healthy older adults. This relative preservation contrasts with the specific and substantial deficits in visual feature binding found in several recent studies of Alzheimer's disease.
Older adults have reliably been shown to exhibit a deficit for associating a variety of different types of stimuli in episodic memory, including verbal and non-verbal, and static and dynamic information (Naveh-Benjamin, Citation2000; Old & Naveh-Benjamin, Citation2008a, Citation2008b). The deficit presents as a greater age-related decline in memory for the associations between features, such as names and faces, than for individual features. A general age-related decline in visual working memory performance has also been observed (e.g. Johnson, Logie, & Brockmole, Citation2010; Park et al., Citation2002). These findings have led to the hypothesis that older adults may have difficulty temporarily maintaining combinations of features such as colours, shapes, and locations (temporary binding). Such deficits could manifest as an age-related difficulty in recalling, for example, that the car was red and the van was blue, rather than vice versa, or whether the round or the oval white pill had just been taken.
Multiple studies have reported evidence for an age-related deficit in temporarily binding objects to spatial locations (Borg, Leroy, Favre, Laurent, & Thomas-Antérion, Citation2011; Brockmole & Logie, Citation2013; Cowan, Naveh-Benjamin, Kilb, & Saults, Citation2006; Fandakova, Sander, Werkle-Bergner, & Shing, Citation2014; Mitchell, Johnson, Raye, & D'Esposito, Citation2000; Mitchell, Johnson, Raye, Mather, & D'Esposito, Citation2000). There is also robust evidence for a binding deficit in Alzheimer's disease patients when only surface visual features such as colour and shape are involved (e.g. Parra, Abrahams, Fabi, et al., Citation2009; Parra, Abrahams, Logie, & Della Sala, Citation2010; Parra, Abrahams, Logie, Mendez, et al. Citation2010). On the other hand, visual binding deficits have been much more elusive in healthy older adults, so much so that, using a change detection paradigm, the evidence is weighted towards older adults being unimpaired in this ability (Brockmole, Parra, Della Sala, & Logie, Citation2008; Parra, Abrahams, Logie, & Della Sala, Citation2009; Read, Rogers, & Wilson, Citation2015; Rhodes, Parra, & Logie, Citation2016). However, Brown and Brockmole (Citation2010) found that a small age-related visual binding deficit may indeed accompany the healthy ageing process, even when using the commonly employed change detection paradigm. In Experiment 2 of that study it was demonstrated that, only in older adults, memory for bound visual objects (coloured shapes) was significantly poorer than memory for the worst performing individual feature (shape). Similarly, Isella, Molteni, Mapelli, and Ferrarese (Citation2015) appeared to observe significant age × memory condition interactions using A′ (but not proportion correct) as their outcome variable (see Allen, Hitch, Mate, & Baddeley, Citation2012), with their older adult group displaying reduced binding memory accuracy. However, they argued for the absence of a specific binding deficit in older adults, because the p-value for the interaction did not meet the .001 level. Furthermore, evidence of age-related temporary visual binding deficits has been reported using more complex paradigms (Chen & Naveh-Benjamin, Citation2012; Peich, Husain, & Bays, Citation2013). Thus, although this form of deficit is by no means robust across studies, it is observable. Given the theoretical and applied importance of this topic, and the inconsistency of findings reported in the literature pertaining to the effects of healthy ageing on temporary visual feature binding, our analyses will highlight the effect sizes and confidence intervals for the key comparisons of interest, in each experiment (Cumming, Citation2012, Citation2014). Rather than simply stating whether or not a statistically significant binding deficit exists, this approach, which has thus far been lacking in the literature, will establish the size of any effect (i.e. binding deficit) that is related to healthy ageing. Additionally, in order to demonstrate that temporary binding is not disproportionately affected by age, one must be able to obtain evidence for the null hypothesis, that there are no interactions between age group and condition. Therefore, we also report Bayes factors for crucial interactions of interest, using the default family of priors outlined by Rouder, Morey, Speckman, and Province (Citation2012).
It is also unclear what cognitive mechanisms may underlie visual feature binding deficits when they are observed (see Allen, Brown, & Niven, Citation2013, for a review). Brown and Brockmole (Citation2010) argued that attention deficits were not able to account for the observed deficit, and this is in parallel to findings in the long-term memory literature (Chen & Naveh-Benjamin, Citation2012; Cowan et al., Citation2006; Kilb & Naveh-Benjamin, Citation2007; Naveh-Benjamin, Guez, & Shulman, Citation2004). Examining the reliability of possible age-related visual feature binding deficits across a variety of contexts has important implications for models of binding in working memory and for the characterisation of healthy cognitive ageing. The present study assesses the extent to which encoding and maintenance limitations may contribute to visual feature binding deficits in young and older adults. We addressed the potential role of three specific processes, which were identified as key variables of interest in the study of temporary visual feature binding. These are encoding duration (Exp. 1: 900/1500 ms), mode of presentation at encoding (Exp. 2: simultaneous/sequential), and interference after encoding (Exp. 3: control/suffix presentation). Cowan et al. (Citation2006) suggested that older adults’ binding deficits could be due to a lack of processing robustness (following Li et al., Citation2004; see also Mitchell, Johnson, Raye, & D'Esposito, Citation2000), emphasising the requirement for detailed investigations of the encoding stage of the visual binding process in younger and older adults. Specifically, processing speed has been shown to exert a strong influence on older adults’ working memory performance (e.g. Salthouse, Citation1992, Citation1994, Citation1996) and could therefore differentially impact older adults’ encoding success and subsequent binding memory performance. In addition, both presentation format at encoding, and suffix interference after encoding, were also important to consider in the present context, given their influence on visual binding that has been identified in the existing literature, particularly with younger adults, as well as their relationship with attentional processes (Allen, Baddeley, & Hitch, Citation2006, Citation2014; Allen, Castellà, Ueno, Hitch, & Baddeley, Citation2015; Ueno, Allen, Baddeley, Hitch, & Saito, Citation2011; Ueno, Mate, Allen, Hitch, & Baddeley, Citation2011). Despite their importance in the recent binding literature, to the best of our knowledge, serial position effects in sequential presentation conditions and suffix interference effects have yet to be investigated in healthy older adults’ visual binding. By investigating all of these variables, we will gain further insights into visual feature binding in both younger and older adults, and important evidence regarding the potential visual binding deficit in healthy ageing.
Experiment 1
In one previous study that demonstrated an age-related temporary visual binding deficit, the total encoding time was longer (1500 ms; Brown & Brockmole, Citation2010, Exp. 2) than had been used in previous similar experiments that did not reveal an age-related binding deficit (900 ms, Brown & Brockmole Exp. 1; Brockmole et al., Citation2008). Indeed, it is possible to predict that encoding time could influence the presence or absence of binding deficits. Recent studies suggest that temporary visual feature binding occurs rather automatically at a low level of the working memory system (Baddeley, Allen, & Hitch, Citation2011; Logie, Brockmole, & Vandenbroucke, Citation2009) but that, by 1500 ms, a stable representation of the object has been encoded into visual short-term storage (Logie, Brockmole, & Jaswal, Citation2011; see also Peich et al., Citation2013). Thus, one could predict enhanced visual working memory for features and/or conjunctions with longer encoding durations, and such enhancement could benefit older adults to a greater extent than younger adults. Rhodes et al. (Citation2016) recently investigated the effect of a rather long encoding time (2500 ms) for comparison with the conventional 900 ms, and found that this did not differentially affect the binding performance of older adults. Nevertheless, it is important to compare directly the 900 and 1500 ms encoding conditions, given the differential findings that have been reported in the literature previously, and in light of the evidence suggesting that 1500 ms is an important threshold for developing stable bound representations, at least in younger adults (Logie et al., Citation2011).
Furthermore, it is possible to predict that the slightly longer encoding duration could be problematic for older adults. Younger adults may disproportionately benefit from the extra available time, or older adults’ performance could actually suffer. As declining frontal lobe functions may underlie cognitive ageing (Braver & West, Citation2008), one could predict that the less efficient use of task-limiting strategies could be observed in older adults when more time is available for encoding (Sander, Werkle-Bergner, & Lindenberger, Citation2011). Participants may supplement fast, relatively automatic, perceptual attention-driven binding processes (Hu, Hitch, Baddeley, Zhang, & Allen, Citation2014; Murre, Wolters, & Raffone, Citation2006) with higher level executive processes such as inhibition and strategy use (Hu et al., Citation2014; Logie et al., Citation2011; Naveh-Benjamin, Brav, & Levy, Citation2007; Sander, Lindenberger, & Werkle-Bergner, Citation2012). Task-limiting strategies could include focusing on a subset of stimuli at the expense of full, successful encoding and/or retention of the array. We hypothesised, then, that stimuli display duration may influence the presence or absence of age-related binding impairments.
Methods
Design
A 2 × 2 × 3 mixed factorial design was used to investigate the effects of age group (younger, older), encoding format (short-900 ms/long-1500 ms; within participants), and memory block type (colour, shape, binding; within participants) on memory performance. A single-probe change detection paradigm was employed, using A′ as the dependent measure. As is typical for change detection paradigms, a hit was defined as the correct detection of change. A′ ranges between 0 and 1, and .5 indicates chance level performance (Stanislaw & Todorov, Citation1999). To enhance comparability with future research, but also for ease of inclusion in future meta-analyses, Cohen's d was selected as the main standardised effect size measure (Cumming, Citation2012, Citation2014).
Participants
There were 48 participants in total. The younger sample comprised 8 males and 16 females, recruited from the University of Edinburgh community, aged 18–25 years (M = 19.92, SD = 1.91; mean years of education = 14.50, SD = 1.60). Their mean estimated full-scale IQ [Test of Premorbid Functioning – UK (ToPF); Pearson, Citation2009] was 101.17 (SD = 7.04). Across all experiments, the older adults volunteered on the basis of being healthy, living independently in the community, and agreeing to visit the University to participate. There were 13 male and 11 female older adults, aged 70–86 years (M = 76.08, SD = 4.24; mean years of education = 15.00, SD = 3.36). Their estimated IQ was 115.63 (SD = 7.44). The latter was significantly different between younger and older adults, t(46) = 6.91, p < .001, although this was in the opposite direction of any expected age effects, and likely reflects greater verbal knowledge in older adults (Park et al., Citation2002). Indeed, the number of years of formal education was not significantly different, t(33) = .66, p = .52. The Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, Citation1975) was used to determine that all older participants were cognitively healthy (M = 28.79, SD = 1.14, min = 27, max = 30). All participants reported normal or corrected-to-normal vision, and no memory problems. For this and the following experiments, the participants were unique to each experiment and had not taken part in any of the other experiments. Participants received either an honorarium or course credit.
Materials
Stimulus displays comprised three coloured shapes presented on a grey background. Each object was created from a pool of six colours (red, yellow, blue, green, cyan, purple) and six shapes (circle, triangle, diamond, heart, arrow, cross), by randomly selecting one colour and one shape, without replacement. Test arrays comprised either a single feature (a colour blob or a blank, grey shape with a black outline for the colour and shape memory blocks, respectively) or a coloured shape (for the binding memory block). Stimuli were presented on a 51 cm LCD monitor with a screen refresh rate of 60 Hz. Each stimulus measured approximately 2.2 cm2, and viewing distance was not constrained.
Procedure
All participants completed the ToPF (Pearson, Citation2009), and older participants also completed the MMSE (Folstein et al., Citation1975). The main task was then administered using a blocked, counterbalanced procedure. There were three blocks of trials, which varied by memory test type (colour, shape, binding), for each of the two encoding time conditions (short, long). The blocked procedure was adopted so as to minimise any potential influence of higher level cognitive mechanisms (e.g. confusion over task instructions, particularly across different memory conditions). Participants completed all three blocks of one encoding condition followed by the other. Encoding conditions and memory blocks were counterbalanced, with the constraint that each participant completed the different memory tasks in the same order for each encoding condition. Each trial block comprised six practice trials followed by 36 experimental trials. Trial order was randomised across participants.
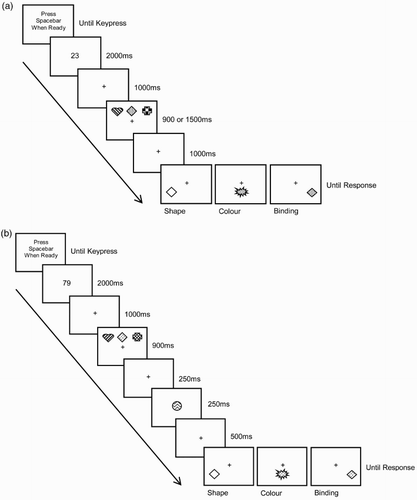
(a) illustrates the paradigm. Participants pressed the space bar to begin a trial, whereupon a randomly generated two-digit number (between 20 and 99) was displayed for 2000 ms. Immediately, participants would begin articulating this number out loud continuously and consistently, throughout the trial, until they made a key-press response. This secondary task was intended to suppress articulation and verbal recoding of the stimuli (Baddeley, Citation2007; Larsen & Baddeley, Citation2003). After presentation of the two-digit number, a central fixation cross appeared for 1000 ms which was then accompanied by three coloured shapes, presented in a row in three fixed locations (left, centre, right), for either 900 (short) or 1500 ms (long), above the cross. After a 1000 ms delay, a single test item appeared below the fixation cross in one of three fixed locations (left, centre, right; randomly selected, but appearing in each position equiprobably). Participants responded yes or no, by pressing the z or m keys on the keyboard, to indicate whether or not the item had appeared in the memory array. Following previous studies (e.g. Allen et al., Citation2006; Wheeler & Treisman, Citation2002), target trials involved presenting a colour, shape, or colour–shape combination at test. In lure trials, new colours or shapes were introduced from the pool in feature memory blocks, and new colour–shape combinations were introduced in the binding memory blocks by swapping features that had appeared in the memory array (see also Brown & Brockmole, Citation2010). Half of the trials featured targets at test, while the other half featured lures.
Figure 1. (a) Experiment 1 procedure. Participants carried out six trial blocks (i.e. each memory block type – colour, shape, binding – under each encoding time condition – 900, 1500 ms). (b) Experiment 3 procedure – suffix condition. Each participant carried out each memory type (colour, shape, binding) under each interference condition (control, suffix). The control condition was the same as the 900 ms condition of Experiment 1. In all experiments, stimuli were drawn from a pool of six different colours and shapes, and are not drawn to scale here. Fill effects represent different colours.
Results
displays the mean A′ scores from each condition. These data were analysed using a 2 (age group) × 2 (encoding time) × 3 (memory type) mixed analysis of variance (ANOVA). There were effects of age group, F(1, 46) = 27.14, MSE = .008, p < .001, = .37, encoding type, F(1, 46) = 7.42, MSE = .002, p = .009,
= .14, and memory task type, F(2, 79.7) = 44.59, MSE = .005, p < .001,
= .49. Collapsed across both age groups, planned comparisons (paired t-tests) showed that performance was superior in the colour condition (M = .94, SD = .05) relative to shape (M = .88, SD = .06), t(47) = 8.09, p < .001, but shape and binding (M = .86, SD = .06) were performed similarly,Footnote1 t(47) = 1.34, p = .19. There was an interaction between encoding condition and age group, F(1, 46) = 4.10, MSE = .002, p = .049,
= .08; collapsed across memory type, older adults benefited from the long (M = .88, SD = .04) compared with the short (M = .85, SD = .04) encoding time, t(23) = 2.92, p = .008, while younger adults performed similarly across both encoding conditions (Mshort = .92, SD = .04; Mlong = .92, SD = .04; t(23) = .6, p = .56). All other interactions were non-significant (all p > .68).
Table 1. Change sensitivity data (mean A′ scores; with standard deviations) for each condition across all three experiments.
Focusing on comparing binding memory performance with that of the worst performing individual feature condition, shape memory, we assessed the size of the effect of retaining bound relative to individual features, using the A′ scores.Footnote2 The mean of the paired differences was calculated across shape and binding memory, and a negative value indicates poorer performance in binding. We first calculated , by dividing the mean of the paired differences between shape and binding performance (Mz) by the standard deviation of the difference scores (SDz; Cohen, Citation1988). We then calculated Cohen's d using
(Cohen, Citation1988). Cohen suggested the convention that an effect size of .2 be interpreted qualitatively as a small effect. A value of .5 is indicative of a medium effect, which would be noticeable when visually inspecting the data, while .8 reflects a large effect.
In younger adults in the short encoding condition, the mean of the paired differences was −.01 (SD = .07 [95% confidence interval: −.04, .01]; d = −.30). In the long encoding condition, the mean of the paired differences was similar at −.02 (SD = .08 [−.05, .02]; d = −0.32). In the older adults, the mean of the paired differences between shape and binding memory in the short encoding condition was −.02 (SD = 0.12 [−.08, .03]; d = −0.25). In the long encoding condition this was −.004 (SD = 0.11 [−0.05, 0.04]; d = −.05). Thus, the effect size is small in both age groups under both encoding conditions, and is negligible in the long encoding condition in older adults.
The argument that there is no disproportionate effect of age on temporary visual feature binding has previously been made on the basis of failure to reject the null hypothesis. Bayes factors offer an intuitive, and increasingly popular, way to state evidence for one proposition (or model) relative to another (Edwards, Lindman, & Savage, Citation1963). In this case, models are defined by the inclusion or omission of main or interaction effects. As mentioned above, we used the default family of priors outlined by Rouder et al. (Citation2012), which place greater prior probability on effects of small magnitude (i.e. symmetrically around zero) but do not rule out larger effects. Our Bayesian ANOVA was conducted using the BayesFactor package in R (Morey & Rouder, Citation2015; R Core Team, Citation2015). The analysis compared a full model, with all main effects and interactions between experimental variables, to reduced models omitting a specific component at a time (for example, a model without an age group × memory type interaction).Footnote3 The resulting Bayes factor therefore reflects the relative evidence for retaining the effect in the full model versus the reduced model, which does not represent the effect.
Relative to a full model, with main effects of age group, memory type, and presentation time, and the interactions between these variables, the reduced model, which omits the age × memory type interaction, was favoured by a factor of approximately 13 to 1. Thus, Experiment 1 provides fairly strong evidence against a differential effect of age across the three memory types. There was also evidence against the three-way interaction, as the reduced model without this component was preferred by over 6 to 1.
Discussion
As expected, younger adults outperformed the older adults overall, indicating an age-related deficit in visual working memory (Brown & Brockmole, Citation2010; Johnson et al., Citation2010; Park et al., Citation2002). Importantly, however, there was little evidence of a specific deficit in the temporary retention of bound visual objects, relative to memory for the most difficult individual feature condition (shape). The standardised effect sizes also support this conclusion. While there was evidence of a small binding-related effect size in the short encoding condition, the younger adults also exhibited this across both encoding conditions; therefore, there are no grounds to argue for a specific age-related binding deficit within this particular experiment. We were also able to gauge the strength of evidence for interactions between age and memory condition, using Rouder et al.’s (Citation2012) default Bayes factors. This analysis showed that models without interactions between age group and memory type were more likely, relative to the saturated, full model, which included them. When considered in relation to the wider working memory literature that is weighted against an age-related deficit in temporary surface visual feature binding using similar paradigms (Brockmole et al., Citation2008; Brown & Brockmole, Citation2010, Exp. 1; Parra, Abrahams, Fabi, et al., Citation2009; Rhodes et al., Citation2016), these results are in keeping with the suggestion that the performance of healthy older adults in binding conditions is primarily driven by their ability to detect changes in individual features, and does not reflect an impairment specifically of temporary binding of colour–shape combinations.
These results also provide evidence regarding the potential role of encoding time in older adults’ temporary visual feature binding performance (Brown & Brockmole, Citation2010). The results clearly showed that encoding time cannot account for the differential findings in the previous literature on visual feature binding. Although older adults benefitted more than younger adults from a longer encoding time overall (e.g. Salthouse, Citation1996), the presence or absence of age-related visual binding deficits in working memory appears to be unrelated to the encoding duration, at least within the present experimental conditions (see also Rhodes et al., Citation2016).
Experiment 2
Sequential presentation appears to disrupt temporary visual feature binding in both younger and older adults (Allen et al., Citation2006; Brown & Brockmole, Citation2010; see also Gorgoraptis, Catalao, Bays, & Husain, Citation2011; Jaswal & Logie, Citation2011). As sequential presentation requires creating new bindings while maintaining previously encountered ones, this disruption may result from a fragility of bound material in visual working memory (Baddeley et al., Citation2011; Logie et al., Citation2009; Ueno, Allen, et al., Citation2011). That is, relative to individual features in visual working memory, bound objects might be more readily overwritten by new information. We would therefore expect to replicate a general decrement in binding performance, when stimuli are presented sequentially. It is possible that older adults may be more vulnerable to this manipulation. Allen et al. (Citation2014) have shown that visual working memory for sequentially presented objects involves two attentional components, which may be thought of as internal, or top-down, attentional control, and externally driven, bottom-up attention (Chun, Golomb, & Turk-Browne Citation2011). If older adults exhibit limited executive attentional resources (Braver & West, Citation2008; Buckner, Citation2004), then sequential presentation could cause difficulty. That said, Brown and Brockmole (Citation2010; see also Read et al., Citation2015) provided evidence that older adults’ visual binding was not differentially disrupted by sequential presentation, suggesting retention of the capacity to encode individual features and bindings serially.
A new avenue of exploration, however, was specifically to examine older adults’ performance across the different serial positions during sequential presentation. Allen et al. (Citation2006, Citation2014) showed that, in younger adults, memory for bound representations exhibited a particular deficit relative to individual feature memory in the earlier positions in the sequence while, for the final position in the sequence, bindings were retained as well as individual features. We therefore predicted that the poorest performance would be observed at an earlier serial position, which depends upon internally guided executive control. We could further predict the same pattern of serial position deficit in older adults, if they create and maintain serially presented bindings in the same way as younger adults. It is possible, however, to predict a different serial position curve in older adults. In a dual-task verbal working memory study, Foos (Citation1989) reported similar serial position curves to Allen et al. (Citation2006), in that the final of three items was best remembered. In older adults, however, a clear deficit existed for the middle item, whereas there was no effect of age for items 1 and 3. We could therefore predict a particularly clear age-related deficit at the middle serial position in our task, which could potentially be more pronounced for bindings.
Methods
Design
A 2 × 2 × 3 mixed factorial design was used to investigate the effects of age group (younger, older), encoding format (simultaneous, sequential; within participants), and memory block type (colour, shape, binding; within participants) on A′, the main dependent measure of memory performance.
Participants
There were 48 participants in total. The younger sample comprised 8 males and 16 females (18–25 years, M = 22.13, SD = 1.75; mean years of education = 16.13, SD = 3.35; ToPF-estimated IQ = 110.00, SD = 5.88). The older sample comprised 8 males and 16 females (70–83 years, M = 77.63, SD = 4.15; mean years of education = 16.08, SD = 3.01; ToPF-estimated IQ = 119.38, SD = 5.67). Estimated IQ was significantly higher in the older than the younger group, t(46) = 5.62, p < .001, but number of years of formal education was not significantly different, t(46) = .05, p = .96. All older participants were cognitively healthy, as determined using the MMSE (Folstein et al., Citation1975; M = 28.33, SD = .92; min = 27, max = 30). All participants reported normal or corrected-to-normal vision, and no memory problems. All participants received either an honorarium or course credit.
Materials and procedure
The materials and procedure were the same as described for the 1500 ms condition of Experiment 1 (see (a)) except that, in the sequential condition, each object was presented individually, in turn, across the three positions at the top of the screen, for 500 ms each. The encoding conditions and memory task blocks were counterbalanced as per Experiment 1.
Results
displays the mean A′ scores for each condition. The data were analysed using a 2 (age group) × 2 (encoding format) × 3 (memory type) mixed ANOVA. There were effects of age group, F(1, 46) = 53.59, MSE = .011, p < .001, = .54, and memory type, F(2, 79.9) = 64.27, MSE = .005, p < .001,
= .58, but no significant effect of encoding format, F(1, 46) = 3.09, MSE = .004, p = .086,
= .06. Collapsed across age group, planned comparisons showed that, as in Experiment 1, performance was most accurate in the colour condition (M = .95, SD = .05) relative to shape (M = .88, SD = .08), t(47) = 8.63, p < .001. Performance was also better in the shape condition than in binding (M = .84, SD = .09), t(47) = 3.34, p = .002. However, there was a significant interaction between memory type and age group,Footnote4 F(2, 92) = 4.12, MSE = .004, p = .019,
= .08. Planned comparisons, collapsed across encoding conditions, showed that, in younger adults, memory for colours (M = .98, SD = .02) was better than shapes (M = .93, SD = .04), t(23) = 6.74, p < .001, and memory for shapes was better than bindings (M = .90, SD = .06), t(23) = 3.34, p = .003. In older adults, whereas memory for colours (M = .91, SD = .04) was better than shapes (M = .83, SD = .07), t(23) = 6.74, p < .001, the difference between shapes and bindings (M = .79, SD = .08) was not significant, t(23) = 1.93, p = .066. The remaining two-way interactions were non-significant (all p > .24), but the three-way interaction was significant, F(2, 92) = 4.24, MSE = .003, p = .017,
= .08.
To further investigate the three-way interaction, separate 2 (encoding format) × 3 (memory type) ANOVAs were carried out within each age group. In the younger adults, there were effects of encoding format, F(1, 23) = 7.67, MSE = .002, p = .011, = .25, and memory type, F(2, 46) = 39.55, MSE = .002, p < .001,
= .63, as well as the two-way interaction, F(2, 32.8) = 6.82, MSE = .003, p = .007,
= .23. As expected, while there was no difference between shape and binding memory performance under simultaneous encoding format conditions, t(23) = .34, p = .74, binding memory performance was poorer than shape memory when stimuli were sequentially encoded, t(23) = 3.97, p = .001. In contrast, in older adults, only the effect of memory type was significant, F(2, 46) = 32.59, MSE = .006, p < .001,
= .59 (all other p > .39), as discussed above.Footnote5
To further qualify the pattern of findings, in the younger adults, the mean of the paired differences between shape and binding memory accuracy in the simultaneous encoding condition was −.01 (SD = .06) with a 95% CI of [−.03, .02] (d = −.11). Within the sequential encoding condition, this was −.06 (SD = .08 [−.09, −.03]; d = –1.12). In the older adults, the mean of the paired differences between shape and binding memory in the simultaneous encoding condition was −.06 (SD = .14 [−.12, .004]; d = −.56), whereas in the sequential encoding condition this was −.02 (SD = .11 [−.06, .03]; d = −.20). Thus, the effect of feature binding increases from very small in the simultaneous condition to large in the sequential condition in younger adults, whereas the effect size is small to medium across both conditions in older adults.
The Bayesian ANOVA on the data from Experiment 2 revealed evidence for the age group × memory type interaction. The full model was marginally more likely, given the data, than a model omitting this interaction (B = 0.526, approximately 2 to 1 support for the interaction). However, removing the colour condition from the analysis—and contrasting shape and binding only—leads to favouring the reduced model, and hence the absence of the age × memory type interaction, by a factor of over 4 to 1.
For the three-way interaction, the full model is preferred over the reduced model whether the colour condition is included in the analysis (B = 0.401, approximately 2.5 to 1 support for the interaction) or not (B = 0.344, approximately 3 to 1 support for the interaction). However, in this case, it is clear that the slight evidence for the three-way interaction is borne out of younger adults’ larger binding cost in the sequential condition (see above).
Serial position analyses
In order to assess the impact of serial position on performance, we analysed the accuracy (proportion correct) data that resulted from the target trials in the sequential presentation condition (Allen et al., Citation2006, Citation2014). A 2 (age group) × 3 (memory type) × 3 (serial position; 1, 2, 3) mixed ANOVA revealed effects of age group, F(1, 46) = 31.77, MSE = .107, p < .001, = .41, memory type, F(2, 92) = 9.19, MSE = .043, p < .001,
= .17, and serial position, F(2, 92) = 11.37, MSE = .062, p < .001,
= .20. There were also interactions between age group and position (see (a)), F(2, 92) = 6.51, MSE = .062, p = .002,
= .13, and between memory type and position (see (b)), F(4, 184) = 3.59, MSE = .031, p = .008,
= .07. All other effects, including the three-way interaction, were non-significant (all p > .29).
Figure 2. Proportion correct data (±SE) from Experiment 2 sequentially presented target trials. (a) Interaction between age group and serial position. (b) Interaction between memory test type and serial position.
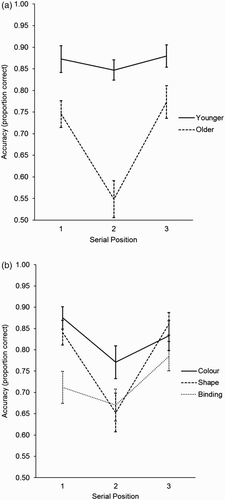
To follow up on the age group × position interaction, planned comparisons investigated the serial position effect (collapsed across memory blocks) within each age group. Within the younger adults, accuracy was not significantly different between serial positions 1 and 2, t(23) = .70, p = .49, and between positions 2 and 3, t(23) = .99, p = .33. In the older adults, however, accuracy was much poorer in serial position 2 as compared with both positions 1, t(23) = 4.73, p < .001, and 3, t(23) = 4.68, p < .001. Thus, while younger adults were unaffected by the serial position of the target object, older adults performed significantly worse, and at chance level, for the middle stimulus in the sequence, across all memory conditions.
Planned comparisons were also used to follow up the effects of memory test type within each serial position, collapsed across age groups. Within position 1, colour and shape were not significantly different, t(47) = 1.35, p = .18, whereas shape was performed much better than binding, t(47) = 3.13, p = .003. Within position 2, colour was performed better than shape, t(47) = 2.93, p = .005, but shape and binding were performed similarly, t(47) = .39, p = .70. Within position 3, colour and shape were not significantly different, t(47) = .77, p = .44, whereas shape was marginally significantly better than binding, t(47) = 2.01, p = .051. As predicted, a binding deficit is greatest and most reliable only at the earliest position in the sequence ((b)), and this was the case for both age groups.
Discussion
The results are consistent with previous literature showing that, in younger adults, temporary visual binding is poorer when stimulus presentation is sequential rather than simultaneous (Allen et al., Citation2006; Brown & Brockmole, Citation2010). The effect of feature binding was large under the sequential condition, but only very small in the simultaneous condition. Results also showed that, for both age groups, the earliest bound item in the sequence is especially vulnerable (Allen et al., Citation2006), reflecting the fragility of bindings in memory, relative to individual features (Allen et al., Citation2006; Baddeley et al., Citation2011).
There was little evidence for an age-related binding deficit in the present experiment, and this was underlined by the effect sizes observed across both encoding conditions, which again were only small to medium in older adults. This result is therefore consistent with the present Experiment 1, and with previous findings in the literature (Brockmole et al., Citation2008; Brockmole & Logie, Citation2013; Parra, Abrahams, Fabi, et al., Citation2009). The present findings also support Brown and Brockmole's (Citation2010) conclusions that any binding deficits in older adults do not result from, nor are exacerbated by, sequential presentation of the memory array (see also Chen & Naveh-Benjamin, Citation2012; Read et al., Citation2015). As the observed small effect of feature binding was not dependent on encoding format (and therefore present in the simultaneous condition), age and presentation format may also impact upon shape memory. However, considering that binding deficits are characterised by deficits over and above those in individual feature memory, in this case shape, then one must conclude that there is only a small to medium effect of feature binding on older adults’ working memory and that sequential presentation format does not drive its presence.
A particularly interesting finding was the marked serial position curve in older adults, who exhibited specific difficulty remembering the middle stimulus in the sequences, across all memory types. In contrast, the younger adults produced a much flatter curve, possibly as the sequence length was within their working memory capacity. Older adults may have been more likely to lose the middle items due to increased susceptibility to interference (Braver & West, Citation2008; Lustig, Hasher, & Zacks, Citation2007), given that the middle objects were unique in being subjected to interference from both a previously encoded item (position 1) and the requirement to create a new representation (position 3). For Experiment 3 we go on to directly assess the role of interference in visual feature binding memory. However, it is important to note that the age-related limitation for serial position 2 could reflect a different process than the binding interference effect observed at position 1 for both age groups. It is more generalised (i.e. across all memory types) and also apparently exerts a much stronger effect, bringing performance near to chance. In older adults with reduced capacity, the middle item may have been differentially lost over time due to its lack of rehearsal (relative to position 1) and recency (relative to position 3; Foos, Citation1989; Foos & Wright, Citation1992). To elaborate, due to a capacity limit in internally guided executive attention, or processing speed deficits (Salthouse, Citation1996; Vaughan & Hartman, Citation2010), older adults may have strategically focused internal attention on the first item (see Hu et al., Citation2014), while item 3 was successfully served by external attention (Allen et al., Citation2014; Hu et al., Citation2014). Although this would have allowed relatively successful performance for two of the three items, item 2 would be particularly vulnerable. We suggest that this would be a useful focus for future work investigating visual working memory limitations with ageing.
Experiment 3
To our knowledge, the impact of a to-be-ignored suffix on memory for features and bindings has not yet been investigated within the ageing context. In younger adults, Ueno, Allen, et al. (Citation2011) and Ueno, Mate, et al. (Citation2011) (see also Allen et al., Citation2015) showed that binding maintenance is disrupted or overwritten by subsequent presentation of a to-be-ignored suffix. This perceptually driven interference particularly impacts on recently encountered items and/or those that participants are attempting to prioritise in memory (Hu et al., Citation2014). It has been claimed that older adults’ visual working memory may suffer interference from irrelevant information at encoding (Braver & West, Citation2008; Lustig et al., Citation2007; Pilotti, Beyer, & Yasunami, Citation2002). Therefore, if an underlying cause of age-related binding deficits is the susceptibility to interference, then a suffix should result in or exacerbate such a deficit. Specifically, we could predict a greater binding deficit, in terms of poorer performance in binding memory relative to shape memory, in older adults when carrying out the suffix interference condition.
Methods
Design
A 2 × 2 × 3 mixed factorial design was used to investigate the effects of age group (younger, older), suffix condition (control, suffix; within participants), and memory test type (colour, shape, binding; within participants) on change detection performance (A′).
Participants
There were 48 participants. Younger participants comprised 8 males and 16 females, (18–25 years, M = 20.50, SD = 1.53; mean years of education = 16.04, SD = 1.43; ToPF-estimated IQ = 107.25, SD = 6.32). Older participants comprised 6 males and 18 females (70–88 years, M = 75.46, SD = 4.86; mean years of education = 15.21, SD = 4.08; ToPF-estimated IQ = 116.42, SD = 7.57). As in the first two experiments, estimated IQ was significantly higher in the older than in the younger group, t(46) = 4.56, p < .001, but there was no significant difference in years of education, t(28.6) = .95, p = .35. Older adults’ mean score on the MMSE (Folstein et al., Citation1975) was 29.08 (SD = 1.14; min = 26, max = 30). All participants received either an honorarium or course credit.
Materials and procedure
The stimuli and procedure were as reported for Experiment 1, except that the six trial blocks varied by suffix condition (control, interference) as well as memory test type (colour, shape, binding). Conditions were counterbalanced as per Experiment 1.
The control condition was administered as per the 900 ms condition in Experiment 1. In the suffix condition, once the memory array had disappeared, and after a further 250 ms, a new coloured shape was presented in place of the fixation cross (see (b)). This suffix was created by combining a colour and a shape from the excess items of the feature pool; specifically, the object was plausible as an object related to the memory set (Ueno, Allen, et al., Citation2011; Ueno, Mate, et al., Citation2011), but neither of the features would have appeared within the memory array for that trial. Participants were informed that the suffix was to be viewed but ignored. The fixation cross replaced the suffix for the remaining 500 ms of the delay period, before presentation of the test item.
Results
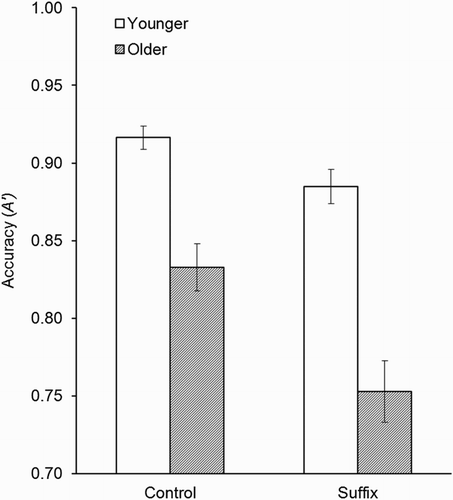
presents the A′ data, which were analysed using a 2 (age group; young, older) × 2 (suffix condition; control, suffix) × 3 (memory type; colour, shape, binding) mixed ANOVA. There were main effects of age, F(1, 46) = 37.43, MSE = .022, p < .001, = .45, in which the younger adults (M = .90, SD = .04) outperformed the older adults (M = .79, SD = .08), and of suffix, F(1, 46) = 35.79, MSE = .006, p < .001,
= .44, in which the suffix reduced performance overall (Mcontrol = .88, SD = .07; Msuffix = .82, SD = .10). There was a main effect of memory type, F(1.7, 77.0) = 71.64, MSE = .009, p < .001,
= .61. Collapsed across age group and suffix condition, planned comparisons revealed that the colour condition was performed better than shape, t(47) = 12.78, p < .001, but there was no difference between shape and binding memory performance, t(47) = 1.20, p = .24. The interactions between suffix and age group, F(1, 46) = 6.74, MSE = .006, p = .013,
= .13, memory type and age group,Footnote6 F(2, 92) = 11.70, MSE = .007, p < .001,
= .20, and suffix and memory type, F(1.7, 78.7) = 4.29, MSE = .005, p = .022,
= .09, were all significant, while the three-way interaction was notFootnote7 (p = .81).
Planned comparisons were used to follow up on the suffix × age group interaction. There were significant suffix effects in both younger (Mcontrol = .92, SD = .04; Msuffix = .88, SD = .05), t(23) = 3.32, p < .01, and older adults (Mcontrol = .83, SD = .07; Msuffix = .75, SD = .10), t(23) = 4.99, p < .001; however, this effect was stronger in older adults (see ).
Figure 3. Interaction between age group and suffix condition in the Experiment 3 A′ data (±SE). (Data are collapsed across memory test type.)
The suffix × memory type interaction was driven by the smaller effect of the suffix within the colour memory task, most likely an artefact of a ceiling effect in this condition. Indeed, there is no suffix × memory type interaction when only shape and binding blocks are included in the analysis, F(1, 46) = .15, MSE = .006, p = .70, = .003.
Following up the age group × memory type interaction, as expected, colour memory was better than shape in both younger (Mcolour = .97, SD = .03; Mshape = .85, SD = .06), t(23) = 9.05, p < .001, and older adults (Mcolour = .90, SD = .05; Mshape = .77, SD = .09), t(23) = 8.95, p < .001. In younger adults, binding (Mbinding = .88, SD = .06) was performed slightly better than shape memory, t(23) = 2.23, p = .036. However, close inspection of the data in reveals that, rather than binding memory performance being better in younger adults in Experiment 3, shape memory performance is slightly worse than in the previous two experiments. In older adults, binding (Mbinding = .71, SD = .14) was significantly poorer than shape memory, t(23) = 2.74, p = .012. This interaction still existed when only shape and binding memory blocks were included in the analysis, F(1, 46) = 12.04, MSE = .009, p = .001, = .21.
In the younger adults, the mean of the paired differences between shape and binding memory accuracy in the control condition was .03 (SD = .07) with a 95% CI of [.002, .06] (d = .63). Within the suffix interference condition, this was .03 (SD = .09 [−.01, .07]; d = .43). In the older adults, the mean of the paired differences between shape and binding memory in the control condition was −.06 (SD = .12 [−.11, −.008]; d = −.69), whereas in the suffix condition this was −.08 (SD = .18 [−.15, .001]; d = −.59). Thus, there was a medium positive effect of binding in younger adults, whereas there was a medium negative effect of feature binding in older adults, regardless of the interference condition.
For Experiment 3, the presence of the two-way interaction between age group and memory type was strongly favoured over its omission (by over 14,000 to 1). Although restricting the analysis to the shape and binding conditions greatly reduced the strength of evidence for this interaction, it was still favoured by a factor of over 100 to 1. There was no suggestion that the visual suffix modulated this, as a model omitting the three-way interaction was favoured by over 7 to 1. This was also true when restricting analysis to the shape and binding memory conditions only (B = 3.436).
Discussion
The findings from Experiment 3 showed an age-related deficit for temporary visual feature binding. This goes against much of the pre-existing literature on temporary visual feature binding in older adults, which has shown this ability to be impervious to healthy ageing (Brockmole et al., Citation2008; Brown & Brockmole, Citation2010, Exp. 1; Parra, Abrahams, Logie, et al., Citation2009). It does, however, support the limited evidence that previously existed using the change detection paradigm (Brown & Brockmole, Citation2010, Exp. 2, see also Isella et al., Citation2015), as well as evidence using alternative paradigms (Chen & Naveh-Benjamin, Citation2012; Peich et al., Citation2013).
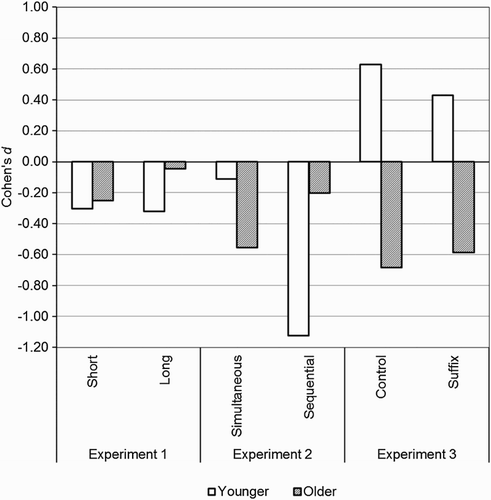
Inspection of the younger adult data presented in highlights that the one clear difference between the findings of Experiment 3 and those from the previous two experiments is that, in Experiment 3, the younger adults showed poorer performance for shape compared with binding memory while, in Experiments 1 and 2, binding performance was poorer than shape. However, in younger adults, binding performance was relatively stable across the three experiments, whereas shape performance decreased slightly in Experiment 3. Thus, the effect in younger adults may be driven by poorer shape memory performance rather than better binding memory performance. Importantly, though, in older adults the observed effect sizes in Experiment 3 are consistent with the present Experiments 1 and 2, as these also demonstrated small to medium effects of feature binding in older adults (see ).
Figure 4. Effect sizes (Cohen's d) for feature binding memory compared with shape memory, for the two age groups in each experiment.
The experiment also produced interesting findings regarding interference effects in visual working memory. We did not replicate a specific suffix effect on binding memory in younger adults (Ueno, Allen, et al., Citation2011; Ueno, Mate, et al., Citation2011). A smaller array size was used due to the focus upon older adults in the present study, suggesting that memory array size may be important for this effect. Future work could explore the factors determining the magnitude of suffix interference effects and the role of capacity on interference effects in binding. Importantly, there were interference effects across all forms of memory in both age groups, but older adults exhibited a greater general suffix effect (Braver & West, Citation2008; Lustig et al., Citation2007; Pilotti et al., Citation2002). Older adults are therefore more susceptible to interference in visual working memory, even when the object is to be ignored (Pilotti et al., Citation2002). Finally, although older adults exhibited greater interference effects than younger adults, their binding performance was not dependent upon interference, as the effect clearly existed across both control and interference conditions.
General discussion
The three experiments reported here were focused upon understanding the role of encoding and maintenance processes in temporary memory for visual features and feature bindings. To summarise the main results, all three experiments provide evidence of a small to medium effect on feature binding performance, relative to individual feature memory, in older adults. However, small to large effects were also observed in the younger adults in Experiments 1 and 2, with a large effect in this group when faced with sequential presentation of the memory array. Indeed, our Bayesian analysis of the data from Experiments 1 and 2 supported the absence of an age group × memory condition interaction when contrasting binding and shape performance, thus going beyond previous failures to reject the null hypothesis (although see Rhodes et al., Citation2016). For Experiment 3, the weight of evidence was in favour of a disproportionate binding cost for older adults. However, effect sizes showed that the binding cost for older adults was no larger in Experiment 3 than was observed in any of the previous two experiments. It is therefore questionable the extent to which we may describe such a pattern of performance as reflecting an age-related binding deficit. Our experiments also highlighted age-related vulnerabilities in visual working memory more generally; namely, older adults differentially benefited from more encoding time, they exhibited a specific, marked impairment in memory for both single features and for colour–shape bindings presented in the middle of three positions in sequential presentation, and they experienced a greater suffix interference effect.
There has been some debate regarding whether or not healthy older adults show impaired temporary binding of surface visual features and the present findings contribute new evidence in this regard. Particularly given the inconsistencies in this literature (i.e. presence/absence of age-related binding deficits), it seems prudent and timely to use an effect size approach to answering this question (Cumming, Citation2012, Citation2014). Thus, rather than asking whether or not such a deficit exists, we may reframe this question to ask whether or not we have consistent evidence for a larger effect size of feature binding relative to individual feature memory in older adults as compared with younger adults. Effect size data may also usefully contribute to future meta-analyses as well as being readily comparable across similar studies. There have been recent observations of age-related binding deficits in visual working memory (Brown & Brockmole, Citation2010, Exp. 2; Chen & Naveh-Benjamin, Citation2012; Peich et al., Citation2013; see also Isealla et al., Citation2015). However, these findings contrast with other previously published experiments using change detection paradigms (Brockmole et al., Citation2008; Brown & Brockmole, Citation2010, Exp. 1; Parra, Abrahams, Fabi, et al., Citation2009; Read et al., Citation2015; Rhodes et al., Citation2016). Taken together, we would argue that the present evidence clearly shows that older adults do consistently demonstrate a small to medium visual feature binding effect size, but that younger adults tend to show this too (present Experiments 1 and 2). We therefore only have minimal evidence for an age-related binding deficit, at least using a change detection paradigm. Certainly, where younger and older adults do not show parallel effects (e.g. present Exp. 3, Brown & Brockmole, Exp. 2), the effect in older adults appears only to be small to medium, in terms of paired differences between binding and shape memory performance. Finally, the cross-experiment analyses reported in Appendix show the consistency that exists across the samples used in the present three experiments, and support the argument that age-related binding deficits are not reliable in healthy older adults.
Researchers have argued that temporary visual feature binding is a relatively automatic process, carried out at a low level of the working memory system (Baddeley et al., Citation2011; Logie, Citation2011; Logie et al., Citation2009). Thus, this process may be relatively resistant to decline in healthy ageing. One conclusion, when taking all of the evidence into consideration, is that binding in visual working memory is less susceptible to ageing than is object-location binding in working memory (Brockmole & Logie, Citation2013; Cowan et al., Citation2006; Mitchell, Johnson, Raye, Mather, et al., Citation2000), and associative long-term memory (e.g. Naveh-Benjamin, Citation2000; Old & Naveh-Benjamin, Citation2008a). Furthermore, temporary visual feature binding is markedly impaired in individuals suffering from Alzheimer's disease (AD; Parra, Abrahams, Fabi, et al., Citation2009; Parra, Abrahams, Logie, & Della Sala, Citation2010; Parra, Abrahams, Logie, Mendez, et al., Citation2010). This suggests that the underlying pathology of this disease compromises the processes involved in an ability that is relatively unaffected by healthy ageing, and offers the possibility of a specific cognitive marker for the disease (e.g. Della Sala, Parra, Fabi, Luzzi, & Abrahams, Citation2012; Parra, Abrahams, Fabi, et al., Citation2009; Parra, Della Sala, Logie, & Morcom, Citation2014; for reviews see Allen et al., Citation2013; Logie, Horne, & Petit, Citation2015; Logie, Parra, & Della Sala, Citation2015). We would therefore argue that temporary visual feature binding is relatively well preserved in healthy compared with pathological ageing, and in comparison with other forms of binding, such as long-term associative binding in healthy older adults (Old & Naveh-Benjamin, Citation2008a).
Yet, age-related deficits, although relatively small, are observable. Indeed, Peich et al. (Citation2013) recently observed binding deficits with healthy ageing, in the form of mis-binding errors. Taking all of the available literature into account, they concluded that there is a quantitative difference between the extent of the visual feature binding deficit observed in healthy older adults and those affected by AD; our findings fully support such a conclusion and contribute standardised effect size data. As the deficit is clearly quantitatively smaller in healthy ageing than has been shown in previous studies of temporary surface visual feature binding in AD, whether or not older adults’ visual feature binding performance may be differentiated from that of younger adults likely depends on the methodological approach being used (Chen & Naveh-Benjamin, Citation2012; Peich et al., Citation2013), and detection of an age-related binding impairment may require very large sample sizes (Brockmole & Logie, Citation2013). Further research into visual working memory as well as visual feature binding more specifically, focusing on the encoding deficits presently observed (related to encoding duration, serial position, and suffix presentation) should be useful in helping to clarify the cognitive mechanisms that underlie abilities and limitations in healthy cognitive ageing, but also in AD.
In the context that the presence of a temporary visual feature binding deficit has been suggested as a marker of AD, our findings clearly show that, using the change detection paradigm, only small to medium effect sizes are observable in healthy older adults’ performance. Furthermore, this is consistent across a series of experiments, and is often comparable to the direction and sizes of effects observed in younger adults. Cumming (Citation2012, Citation2014) highlighted that we should move away from our reliance upon the dichotomous (accept/reject) approach afforded by null hypothesis significance testing, as this is often untrustworthy when used across different experiments and samples of the population. Rather, a preferable approach is to use simpler experimental paradigms that allow for the estimation of effect sizes over numerous studies. In the case of the debate regarding age-related visual feature binding deficits, we have shown that effect sizes are useful in establishing the ability of older relative to younger adults. It is recommended that future research should aim to establish the effect sizes associated with visual feature binding in pathological ageing, and with other forms of binding in the healthy ageing context.
Data source
The underlying research materials for this article can be accessed via the UK Data Service, doi:10.5255/UKDA-SN-850670.
Supplementary_Material.pdf
Download PDF (429.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Louise A. Brown http://orcid.org/0000-0003-3520-6175
Richard J. Allen http://orcid.org/0000-0002-1887-3016
Additional information
Funding
Notes
1. There is some discrepancy in the associative memory literature regarding the data that are compared in order to test for a memory deficit. In the long-term memory literature, associative memory scores are typically compared with the averaged performance of the two individual feature conditions. We have focused upon comparing shape, the worst performed individual feature condition, with binding performance primarily because (1) an averaged score is an artificial amalgamation of performance across two different single feature types and (2) while helping to overcome floor effects in the binding condition, the best performed individual feature condition – in this case, colour – can result in ceiling effects. Furthermore, this approach has been commonly used in the working memory literature (e.g. Allen et al., Citation2006; Brockmole et al., Citation2008; Ueno, Allen, et al., Citation2011). However, for comparison with the wider literature, the analysis with averaged individual feature performance was also carried out, and an age-related binding deficit was not significant (p = .96).
2. We also analysed Cohen's d using the proportion correct measure. We observed similar effect sizes as with A′, and the same general pattern as depicted in .
3. We used the anovaBF function with the whichModels argument set to top and the number of Monte Carlo samples set to 50,000. An additional 10,000 iterations were run until the proportional error associated with each Bayes factor was less than 5%. The full results from the Bayesian analyses from each experiment are available in the Supplementary file.
4. This interaction is not significant when comparing binding with the averaged colour-shape memory blocks (p = .20).
5. The 2 (age group) × 2 (encoding format) × 2 (memory type; shape, binding) ANOVA on the A′ data revealed qualitatively the same findings regarding binding as did the analysis that included the colour memory condition, in that the three-way interaction remained significant, F(1, 46) = 5.17, MSE = .005, p = .028, = .10.
6. The analysis involving the averaged colour and shape blocks for comparison with binding also revealed this significant interaction, F(1, 46) = 8.77, MSE = .004, p = .005, = .16.
7. The age group × memory type interaction remains when only the data from the control condition were analysed, F(2, 92) = 9.10, MSE = .004, p < .001, = .17, and with a range of other measures that were also analysed, including proportion correct, F(2, 92) = 5.00, MSE = 74.36, p = .009,
= .10, and corrected recognition (hits–false alarms), F(2, 92) = 6.68, MSE = .029, p = .002,
= .13. Furthermore, the two-way interaction remained in an analysis of covariance on the A′ scores, which adjusted for the effects of sex, years of education, and Test of Premorbid Functioning (IQ estimate) score, F(1, 43) = 10.94, MSE = .009, p = .002,
= .20. Finally, the three-way interaction is still not significant when only the shape and binding memory blocks are included in the analysis (p = .81).
References
- Allen, R. J., Baddeley, A. D., & Hitch, G. J. (2006). Is the binding of visual features in working memory resource-demanding? Journal of Experimental Psychology: General, 135, 298–313. doi:10.1037/0096-3445.135.2.298
- Allen, R. J., Baddeley, A. D., & Hitch, G. J. (2014). Evidence for two attentional components in visual working memory. Journal of Experimental Psychology: Learning, Memory, & Cognition, 40, 1499–1509. doi:10.1037/xlm0000002
- Allen, R. J., Brown, L. A., & Niven, E. (2013). Aging and visual feature binding in working memory. In H. St Clair-Thompson (Ed.), Working memory: Developmental differences, component processes, and improvement mechanisms (pp. 83–96). Hauppauge, NY: Nova Science.
- Allen, R. J., Castellà, J., Ueno, T., Hitch, G. J., & Baddeley, A. D. (2015). What does visual suffix interference tell us about spatial location in working memory? Memory & Cognition, 43, 133–142. doi:10.3758/s13421-014-0448-4
- Allen, R. J., Hitch, G. J., Mate, J., & Baddeley, A. D. (2012). Feature binding and attention in working memory: A resolution of previous contradictory findings. The Quarterly Journal of Experimental Psychology, 65, 2369–2383. doi:10.1080/17470218.2012.687384
- Baddeley, A. D. (2007). Working memory, thought and action. Oxford: Oxford University Press.
- Baddeley, A. D., Allen, R. J., & Hitch, G. H. (2011). Binding in visual working memory: The role of the episodic buffer. Neuropsychologia, 49, 1393–1400. doi:10.1016/j.neuropsychologia.2010.12.042
- Borg, C., Leroy, N., Favre, E., Laurent, B., & Thomas-Antérion, C. (2011). How emotional pictures influence visuospatial binding in short-term memory in ageing and Alzheimer's disease? Brain and Cognition, 76, 20–25. doi:10.1016/j.bandc.2011.03.008
- Braver, T. S., & West, R. L. (2008). Working memory, executive control, and aging. In F. I. M. Craik & T. A. Salthouse (Eds.), The handbook of aging and cognition (3rd ed., pp. 311–372). New York, NY: Psychology Press.
- Brockmole, J. R., & Logie, R. H. (2013). Age-related change in visual working memory: A study of 55,753 participants aged 8 to 75. Frontiers in Perception Science, 4(12). doi:10.3389/fpsyg.2013.00012
- Brockmole, J. R., Parra, M. A., Della Sala, S., & Logie, R. H. (2008). Do binding deficits account for age-related decline in visual working memory? Psychonomic Bulletin & Review, 15, 543–547. doi:10.3758/PBR.15.3.543
- Brown, L. A., & Brockmole, J. R. (2010). The role of attention in binding visual features in working memory: Evidence from cognitive ageing. The Quarterly Journal of Experimental Psychology, 63, 2067–2079. doi:10.1080/17470211003721675
- Buckner, R. L. (2004). Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron, 44, 195–208. doi:10.1016/j.neuron.2004.09.006
- Chen, T., & Naveh-Benjamin, M. (2012). Assessing the associative deficit of older adults in long-term and short-term/working memory. Psychology and Aging, 27, 666–682. doi:10.1037/a0026943
- Chun, M. M., Golomb, J. D., & Turk-Browne, N. B. (2011). A taxonomy of external and internal attention. Annual Review of Psychology, 62, 73–101. doi:10.1146/annurev.psych.093008.100427
- Cohen, J. (1988). Statistical power analysis for the behavioural sciences (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum Associates.
- Cowan, N., Naveh-Benjamin, M., Kilb, A., & Saults, J. S. (2006). Life-span development of visual working memory: When is feature-binding difficult? Developmental Psychology, 42, 1089–1102. doi:10.1037/0012-1649.42.6.1089
- Cumming, G. (2012). Understanding the new statistics: Effect sizes, confidence intervals, and meta-analysis. New York, NY: Routledge.
- Cumming, G. (2014). The new statistics: Why and how. Psychological Science, 25, 7–29. doi:10.1177/0956797613504966
- Della Sala, S., Parra, M. A., Fabi, K., Luzzi, S., & Abrahams, S. (2012). Short-term memory binding is impaired in AD but not in non-AD dementias. Neuropsychologia, 50, 833–840. doi:10.1016/j.neuropsychologia.2012.01.018
- Edwards, W., Lindman, H., & Savage, L. J. (1963). Bayesian statistical inference for psychological research. Psychological Review, 70, 193–242. doi:10.1037/h0044139
- Fandakova, Y., Sander, M. C., Werkle-Bergner, M., & Shing, Y. L. (2014). Age differences in short-term memory binding are related to working memory performance across the lifespan. Psychology and Aging, 29, 140–149. doi:10.1037/a0035347
- Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. doi:10.1016/0022-3956(75)90026-6
- Foos, P. W. (1989). Adult age differences in working memory. Psychology and Aging, 4, 269–275. doi:10.1037/0882-7974.4.3.269
- Foos, P. W., & Wright, L. (1992). Adult age differences in the storage of information in working memory. Experimental Aging Research, 18, 51–57. doi:10.1080/03610739208253911
- Gorgoraptis, N., Catalao, R. F. G., Bays, P. M., & Husain, M. (2011). Dynamic updating or working memory resources for visual objects. The Journal of Neuroscience, 31, 8502–8511. doi:10.1523/JNEUROSCI.0208-11.2011
- Hu, Y., Hitch, G. J., Baddeley, A. D., Zhang, M., & Allen, R. J. (2014). Executive and perceptual attention play different roles in visual working memory: Evidence from suffix and strategy effects. Journal of Experimental Psychology: Human Perception and Performance, 40, 1665–1678. doi:10.1037/a0037163
- Isella, V., Molteni, F., Mapelli, C., & Ferrarese, C. (2015). Short term memory for single surface features and bindings in ageing: A replication study. Brain and Cognition, 96, 38–42. doi:10.1016/j.bandc.2015.02.002
- Jaswal, S., & Logie, R. H. (2011). Configural encoding in visual feature binding. Journal of Cognitive Psychology, 23, 586–603. doi:10.1080/20445911.2011.570256
- Johnson, W., Logie, R. H., & Brockmole, J. R. (2010). Working memory tasks differ in factor structure across age cohorts: Implications for dedifferentiation. Intelligence, 38, 513–528. doi:10.1016/j.intell.2010.06.005
- Kilb, A., & Naveh-Benjamin, M. (2007). Paying attention to binding: Further studies assessing the role of reduced attentional resources in the associative deficit of older adults. Memory & Cognition, 35, 1162–1174. doi:10.3758/BF03193486
- Larsen, J. D., & Baddeley, A. (2003). Disruption of verbal STM by irrelevant speech, articulatory suppression, and manual tapping: Do they have a common source? The Quarterly Journal of Experimental Psychology, 56, 1249–1268. doi:10.1080/02724980244000765
- Li, S.-C., Lindenberger, U., Hommel, B., Aschersleben, G., Prinz, W., & Baltes, P. B. (2004). Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychological Science, 15, 155–163. doi:10.1111/j.0956-7976.2004.01503003.x
- Logie, R. H. (2011). The functional organization and capacity limits of working memory. Current Directions in Psychological Science, 20, 240–245. doi:10.1177/0963721411415340
- Logie, R. H., Brockmole, J. R., & Jaswal, S. (2011). Feature binding in visual working memory is unaffected by task-irrelevant changes of location, shape and color. Memory and Cognition, 39, 24–36. doi:10.3758/s13421-010-0001-z
- Logie, R. H., Brockmole, J. R., & Vandenbroucke, A. R. E. (2009). Bound feature combinations in visual short-term memory are fragile but influence long-term learning. Visual Cognition, 17, 160–179. doi:10.1080/13506280802228411
- Logie, R. H., Horne, M. J., & Pettit, L. D. (2015). When cognitive performance does not decline across the lifespan. In R. H. Logie & R. Morris (Eds.), Working memory and ageing (pp. 21–47). Hove: Psychology Press.
- Logie, R. H., Parra, M. A., & Della Sala, S. (2015). From cognitive science to dementia assessment. Policy Insights from the Behavioral and Brain Sciences, 2, 81–91. doi:10.1177/2372732215601370
- Lustig, C., Hasher, L., & Zacks, R. (2007). Inhibitory deficit theory: Recent developments in a “new view". In D. S. Gorfein & C. M. MacLeod (Eds.), The place of inhibition in cognition (pp. 145–162). Washington, DC: American Psychological Association. doi:10.1037/11587-008
- Mitchell, K. J., Johnson, M. K., Raye, C. L., & D'Esposito, M. (2000). fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Cognitive Brain Research, 10, 197–206. doi:10.1016/S0926-6410(00)00029-X
- Mitchell, K. J., Johnson, M. K., Raye, C. L., Mather, M., & D'Esposito, M. (2000). Aging and reflective processes of working memory: Binding and test load deficits. Psychology and Aging, 15, 527–541. doi:10.1037//0882-7974.15.3.527
- Morey, R. D., & Rouder, J. N. (2015). BayesFactor: Computation of Bayes factors for common designs [computer software manual]. Retrieved from http://CRAN.R-project.org/package=BayesFactor (R package version 0.9.11-1).
- Murre, J. M. J., Wolters, G., & Raffone, A. (2006). Binding in working memory and long term memory: Towards an integrated model. In H. D. Zimmer, A. Mecklinger, & U. Lindenberger (Eds.), Handbook of binding and memory: Perspectives from cognitive neuroscience (pp. 221–250). Oxford: Oxford University Press.
- Naveh-Benjamin, M. (2000). Adult age-differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition, 26, 1170–1187. doi:10.1037/0278-7393.26.5.1170
- Naveh-Benjamin, M., Brav, T. K., & Levy, O. (2007). The associative memory deficit of older adults: The role of strategy utilization. Psychology and Aging, 22, 202–208. doi:10.1037/0882-7974.22.1.202
- Naveh-Benjamin, M., Guez, J., & Shulman, S. (2004). Older adults’ associative deficit in episodic memory: Assessing the role of decline in attentional resources. Psychonomic Bulletin & Review, 11, 1067–1073. doi:10.3758/BF03196738
- Old, S. R., & Naveh-Benjamin, M. (2008a). Differential effects of age on item and associative measures of memory: A meta-analysis. Psychology and Aging, 23, 104–118. doi:10.1037/0882-7974.23.1.104
- Old, S. R., & Naveh-Benjamin, M. (2008b). Memory for people and their actions: Further evidence for an age-related associative deficit. Psychology and Aging, 23, 467–472. doi:10.1037/0882-7974.23.2.467
- Park, C., Lautenschlager, G., Hedden, T., Davidson, N. S., Smith, A. D., & Smith, P. K. (2002). Models of visuospatial and verbal memory across the adult life span. Psychology and Aging, 17, 299–320. doi:10.1037/0882-7974.17.2.299
- Parra, M. A., Abrahams, S., Fabi, K., Logie, R. H., Luzzi, S., & Della Sala, S. (2009). Short-term memory binding deficits in Alzheimer's disease. Brain, 132, 1057–1066. doi:10.1093/brain/awp036
- Parra, M. A., Abrahams, S., Logie, R. H., & Della Sala, S. (2009). Age and binding within-dimension features in visual short-term memory. Neuroscience Letters, 449, 1–5. doi:10.1016/j.neulet.2008.10.069
- Parra, M. A., Abrahams, S., Logie, R. H., & Della Sala, S. (2010). Visual short-term memory binding in Alzheimer's disease and depression. Journal of Neurology, 257, 1160–1169. doi:10.1007/s00415-010-5484-9
- Parra, M. A., Abrahams, S., Logie, R. H., Mendez, L. G., Lopera, F., & Della Sala, S. (2010). Visual short-term memory binding deficits in familial Alzheimer's disease. Brain, 133, 2702–2713. doi:10.1093/brain/awq148
- Parra, M. A., Della Sala, S., Logie, R. H., & Morcom, A. (2014). Neural correlates of shape-color binding in visual working memory. Neuropsychologia, 52, 27–36. doi:10.1016/j.neuropsychologia.2013.09.036
- Pearson Education, Inc. (2009). Test of premorbid functioning – UK edition. London: Pearson Assessment.
- Peich, M., Husain, M., & Bays, P. M. (2013). Age-related decline of precision and binding in visual working memory. Psychology and Aging, 28, 729–743. doi:10.1037/a0033236
- Pilotti, M., Beyer, T., & Yasunami, M. (2002). Top-down processing and the suffix effect in young and older adults. Memory & Cognition, 30, 89–96. doi:10.3758/BF03195268
- R Core Team. (2015). R: A language and environment for statistical computing [computer software manual]. Vienna: R Foundation for Statistical Computing. Retrieved from http://www.R-project.org/
- Read, C. A., Rogers, J. M., & Wilson, P. H. (2015). Working memory binding of visual object features in older adults. Aging, Neuropsychology, and Cognition. doi:10.1080/13825585.2015.1083937
- Rhodes, S., Parra, M. A., & Logie, R. H. (2016). Ageing and feature binding in visual working memory: The role of presentation time. The Quarterly Journal of Experimental Psychology, 69, 654–668. doi: 10.1080/17470218.2015.1038571
- Rouder, J. N., Morey, R. D., Speckman, P. L., & Province, J. M. (2012). Default bayes factors for ANOVA designs. Journal of Mathematical Psychology, 56, 356–374. doi:10.1016/j.jmp.2012.08.001
- Salthouse, T. A. (1992). The influence of processing speed on adult age differences in working memory. Acta Psychologica, 79, 155–170. doi:10.1016/0001-6918(92)90030-H
- Salthouse, T. A. (1994). The aging of working memory. Neuropsychology, 8, 535–543. doi:10.1037/0894-4105.8.4.535
- Salthouse, T. A. (1996). The processing-speed theory of adult age differences in cognition. Psychological Review, 103, 403–428. doi:10.1037/0033-295X.103.3.403
- Sander, M. C., Lindenberger, U., & Werkle-Bergner, M. (2012). Lifespan age differences in working memory: A two-component framework. Neuroscience and Biobehavioral Reviews, 36, 2007–2033. doi:10.1016/j.neubiorev.2012.06.004
- Sander, M. C., Werkle-Bergner, M., & Lindenberger, U. (2011). Binding and strategic selection in working memory: A lifespan dissociation. Psychology and Aging, 26, 612–624. doi:10.1037/a0023055
- Stanislaw, H., & Todorov, N. (1999). Calculation of signal detection theory measures. Behavior Research Methods, Instruments, & Computers, 31, 137–149. doi:10.3758/BF03207704
- Ueno, T., Allen, R. J., Baddeley, A. D., Hitch, G. J., & Saito, S. (2011). Disruption of visual feature binding in working memory. Memory & Cognition, 39, 12–23. doi:10.3758/s13421-010-0013-8
- Ueno, T., Mate, J., Allen, R. J., Hitch, G. J., & Baddeley, A. D. (2011). What goes through the gate? Exploring interference with visual feature binding. Neuropsychologia, 49, 1597–1604. doi:10.1016/j.neuropsychologia.2010.11.030
- Vaughan, L., & Hartman, M. (2010). Aging and visual short-term memory: Effects of object type and information load. Aging, Neuropsychology, & Cognition, 17, 35–54. doi:10.1080/13825580903009063
- Wheeler, M. E., & Treisman, A. M. (2002). Binding in short-term visual memory. Journal of Experimental Psychology: General, 131, 48–64. doi:10.1037//0096-3445.131.1.48
Appendix. Cross-experiment analyses
As Experiment 1 showed that encoding time does not interact with memory test type, the parallel data from all three experiments were pooled together in order to assess (a) whether an age-related binding deficit exists under control conditions only and (b) the consistency of performance across the three experiments. Data were taken from all 48 participants of Experiment 1, but only from the encoding time condition that was carried out first for each participant. For Experiments 2 and 3, the selected data were from the control condition only, and from only the 24 participants who carried out the control condition first. Thus, we compared the control conditions across all three experiments that could usefully be compared without contamination by other experimental conditions (sequential presentation, suffix presentation). In all cases only the shape and binding memory performance data were selected in order to focus upon the most crucial memory conditions for comparison. This resulted in a 2 (age group) × 2 (memory type: shape, binding) × 3 (experiment) mixed factorial design, using the A′ scores. There were effects of memory, F(1, 90) = 5.70, MSE = .004, p = .019, = .06 [with shape performance (M = .87, SD = .08) slightly better than binding performance (M = .84, SD = .10)], age group, F(1, 90) = 67.52, MSE = .007, p < .001,
= .43 [with younger adults (M = .90, SD = .05) outperforming older adults (M = .81, SD = .07)], and an age × experiment interaction, F(2, 90) = 3.84, MSE = .007, p = .025,
= .08. Independent t-tests on the data collapsed across memory condition reflected that there was generally slightly poorer performance in both Experiments 2 (M = .78, SD = .07) and 3 (M = .78, SD = .09) relative to Experiment 1 (M = .83, SD = .05) in older adults (both p < .041), while there was no difference between Experiment 1 and the other two experiments in the younger adults (both p > .28). All other effects were non-significant (all p > .12). Specifically regarding the age × memory condition interaction, this was not significant, F(1, 90) = 2.17, MSE = .004, p = .145,
= .02, with younger adults scoring very similar across the shape (M = .91, SD = .06) and binding (M = .89, SD = .06) conditions, and older adults also showing very little evidence of decline (Mshape = .82, SD = .07; Mbinding = .79, SD = .10). A Bayesian ANOVA on this cross-experiment dataset also showed that, when omitting the age × memory condition interaction from the full model, the reduced model is favoured (B = 1.77). The results of these cross-experiment analyses therefore demonstrate no general age-related binding deficit, and no age-related binding deficit dependent on particular samples.
