Abstract
The influence of external factors other than nutrients on biofilm development and composition was studied with a combination of optical (Confocal Laser Scanning Microscopy, PAM fluorometry) and chemical methods (EPS extraction, HPLC, TOC determination). The development of algal-bacterial biofilms was followed from bare surfaces to mature biofilms in two water reservoirs on Dutch filtration dunes. Biofilms developed under the influence of grazing, light limitation or undisturbed by either of these two factors. Biofilms appeared similar at the beginning of the colonization period at the three sites and laser microscopy observations revealed the predominance of bacteria and capsular EPS (extracellular polymeric substances) in young biofilms. After 3 weeks development, the biofilms had a higher number of taxa; undisturbed biofilms presented the highest biomass, the longest developmental period and showed a significant correlation between organic carbon, chlorophyll and EPS production, indicating a close coupling between autotrophic carbon production and EPS. In light-limited biofilms, this coupling was weaker (although the organic carbon content was similar to that of the undisturbed biofilms) and a lower algal biomass was reached. Light-limited biofilms were mostly composed of diatoms, which are more efficient in low irradiances than green microalgae. Biofilms grazed by the snail Potamopyrgus antipodarum presented the lowest biomass level, but the highest proportion of EPS. Grazing seemed to favour the predominance of EPS-rich algae, as well as firmly attached diatoms. Although filamentous cyanobacteria were found in mature biofilms at the three locations, they were more abundant in the grazed biofilms. The differences in carbon uptake with respect to its allocation indicated that external factors influencing biofilm development affect the cycling and transport of carbon in biofilms and hence influence the effect of biofilm metabolism on the overlying water quality.
Introduction
Biofilms commonly cover submersed surfaces in shallow freshwater systems and have the capacity to modify the transport and accumulation of substances such as nutrients and suspended particles in the water (Lowe & Pan, Citation1996). Autotrophic biofilms have been the subject of numerous studies focusing on their role as regulators of the organic carbon content in shallow environments (Battin et al., Citation1999), as a crucial link at the base of the trophic web (Steinman, Citation1996) and in purifying water (Percival et al., Citation2000) or, conversely, the nuisance caused by biofouling (Gawne et al., Citation1998).
Biofilm-forming organisms, like diatoms, are also currently used as water quality indicators (Prygiel et al., Citation1999) and differences in biofilm composition between sites are mostly attributed to differences in the chemical composition of the water, such as nutrient concentration and pollution levels. Nevertheless, other external factors like grazing (Hillebrand, Citation2003), disturbance (Biggs & Smith, Citation2002) and irradiance (Pillsbury & Lowe, Citation1999) have been found to play a crucial role determining biofilm composition.
The development of biofilms has normally been studied taking exclusively one of the microbial compartments into account, either the microalgae (Liboriussen & Jeppensen, Citation2003 and references therein), or the bacteria (Jackson et al., Citation2001). Natural algal-bacterial biofilms, however, function as a more complex system than the sum of the species present, since the microorganisms interact and develop interdependently (Barranguet et al., Citation2003). Moreover, the microorganisms in biofilms are embedded in a polysaccharide matrix of extracellular polymeric substances (EPS), which gives cohesion to the biofilm and facilitates the interactions between the biofilm components (Flemming & Wingender, Citation2001). EPS have been shown to act as a defence against grazing in certain biofilm-forming heterotrophic bacteria (Matz et al., Citation2002) and in biofilm-forming cyanobacteria (Pajdak-Stos et al., Citation2001), but their role has never been investigated in multispecies algal-bacterial biofilms.
Little information is available on the changes in biofilm composition with age, from bare surfaces to mature biofilms, with reference to the carbon allocation (extracellular-intracellular) between the biofilm components (Smith & Underwood, Citation1998; Decho, Citation2000). Changes in biofilm composition with age will bring about changes in the way the biofilm modifies the quality of the overlying water and uses nutrients, ultimately changing the transfer of substances between the benthos and the water column (Sekar et al., Citation2002; Van der Grinten et al., Citation2004).
The aim of this paper was to study the development of natural biofilms at water reservoirs from infiltration dunes used for the preparation of drinking water. Changes in the contribution of algae, EPS and bacteria to the total carbon content in biofilms developing from bare surfaces to mature biofilms were assessed. We compared the development of biofilms in these water bodies at three different sites were biofilms grow undisturbed, or are grazed or light limited, but all under similar water masses. We analysed the differences caused by these three conditions on biofilm structure and composition, related to the distribution of the organic fraction within and between the different biofilm compartments.
Materials and methods
The samples were taken between May and July 2001 on the filtration dunes of Leiduinen, The Netherlands (52 20′ 44′′ N; 4 34′ 38′′ E), which are used for the preparation of drinking water. Water from the River Rhine is transported to the dunes after pre-treatment and filtration occurs under gravity. The water quality is further improved and stabilized by degradation of nitrate. The water from the lower storage canals is collected at a central basin before further purification. One sampling site was located in one of the lower storage canals, Nieuw Kanaal (NK), and the other in the central basin where all filtered water is collected: the Oranjekom (OK).
Both locations are sampled weekly by the GWA (Gemeente Warterleiding Amsterdam), the local drinking water company, which provided data on the main physical and chemical parameters summarized in . The water undergoes the same pre-treatment at both sites, although there are differences in their biological communities. At NK, cyanobacterial blooms occur during the summer in the phytoplankton, but not in the benthos. Biofilms at NK are heavily grazed by the snail Potamopyrgus antipodarum (Gray), which occurs at high densities during the summer, while this snail is much less abundant at OK (de Bokx, Citation1997) and cyanobacterial blooms have not been recorded. OK is surrounded by forests, and floating mats of plant detritus often accumulate by wind action, reducing light penetration by 50 – 70%. Consequently, OK was sampled at two points: OK west (without floating mats) and OK east (with mats). Hence, biofilms were studied under three contrasting conditions: at NK, the biofilms were heavily grazed; at OK, they were not grazed, but biofilms on the east side were heavily shaded, while those on the west side had undisturbed conditions for biofilm development.
Table 1 . Physical and chemical variables during May to July 2002 in two water bodies sampled in Leiduinen: Nieuw Kanaal (NK) and Oranjekom (OK). k: light attenuation coefficient calculated from PAR measurements in absence of floating mats.
Biofilm development was monitored using glass discs (1.5 cm2) as an artificial substrate. The discs were held by polyethylene blocks (on opposite sides, five discs per side) that hung from floating polyethylene racks (five per location), creating a vertical alignment of the discs at about 25 cm below the water surface, where the depth was 1.5 m. The racks were arranged in a straight line with one side facing east and the other west to minimize differences in the daylight received by the two sides. Samples were taken after 3, 6, 12 and 17 days and 3, 4, 5, 6, 7, 8 and 10 weeks. Between four and eight blocks were sampled per time at each location, depending on the amount of biofilm biomass present. Irradiance (PAR: photosynthetically available radiation; LI-COR LI 1400), conductivity and temperature (WTW LF92) were measured at both locations and are summarized in .
The biofilms were transported directly to the laboratory in separate cool-boxes filled with water from each location. Fresh samples were taken for Confocal Laser Scanning Microscopy (CLSM), light microscope observations and carbohydrate extraction. Additional replicate samples were frozen (− 80°C) for the determination of organic carbon content and HPLC pigment analyses.
The relative coverage of the different algal species was first checked microscopically at low magnification on intact biofilms. Biofilms were later fixed in 4% formaldehyde (three discs per treatment) to study the algal species composition in more detail. Epifluorescence microscopy was used to check the viability of the algae. Identification of the diatom species was confirmed after cleaning the frustules according to Barber & Haworth (Citation1981) and mounting in high refraction resin.
Measurements in the biofilms
Carbon content of the biofilms was measured using a Total Organic Carbon Analyzer (model 700, O.I. Analytical). Biofilms were scraped from 10 glass discs and suspended in 10 ml Elix water in a 20-ml glass vial. The suspension was homogenized for 1 min (ultramixer, 20,000 rpm) and the volume made up to 100 ml with Elix. Total inorganic and organic carbon (TIC and TOC) were measured under continuous stirring. The reaction time was set to 10 min.
Pigments were extracted from freeze-dried biofilm samples with 95% methanol and 5% ammonium acetate and analysed by HPLC according to Barranguet et al. (Citation1997). EPS were extracted in two steps, first with bidistilled water, which extracts the loose fraction of the EPS, and then with 0.1 M H2SO4, a solvent strong enough to extract the bound fraction (Barranguet et al., Citation2004). The bound EPS seem to be involved in the initial attachment of cells to a surface and so are especially relevant in early developmental stages (Scott et al., Citation1996). Carbohydrate concentrations were estimated using the phenol-sulphuric method (Dubois, Citation1959).
CLSM analysis and PAM fluorometry were used as non-destructive methods to monitor the development of the auto- and heterotrophic biofilm compartments simultaneously, according to Barranguet et al. (Citation2004). For each type of biofilm, three replicate samples were examined. Maximum quantum yield in the dark (Fv/Fm) was measured with a Water-PAM/F (Heinz Walz GmbH, Effeltrich) after 20 min dark adaptation. In CLSM, microalgal biomass was quantified as chlorophyll a by using auto-fluorescent properties of chlorophyll (absorption maximum 668 nm). Bacterial DNA was stained with Syto 9 (Molecular Probes Inc.). EPS was estimated using a fluorescent labelled lectin of Canavalia ensiformis (Con-A. Molecular probe Inc.). After the staining was completed, the discs were rinsed and examined using a Zeiss model LSM 510 Laser scanning microscope equipped with two Helium-Neon lasers (ex. 543 nm and 633 nm) and an Argon laser (ex. 488 nm). All observations were performed using a 20×/0.75 NA objective (Zeiss, Plan-Apochromat®).
Biofilms were scanned at 5-μm intervals, starting at the glass disc surface, resulting in a series of individual images at different depths (z-scan). For image analysis, four 3D-images per glass disc were recorded, producing 12 3D-images per biofilm. For each 3D-image the total contribution of algae, EPS and bacteria as a function of depth were calculated by summing the individual channels per optical section resulting in a z-profile (software by Mark Savenije). The mean contribution of algae, EPS and bacteria per disc was determined by averaging the four z-profiles.
Results
During the early stages of development, the chemical methods used for the extraction of carbon, pigments and EPS were close to their detection limits, and several replicates had to be pooled. Therefore, CLSM was essential for examining early biofilm development. After the biofilms reached a certain thickness (40 μm), CLSM could not be used due to the attenuation of the signal, as reported earlier by Barranguet et al. (Citation2004).
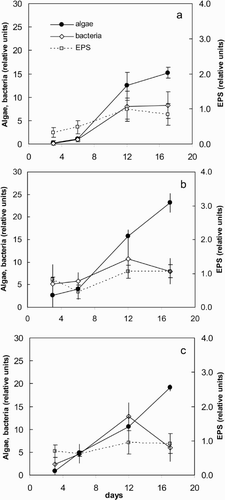
Young biofilms (3 to 6 days old) were characterized by the abundance of EPS (mostly capsular) and heterotrophic bacteria. However, the relative contribution of EPS and heterotrophic bacteria decreased after the first week of biofilm development, when the number of microalgae increased and began to represent a larger proportion of the organic bulk in the biofilms (, , ). This trend was similar in the three locations. Throughout the whole developmental period, Fv : Fm measured with the PAM fluorometer showed stable values between 0.65 and 0.75, without significant differences between sites (t-tests, p > 0.05).
Fig. 1. Relative abundance of algae and bacteria (determined by Confocal Laser Scanning Microscopy) and extracellular polymeric substances (EPS, in relative fluorescence units) in young biofilms growing at the three locations studied at Leiduinen. (a) Nieuw Kanaal (NK); (b) Oranjekom (OK) east; (c) OK west. Error bars represent standard deviations (n = 12).
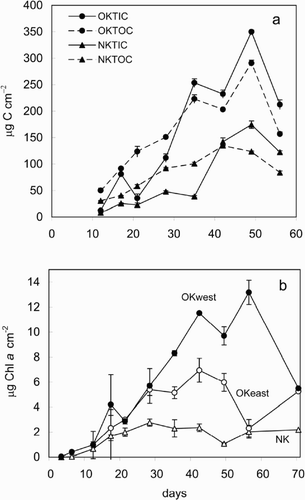
A linear increase in the carbon content of the biofilms at the three locations was measurable from the first week, OK east and west did not show significant differences (t-tests), while NK had lower values of organic and inorganic carbon (). At NK, grazing made the biofilms more encrusting and thinner. For all biofilms, the increase of inorganic carbon was slower than the organic carbon until week 4, when the inorganic carbon concentration increased sharply. A significant correlation between organic carbon and Chl a was found at OK (r 2 = 0.682, p < 0.05), while at NK such correlation was not observed, as the increase in TOC was more gradual and showed a plateau later than Chl a ( and ). Chl a development in the biofilms showed the same temporal increase pattern at the three locations, although the values reached were quite different. As could be predicted from the carbon concentrations, grazing reduced algal biomass considerably at NK and Chl a values stabilized after 21 days of development (). In contrast, OK east and OK west showed similar algal growth until day 28. At this time, Chl a at OK east had reached a plateau while at OK west algal growth continued until day 42 ().
Fig. 2. Carbon and Chl a content of biofilms at different stages of development. (a) Total organic (TOC) and inorganic carbon (TIC) content; (b) Chl a content. Site abbreviations as in . Error bars represent standard deviations (n = 4).
The normalization of the accessory plant pigments to Chl a () provided information about the relative abundance of the different algal classes in the biofilms. The higher ratio of Chl b than of Chl c to Chl a indicated that chlorophytes dominated the algal population at OK west and east at the beginning of biofilm development. After 2 weeks, however, diatoms became the main component with fucoxanthin/Chl a ratios between 0.3 and 0.4 (not shown) and Chl c/Chl a ratios between 0.08 and 0.1 (, , ). The dominance of diatoms was more accentuated at OK east, where the ratios Chl c : Chl a were the highest, while Chl b : Chl a remained significantly lower than at OK west (t-tests; p < 0.01). At NK, the dominance of the chlorophytes in the young biofilms was not so clear (), as the ratio Chl b : Chl a varied widely with time.
Fig. 3. Concentrations of accessory pigments relative to Chl a (both in μg cm− 2) in the biofilms at the three locations studied. (a) OK east; (b) OK west; (c) NK. Error bars represent standard deviations. Error bars as in .
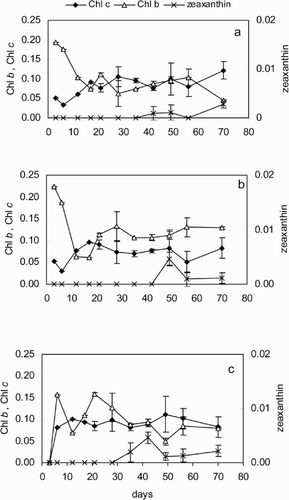
After 4 weeks of biofilm development at all three locations, an increase in the ratio zeaxanthin : Chl a indicated a rise in the proportion of cyanobacteria, as there was no concurrent increase of the Chl b : Chl a ratio (). Microscopic observations confirmed that filamentous cyanophytes [Heteroleibleinia kuetzingii (Schmidle)] were successful late biofilm colonizers and were especially abundant in mature biofilms (more than 4 weeks old) at the three locations. Among the chlorophytes, the colonial Chaetophora sp. was abundant at all locations; the highest coverage of these 0.4 – 0.8 mm diameter colonies, densely embedded in EPS, was at NK.
In spite of the similarities described above, there were taxonomic differences between the microalgal population at sites OK and NK. At NK, the extensive grazing on the biofilms favoured the predominance of the most firmly attached, EPS-rich species, like the diatom Cocconeis placentula Ehrenberg (attached by mucilage under the entire valve face) and Cymbella affinis Kützing (an epilithic species with an abundant production of mucilage). At OK east and west, diatoms attached with short mucous stalks or basal discs, which were less firmly fastened to the substrate, prevailed (Achnanthes minutissima Kützing, Gomphonema sp.). Free moving diatoms, like species of Navicula, were also somewhat more abundant at OK than at NK. The colonial chlorophyte Coleochaete orbicularis Pringsheim, a species frequently growing on the leaves of aquatic plants, was only present at OK, which is consistent with the abundant vegetation that surrounds this reservoir.
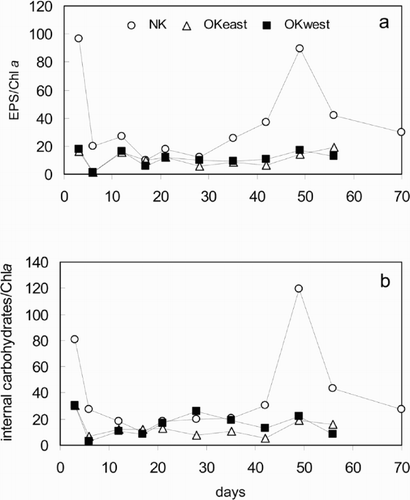
The quantification of EPS confirmed the high concentration at NK, mainly consisting of the bound fraction, with the internal carbohydrates slightly more abundant than the bound EPS (). There was no correlation between the increase of Chl a and carbohydrates, either intracellular (internal) or EPS. Similar to the organic and inorganic carbon content, EPS concentrations continued to increase, after the Chl a concentrations had stabilized (). For OK east, the pattern of EPS production in the biofilms was similar to that of NK, but there was a smaller difference between the concentrations of loose and bound EPS (), consistent with the more abundant diatom fraction. The loose EPS was more closely correlated with the Chl a than the bound EPS (r 2 loose = 0.78; r 2 bound = 0.52), since the latter and the internal carbohydrate fraction, showed a good agreement with Chl a only during the first week of biofilm development.
Fig. 4. Carbohydrate concentrations in the biofilms, divided into three fractions: extracellular loose, extracellular bound and internal. (a) NK; (b) OK east; (c) OK west. Error bars as in .
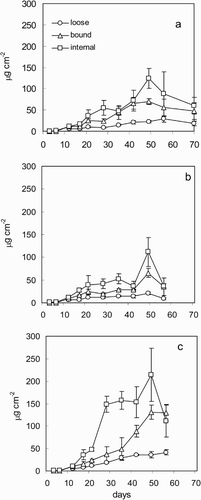
At OK west, there was good agreement between the patterns of carbon increase, Chl a and EPS (r 2 bound vs Chl a = 0.85; r 2 loose vs Chl a = 0.97) while the internal carbohydrates did not correlate significantly with Chl a. The majority of the carbohydrates belonged to the internal fraction, followed by the bound EPS, but these became more abundant only at the end of the colonization period ().
Expressing EPS (loose + bound) as a function of Chl a clearly showed the effect of grazing on the organic matter distribution in biofilms. Although the EPS production was the highest at OK west in absolute terms, the biofilms subject to grazing at NK had significantly higher carbohydrate : Chl a ratios, both for the internal and external carbohydrates (). With time, the abundance of carbohydrates relative to Chl a decreased after day 3, confirming the patterns shown in .
Discussion
The development of the biofilms in the water reservoirs at Leiduinen showed the typical pattern of biofilm growth reported elsewhere in the literature: settlement, growth, stabilization and ultimately biomass decrease through senescence or sloughing (Cooksey & Wigglesworth-Cooksey, Citation1995). Biofilm development typically starts with the formation of a conditioning film of bacteria, which allows for the settlement of larger microorganisms on the new substrate. In this study, eukaryotic microorganisms (green algae and diatoms) were already present in small numbers at both locations after 3 days. EPS, especially capsular ones, are thought to have an important role during the initial stages of biofilm development, since they facilitate the attachment of cells to the substrate (Wingender et al., Citation1999; Decho, Citation2000). Our CLSM observation of the young (3- and 6-day-old) biofilms confirmed that extracellular carbohydrates present were mainly capsular.
After the initial state, which was similar for the three locations studied, the biofilms showed a clear increase in thickness and density, but the relation between carbon concentrations, abundance of microalgae and carbohydrate production showed a different pattern under the situations of grazed (NK), light limited (OK east) or undisturbed growth (OK west). None of the biofilms studied presented signs of nutrient limitation, as indicated by the high and stable values of Fv : Fm (Parkhill et al., Citation2001), which made the forcing factors limiting biofilm growth very clearly identifiable.
The highest biomass and the longest period of biomass increase were found at OK west, together with a coupling between the increase of carbon in the biofilms, algal biomass (Chl a) and EPS, showing a balance between microorganism growth and the production of EPS. Nevertheless, the main proportion of carbohydrates was internal and the concentrations indicated that the biofilms first replenished their internal carbohydrate pool, to be later secreted as EPS. These results are in accordance with de Brouwer & Stal (Citation2002), who found that EPS is a product of overflow metabolism and is continuously produced once the microalgae enter the stationary phase.
When biofilms developed under low light (OK east), the correlation between algal biomass and EPS was weaker, and the concentration of internal carbohydrates relative to EPS was lower; the mature biofilms had a significantly lower microalgal abundance than at OK west, although the total organic carbon contents were similar. Since both stations are in the same water body, we conclude that light limitation was responsible for the increase in the proportion of heterotrophic microorganisms compared to algae. The ratio of diatoms to green algae at OK east was greater than in the other stations, probably because diatoms are better at coping with low irradiances (Pillsbury & Lowe, Citation1999).
The grazed biofilms at NK were the richest in EPS relative to organic carbon, in spite of having the lowest organic concentration per unit area, both as total carbon and algal biomass. Potamopyrgus antipodarum has been reported to graze very effectively on periphyton, decreasing the total microalgal coverage (James et al., Citation2000a), most of its diet being diatoms (James et al., Citation2000b). In our case, the decrease in algal biomass due to grazing was clear, but the proportion of diatoms did not change significantly compared to the other stations, although the diatoms species found were different. We detected a higher proportion of firmly attached diatoms at NK, relative to erect and vertically oriented growth forms. These observations are consistent with the report of Steinman (Citation1996) that the physiognomy of biofilms changes due to the action of grazers, which reduce ramified algal forms.
The biofilms at NK had increased EPS production, which did not correlate with algal biomass, so EPS seem to have been an effective defence against grazing for some of the algae. EPS production has been reported as a defence against grazing in certain microbial species (Padjak-Stos et al., Citation2001; Matz et al., Citation2002). Here we report, for the first time, the general response of the whole biofilm community, which increased the proportion of EPS relative to microalgal biomass with a prevalence of EPS embedded forms, such as green algal colonies. Also filamentous cyanobacteria, which colonized mature biofilms successfully, were the most abundant at NK; this group is currently known as poor source for grazers, due to lower nutritious value, palatability or specific defences against grazers (Juttner & Wu, Citation2000).
In conclusion, biofilm age changes the composition of the different organic fractions, although there is a general trend for bacteria and EPS to dominate at the beginning of the development, with an increase of algal biomass in later developmental stages. External factors can change the carbon allocation between compartments (bacteria, algae, EPS), as well as the composition of an individual compartment. A severe limitation by grazing causes an uncoupling of algal production and the extrusion of carbohydrates, as a result of defence mechanisms against grazing, so that an uncoupling between the intra- and extracellular allocation of carbon ensues. These differences in composition and physiognomy have repercussions for the production and recycling of carbon and nutrients in biofilms and should be taken into account when studying the metabolism of carbon in shallow systems and the power of biofilms to modify the overlying water composition.
Acknowledgments
This study was financed by the European project BIOFILMS, Contract No.: EVK1-CT-1999-00001. We like to thank M. Savenije, W. Takkenberg and E. Manders from the Swammerdam Institute for Life Sciences, University of Amsterdam for their help with the CLSM measurements.
References
References
- Barber , HG and Harrow , EY . 1981 . A guide to the morphology of diatom frustule . Freshwater Biological Association, Scientific Publication , 44 : 1 – 112
- Barranguet , C , Herman , PMJ and Sinke , JJ . 1997 . Microphytobenthos biomass and community composition studied by pigments biomarkers: importance and fate in the carbon cycle of a tidal flat . J. Sea Res. , 38 : 59 – 70
- Barranguet , C , van den Ende , FP , Rutgers , M , Breure , AM , Greijdanus , M , Sinke , JJ and Admiraal , W . 2003 . Copper induced modifications of the trophic relations in riverine algal-bacterial biofilms . Environ. Toxicol. Chem. , 22 : 1340 – 1349
- Barranguet , C , van Beusekom , SAM , Veuger , B , Neu , TR , Manders , EMM , Sinke , JJ and Admiraal , W . 2004 . Studying undisturbed autotrophic biofilms: still a technical challenge . Aq. Micob. Ecol. , 34 : 1 – 9
- Battin , TJ , Buttirini , A and Sabater , F . 1999 . Immobilization and metabolism of dissolved organic carbon by natural sediment biofilms in a Mediterranean and temperate stream . Aquatic Microb. Ecol. , 19 : 297 – 305
- Biggs , BJF and Smith , RA . 2002 . Taxonomic richness of stream benthic algae: Effects of flood disturbance and nutrients . Limnol. Oceanogr. , 47 : 1175 – 1186
- de Bokx EM 1997 Hydrobiologisch onderzoek in de Amsterdamse Waterleidingduinen. Omschrijving van de mosterlocaties op basis van de gevonden macrofauna. Toegevoegde rapportage bij de Eind-rapportage Report of the Gemeente Waterleiding Amsterdam: 47 p
- Cooksey , KE and Wigglesworth-Cooksey , B . 1995 . Adhesion of bacteria and diatoms to surfaces in the sea: a review . Aq. Microb. Ecol. , 9 : 87 – 96
- de Brouwer , JFC and Stal , LJ . 2002 . Daily fluctuations of exopolymers in cultures of the benthic diatoms Cylindrotheca closterium and Nitzschia sp. (Bacillariophyceae) . J. Phycol. , 38 : 464 – 472
- Decho , AW . 2000 . Microbial biofilms in intertidal systems: an overview . Cont. Shelf Res. , 20 : 1257 – 1273
- Dubois , M . 1959 . Colorimetric method for determination of sugars and related substances . Ann. Chem. , 28 : 350 – 356
- Flemming , HC and Wingender , J . 2001 . Relevance of microbial extracellular polymeric substances (EPSs) - Part I: Structural and ecological aspects . Wat. Sci. Tech. , 43 : 1 – 8
- Gawne , B , Wang , Y , Hoagland , KD and Gretz , MR . 1998 . Role of bacteria and bacterial exopolymer in the atttachment of Achnanthes longipes (Bacillariophyceae) . Biofouling , 13 : 137 – 156
- Hillebrand , H . 2003 . Opposing effects of grazing and nutrients on diversity . Oikos , 100 : 592 – 600
- Jackson , CR , Churchill , PF and Roden , EE . 2001 . Successional changes in bacterial assemblage structure during epilithic biofilm development . Ecology , 82 : 555 – 566
- James , MR , Hawes , I and Weatherhead , M . 2000a . Removal of settled sediments and periphyton from macrophytes by grazing invertebrates in the littoral zone of a large oligotrophic lake . Freshwater Biol. , 44 : 311 – 326
- James , MR , Hawes , I , Weatherhead , M , Stanger , C and Gibbs , M . 2000b . Carbon flow in the littoral food web of an oligotrophic lake . Hydrobiologia , 441 : 93 – 106
- Juttner , F and Wu , JT . 2000 . Evidence of allelochemical activity in subtropical cyanobacterial biofilms of Taiwan . Arch. Hydrobiol. , 147 : 505 – 517
- Liboriussen , L and Jeppensen , E . 2003 . Temporal dynamics in epipelic, pelagic and epiphytic algal production in a clear and a turbid shallow lake . Freshwater Biol. , 48 : 418 – 432
- Lowe RL Pan Y 1996 Benthic algal communities as biological monitors In: Algal Ecology (Stevenson, R.J., Bothwell, M.L. and Lowe, R.L., editors) 705 – 739 Academic Press San Diego CA USA
- Matz , C , Deines , P and Jurgens , K . 2002 . Phenotypic variation in Pseudomonas sp. CM10 determines microcolony formation and survival under protozoan grazing . FEMS Microb. Ecol. , 39 : 57 – 65
- McCormick , PV , Louie , D and Cairns , J . 1994 . Longitudinal effects of herbivory on lotic periphyton assemblages . Freshwater Biol. , 31 : 201 – 212
- Pajdak-Stos , A , Fialkowska , E and Fyda , J . 2001 . Phormidium autumnale (Cyanobacteria) defense against three ciliate grazer species . Aq. Microb. Ecol. , 23 : 237 – 244
- Parkhill , JP , Maillet , G and Cullen , JJ . 2001 . Fluorescence-based maximal quantum yield for PSII as a diagnostic of nutrient stress . J. Phycol. , 37 : 517 – 529
- Percival SL Walker JT Hunter PR 2002 Microbiological Aspects of Biofilms and Drinking Water CRC Press LCC
- Pillsbury , RW and Lowe , RL . 1999 . The response of benthic algae to manipulation of light in four acidic lakes in northern Michigan . Hydrobiologia , 394 : 69 – 81
- Prygiel J Whitton BA Burowska J 1999 Use of Algae for Monitoring Rivers III, Agence de l'Eau Artois-Picardie Douai France
- Scott , C , Fletcher , RL and Bremer , GB . 1996 . Observations on the mechanisms of attachment of some marine fouling blue-green algae . Biofouling , 10 : 161
- Sekar , R , Nair , KVK , Rao , VNR and Venugopalan , VP . 2002 . Nutrient dynamics and successional changes in a lentic freshwater biofilm . Freshwater Biol. , 47 : 1893 – 1907
- Smith , DJ and Underwood , GJC . 1998 . Exopolimer production by intertidal epipelic diatoms . Limnol. Oceanogr. , 43 : 1578 – 1591
- Steinman AD 1996 Effects of grazers on freshwater benthic algae In: Algal Ecology (Stevenson, R.J., Bothwell, M.L. & Lowe, R.L., editors) 341 – 373 Academic Press San Diego CA USA
- van der Grinten , E , Janssen , M , Simis , SGH , Barranguet , C and Admiraal , W . 2004 . Phosphate regime structuring species composition in cultured phototrophic biofilms . Freshwater Biol. , 49 : 369 – 381
- Wingender J Neu TR Flemming H 1999 Microbial Extracellular Polymeric Substances: Characterization, Structure, and function, Springer Heidelberg New York