Abstract
Allelopathic effects of several concentrations of fresh tissue and dry powder of two bloom-forming green macroalgae Ulva pertusa and Ulva linza on the red tide microalga Prorocentrum micans were investigated in coexistence culture systems. Preliminary studies on the algicidal effects of one aqueous and four organic solvent extracts from the macroalgae on the microalga were carried out to confirm the existence of allelochemicals in the tissue of these two macroalgae. The effects of filtrate of used macroalgal culture medium were tested on P. micans using a single initial or semi-continuous filtrate addition. Furthermore, the potential effects of the microalga on these two macroalgae were also evaluated. The results of the coexistence experiments demonstrated that the growth of P. micans was strongly inhibited by the addition of both the fresh tissue and the dry powder of both species of Ulva. Aqueous and methanol extracts of both macroalgae had strong growth inhibitory effects on P. micans, while the other three organic solvent extracts (acetone, ether and chloroform) had no apparent effect on its growth. This suggests that the allelochemicals from both macroalgae had relatively high polarities. The filtrate of used macroalgal culture medium exhibited no apparent growth inhibitory effect on the microalga under initial filtrate addition whereas the growth of P. micans was significantly inhibited under semi-continuous filtrate addition, which suggested that continuous release of small quantities of rapidly degradable allelochemicals from the fresh tissue of both macroalgae was effective in inhibiting the growth of P. micans. In contrast, the microalga had no apparent effect on the growth of either macroalgal species in coexistence experiments.
Introduction
The appearance of excessive growth of green macroalgae in response to coastal eutrophication, (termed ‘green tides’ by marine ecologists) is an increasingly common phenomenon along shorelines worldwide (Fletcher et al., Citation1990; Valiela et al., Citation1997; Taylor, Citation1999). Blooms of green macroalgae have been reported to have a harmful impact on intertidal marine ecosystems in a variety of ways, including changes in ecosystem structure (Raffaelli et al., Citation1998; Taylor, Citation1999; Nelson & Lee, Citation2001) and reduction of biodiversity (Valiela et al., Citation1997; Raffaelli et al., Citation1998; Taylor, Citation1999). Moreover, green tides may also have a deleterious effect on local fisheries and aquaculture industries and may contribute to a decrease in tourism revenue due to the presence of aesthetically displeasing and noxious-smelling deposits of drift weed on shorelines (Hernandez et al., Citation1997; Taylor, Citation1999). ‘Red tides’ (caused by blooms of harmful microalgae) are also occurring with increasing frequency in coastal waters worldwide and cause disastrous damage to the fishery and aquaculture industries of many countries (Qi et al., Citation1993; Horner et al., Citation1997; Kim, Citation1997). Because of the severe ecological and economic problems that result from coastal algal blooms, many investigations have sought the mechanisms that enable these bloom-forming algae to outcompete other coastal primary producers and establish dominant populations. Understanding the mechanisms may be helpful in developing strategies for the control of such harmful algal blooms (Hernandez et al., Citation1997; Smayda, Citation1997; Valiela et al., Citation1997; Taylor et al., Citation2001).
The species composition and growth dynamics of primary producers in aquatic ecosystems are known to be strongly affected by parameters such as the availability of nutrients, space, light and competition for these resources, selective grazing and also by allelopathic interactions (Gopal & Goel, Citation1993; Inderjit & Dakshini, Citation1994; van Donk & van de Bund, Citation2002; Gross, Citation2003). A number of authors have demonstrated that allelopathy among aquatic primary producers can profoundly affect the structure and succession of aquatic ecosystems (Keating, Citation1977, Citation1978; Körner & Nicklisch, Citation2002; Schagerl et al., Citation2002; van Donk & van de Bund, Citation2002). Some researchers have utilized the allelopathic effects of certain aquatic plants as a means of biological control of the growth of undesirable algae and weeds in aquatic ecosystems (Szczepanski Citation1977; Sun et al., Citation1989; Nakai et al., Citation1996, Citation1999, Citation2000; Jeong et al., Citation2000). However, most investigators so far have attributed the dominance of bloom-forming green macroalgae over other coastal primary producers to their physiological characteristics, such as their capacity for rapid nutrient uptake, high growth rate and tolerance of a broad range of environmental conditions (Smayda, Citation1997; Valiela et al., Citation1997; Raffaelli et al., Citation1998; Lotze & Schramm, Citation2000). The potential chemical interactions between bloom-forming primary producers in the coastal environment has been generally neglected, partially because green macroalgae are generally assumed to be unlikely to contain or release toxic compounds (Valiela et al., Citation1997) and also because they lack obvious chemical defences (van Alstyne, Citation2001). Studies on the potential allelopathic effects of opportunistic green macroalgae on other coastal bloom-forming organisms may contribute to the understanding of the mechanisms of formation and the ecological consequences of green tides. If these green macroalgae have deleterious effects on other undesirable primary producers, e.g. red tide microalgae, a new control method could be developed against them, which would utilize the large crops of green macroalgae.
Ulva pertusa and Ulva linza (previously Enteromorpha linza; Hayden et al., Citation2003) are two of the most common macroalgae contributing to the phenomenon known as green tides (Fletcher et al., Citation1990; Hernandez et al., Citation1997; Lotze & Schramm, Citation2000; Taylor et al., Citation2001), while Prorocentrum micans is one of the most harmful microalgae responsible for red tides (Adams et al., Citation1968; Qi et al., Citation1993; Zhu et al., Citation1997). The present study investigates the potential algicidal effects of U. pertusa and U. linza on cultures of P. micans. The three main objectives of this study were to provide evidence for the allelopathic effects of these two bloom-forming green macroalge on the red tide microalga, to gain an insight into the chemical interactions between macroalgae and microalgae in coastal areas, and to present evidence for the feasibility of using species of bloom-forming green macroalgae to control the proliferation of red tide microalgae.
Materials and methods
An axenic strain of Prorocentrum micans Ehrenberg was obtained from the Microalga Research Laboratory of the Ocean University of China, Qingdao, China. Ulva pertusa Kjellman and Ulva linza Linnaeus were collected in May 2003 from the coast of Qingdao during a bloom of these two species. The macroalgae were washed carefully in tap water to remove sediment and epiphytes. All three species were cultured in f/2 medium (Guillard & Ryther, Citation1962) at 20°C and an irradiance of 60 μmol m−2 s−1 (12 : 12 h light – dark cycle). All flasks containing the microalga were shaken twice a day to prevent wall growth. These culture conditions were used for all the growth experiments in this study. Two days prior to experiments, the Ulva species were treated with a mixture of penicillin (100 mg l−1), chloramphenicol (0.75 mg l−1), polymixin (0.75 mg l−1) and neomycin (0.9 mg l−1) for 48 h to minimize the effects of bacteria on the interactions between macroalgae and microalga during the experiments. All assay cultures in each experiment of this study were inoculated with exponential phase P. micans cells to give approximately the same starting cell densities.
Preparation of macroalgal extracts
Fresh tissue of each macroalgal species was air dried for 5 days at room temperature and then ground into a fine powder in a mortar. The dry/wet weight ratio of U. pertusa and U. linza were about 1/4 and 1/5, respectively. For the aqueous extract, 20 g dry powder was mixed with 1000 ml distilled water and shaken for 24 h at 20°C in darkness. The mixture was separated by high speed centrifugation and the extraction procedure repeated twice more with the particulate fraction. The combined aqueous extract was then evaporated to dryness under reduced pressure in a rotary evaporator and the residue re-dissolved in 100 ml distilled water to form a stock solution (200 ppt). The same protocol was followed with four organic solvents (HPLC grade methanol, acetone, ether and chloroform) starting with 50 g dry powder (stock solution 500 ppt).
P. micans growth assays
To test the effects of P. micans on each of the Ulva species, they were cultured together for 20 days. Flasks (100 ml) containing 40 ml f/2 medium and 2 × 103 cells ml−1 of P. micans were inoculated with 0.025 g wet weight of U. pertusa or U. linza and grown as above. Every fourth day, the thallus pieces were removed, washed, blotted and weighed to determine the total wet weight of the macroalgae and the cell densities of P. micans were measured. The concentrations of nitrate and phosphate in the medium were also measured, and sufficient of each was added to restore them to the initial concentrations. Macroalgal cultures without P. micans served as controls.
Fresh tissue and dry powder
Exponentially growing cells of P. micans were used to inoculated culture flasks (40 ml f/2, initial cell density 5 × 103 cells ml−1) containing fresh tissue of either U. pertusa or U. linza (0 to 10 g l−1) and these were grown together for 10 days (as above). Every other day, 2 ml samples were collected from each flask and preserved with Lugol's solution for later counting. This volume was replaced by 2 ml of a nutrient solution containing the complete ingredients for 40 ml of f/2 medium to avoid nutrient depletion and competition for nutrients between the microalga and macroalga. After 10 days co-culture, the macroalgal tissue was weighed. This assay protocol was repeated using dry powdered U. pertusa and U. linza added at the rates of 0 to 2.4 g l−1.
Aqueous and organic solvent extracts
Assays were conducted in 25-ml flasks containing 10 ml of P. micans in f/2 (initial cell density 5 × 103 cells ml−1). The stock solutions of each macroalgal extract were diluted (aqueous extracts, 10 – 160 ppt; organic solvent extracts, 25 – 400 ppt), and 100 μl of aqueous extract or 10 μl of organic solvent extract were added to the cultures (giving final concentrations of 0.1 – 1.6 ppt or 0.025 – 0.4 ppt; controls received the same volume of corresponding solvents). After 6 days' incubation, cell numbers of P. micans were counted in 1 ml samples.
Filtered, used culture medium
U. pertusa and U. linza were grown under the standard conditions for 7 days from an inoculum of 40 g l−1. After removal of the macroalgal tissue, the culture medium was filtered through autoclaved membrane filters (0.22 μm pore size), and nutrient concentrations were restored to the initial values. Culture flasks containing 40 ml of the medium were immediately inoculated with P. micans (fresh f/2 as a control). Additional flasks were inoculated and incubated as semi-continuous cultures: every other day, a 10 ml sample was removed for cell counts and 10 ml of the appropriate culture medium added. The assay ran for 10 days.
The cell density of P. micans samples was determined in a Sedgwick-Rafter counting chamber under an Olympus optical microscope. In assays with macroalgal extracts, the addition of distilled water alone did not have any effect on the growth of P. micans in the controls, but the organic solvents reduced the growth of P. micans to different extents (expressed as a percentage of the cell density of the distilled water control: methanol, 90%; acetone, 70%; ether, 85%; chloroform, 60%). As the initial cell counts for the assays differed, the final cell density of P. micans was expressed as a percentage of the cell density of the assay control. From this a value was obtained denoting the biomass of Ulva required to reduce the normal growth of P. micans by 50% (expressed as EC50 – effective concentration, g l−1). Where only initial and final cell counts were made, the mean growth grate (μ, %day−1) of P. micans for each assay was calculated as: μ = 100(Nt-N0)/N0t, where N0 and Nt are the cell density of the microalga at the beginning and the end of the experiment, and t (days) the duration of the experiment.
Results
Effects of co-culture on macroalgal growth
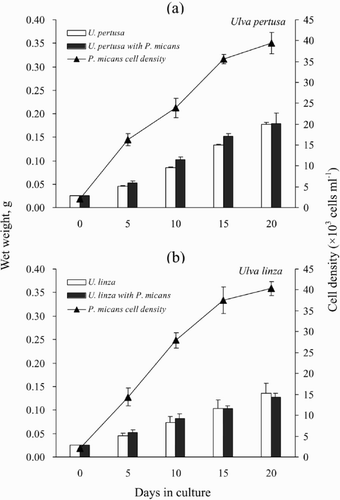
The wet weights of Ulva pertusa and Ulva linza grown in the presence and absence of Prorocentrum micans during the 20-day co-culture experiment are shown in . Despite the high cell density of P. micans during the last 5 days of this experiment (about 4 × 104 cells ml−1), neither the biomass of U. pertusa (), nor that of U. linza () was significantly lower than that in the controls.
Effects of fresh or powdered dry macroalgal tissue on the growth of P. micans
When the initial inoculation of fresh tissue of either U. pertusa or U. linza was greater than 0.625 g l−1, the final cell density and the mean growth rates of P. micans were lower than those of the controls (; ANOVA, p < 0.001). The greater the mass of macroalgal tissue added to the cultures, the lower the final cell density of P. micans (). At the two highest inoculation masses of fresh tissue of either macroalga (5 and 10 g l−1), the cultures of P. micans could not sustain normal growth and, at 10 g l−1, the cultures of P. micans had died by the end of the experiment (). The EC50 values for fresh tissue of U. pertusa or U. linza were 1.8 g l−1 and 2.3 g l−1 respectively.
Fig. 2. Effects of fresh tissue or dry powder of Ulva on the growth of Prorocentrum micans. (a) P. micans cultured with fresh tissue of U. pertusa or U. linza. (b) P. micans cultured with dry powder of U. pertusa or U. linza. The data are presented as means ± SE (n = 4).
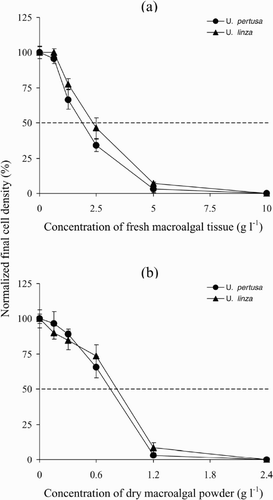
Adding more than 0.15 g l−1 of either species of Ulva in powdered form to the P. micans cultures resulted in significantly reduced final cell density values and mean growth rates (; ANOVA, p < 0.01). At the two highest dosages of powder of either macroalga (1.2 and 2.4 g l−1), cultures of P. micans gradually died and had died completely within 4 days of the addition of 2.4 g l−1 powder. The EC50 values for powdered U. pertusa or U. linza were 0.7 g l−1 and 0.8 g l−1, respectively. These values are the equivalent of 2.9 and 3.9 g l−1 of fresh tissue.
Effects of macroalgal extracts on the growth of P. micans
The final cell density of P. micans grown for 6 days with the addition of aqueous extracts from either U. pertusa or U. linza was significantly reduced (Tukey's, p < 0.01) when the concentration of the aqueous extracts was higher than 0.1 ppt (, ). With the addition of increasing concentrations of extract there was a concomitant decrease in the final cell density of P. micans. The EC50 values for aqueous extracts from U. pertusa or U. linza were 0.7 ppt and 1.0 ppt, respectively.
Fig. 3. Effects of Ulva tissue extracts on the growth of Prorocentrum micans. (a) P. micans cultured with tissue extracts from U. pertusa. (b) P. micans cultured with tissue extracts from U. linza. The data are presented as means ± SE (n = 4).
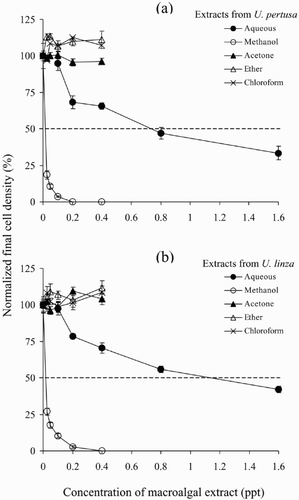
The addition of methanol extract to the assay cultures resulted in a significantly reduced final cell density at all concentrations used (Tukey's, p < 0.001; , ) and again, at increasing concentrations there was a concomitant decrease in the final cell density of P. micans. With doses of 0.2 and 0.4 ppt for U. pertusa and 0.4 ppt for U. linza, cultures of P. micans died completely during the experiment. The EC50 values for methanol extracts from U. pertusa and U. linza were 0.015 ppt and 0.017 ppt, respectively. In contrast, the final cell density of P. micans was not reduced by the addition of any concentration of acetone, ether or chloroform extract from either of the Ulva species. (Tukey's, p > 0.05).
Effects of used culture medium on the growth of P. micans
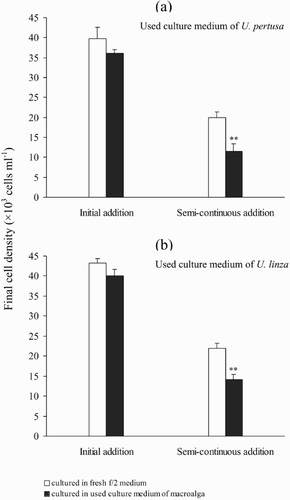
There was no significant difference (Tukey's, p > 0.05) between the final cell density of P. micans cultures treated with an initial dose of filtered used culture medium and those of the controls. However, when cultures were treated with a semi-continuous addition of used culture medium from either of the Ulva spp., the growth of P. micans was significantly reduced (Tukey's, p < 0.01; , ).
Fig. 4. Final cell densities of Prorocentrum micans with initial or semi-continuous addition of used culture medium from Ulva cultures. (a) P. micans cultured in used culture medium of U. pertusa. (b) P. micans cultured in used culture medium of U. linza. Asterisks marked on the histograms indicate statistically significant reductions in the final cell density (*p < 0.05, **p < 0.01 & ***p < 0.001; Tukey post-hoc tests). The data are presented as means ± SE (n = 4).
Discussion
Allelopathy, as defined by Molisch (Citation1937) is the biochemical interaction, both stimulatory and inhibitory, between primary producers or between primary producers and microorganisms. In comparison with the intensive and extensive studies on the allelopathic interactions among terrestrial plants, the knowledge of allelopathy in aquatic plant communities is still fragmentary (Gopal & Goel, Citation1993; Körner & Nicklisch, Citation2002; Gross, Citation2003). This is partially because it is technically difficult to provide direct evidence for allelopathic interactions in aquatic ecosystems under natural conditions. Other processes and factors, such as resource competition and change of environmental parameters, can mask the ecological advantages of certain aquatic organisms over others gained through allelopathy (Keating, Citation1977; Sukenik et al., Citation2002). It is therefore essential that attempts to identify allelopathic interactions among aquatic organisms should be conducted in a controlled system.
In the present study, we conducted laboratory experiments under controlled environmental conditions, preventing nutrient and light competition, and the possible effects of bacteria, to investigate the allelopathic effects of Ulva pertusa and Ulva linza on cultures of Prorocentrum micans. A recognized effect of growing macroalgae in culture is that they may increase the pH of the culture medium, making it unsuitable for the growth of microalgae in co-culture. Schmidt & Hansen (Citation2001) investigated the effect of pH on the immobilization of Heterocapsa triquetra cells by Chrysochromulina polylepis and noted that pH had a dramatic effect on H. triquetra. In our experiments, pH of the culture medium was measured at the beginning and the end of the experiment but the increase in pH was too small to contribute to the growth inhibition of the P. micans cultures. The secretion of allelopathic substances by both U. pertusa and U. linza is the most likely explanation for the observed growth inhibition of P. micans during the co-culture experiments.
The pattern of the results in both the co-culture experiment with fresh tissue and the P. micans assays with the filtrate of used macroalgal culture medium, suggest that both U. pertusa and U. linza release small quantities of rapidly degradable allelopathic substances, so that a continuous allelochemical secretion from the fresh tissue is required to inhibit the growth of P. micans. Nakai et al. (Citation1999) demonstrated that the growth inhibition of cyanobacteria by the macrophyte Myriophyllum spicatum needed a continuous secretion of some unstable, growth-inhibiting compounds, and they also found that the growth of Microcyctis aeruginosa was not inhibited by the initial addition of used culture medium from cultures of M. spicatum, whereas a quasi-continuous addition resulted in growth inhibition. The growth of Scenedesmus acutus was reduced by Chara aspera only when Chara was present in the medium during the experiment (van Donk & van de Bund, Citation2002); when the Scenedesmus was inoculated into medium in which Chara had been grown but then removed prior to the growth experiment, no allelopathic effect could be demonstrated. These phenomena are very similar to ours, suggesting that the continuous secretion by primary producers of small quantities of possibly unstable allelochemicals may not be uncommon in aquatic ecosystems.
The aqueous and the methanol extracts from both species of Ulva exhibited strong inhibitory effects on the growth of P. micans, whereas the extracts with less polar organic solvents had no apparent effect. This suggests that the allelochemicals extracted had relatively high polarities. It is noteworthy that the methanol extracts from both species of Ulva showed the highest allelopathic activity. Based on the EC50 values, the methanol extracts are approximately 50 times more effective than the aqueous extracts.
Although toxic properties have rarely been associated with the bloom-forming green macroalgae, there is increasing evidence that these macroalgae produce chemical defences against herbivores (van Alstyne, Citation2001; van Alstyne et al., Citation2001), and that their extracts have allelopathic properties (Nelson et al., Citation2002). The present study has demonstrated that the bloom-forming green macroalgae U. pertusa and U. linza have detrimental allelopathic effects on the red tide microalga P. micans. Therefore, we speculate that the chemical interactions between the bloom-forming macroalgae and other coastal organisms may play an important role in determining species compositions and dominance patterns during macroalgal blooms, and may partially explain the widespread success of the bloom-forming green macroalgae in coastal areas.
Both green tides and red tides are harmful to marine ecosystems and the coastal economy, and many studies have begun to concentrate on the control of such undesirable algal growth. In this study, it has been demonstrated that two bloom-forming green macroalgae, U. pertusa and U. linza, can secrete some kind of allelopathic substance or substances and that these can inhibit the growth of the red tide microalga P. micans. Our results not only provide insight into chemical interactions between macroalgae and microalgae from coastal areas but also suggest a novel method of utilizing bloom-forming green macroalgae against the possibly more economically deleterious red tide species. The harvesting and processing of the macroalgal biomass as a contribution to the control and management of red tides in confined areas would also contribute to the local economy. Isolation and characterization of the allelochemicals from these two green macroalgae will be carried out in our future research.
Acknowledgements
The authors thank Dr. Daryl Anne Birkett for her great help with manuscript revision, and the colleagues in our laboratory for their kind assistances in collecting and treating U. pertusa and U. linza. This work was supported by NSFC for Talented Youths (397250239) and the Project under Major State Basic Research of China (G1999012011).
References
References
- Adams , JA , Seaton , DD , Buchanan , JB and Longbottom , MR . 1968 . Biological observations associated with the toxic phytoplankton bloom off the East Coast . Nature , 220 : 24 – 25 .
- Fletcher , RL , Cuomo , V and Palomba , I . 1990 . The ‘green tide’ problem, with particular reference to the Venice Lagoon . Br. Phycol. J. , 25 : 87 – 87 .
- Gopal , B and Goel , U . 1993 . Competition and allelopathy in aquatic plant communities . Bot. Rev. , 59 : 155 – 210 .
- Gross , EM . 2003 . Allelopathy of aquatic autotrophs . Crit. Rev. Plant. Sci. , 22 : 313 – 339 .
- Guillard , RRL and Ryther , JH . 1962 . Studies on marine planktonic diatoms. I. Cyclotella nana (Hustedt) and Detonula confervaceae (Cleve) . Can. J. Microbiol. , 8 : 229 – 239 .
- Hayden , HS , Blomster , J , Maggs , CA , Silva , PC , STANHope , MJ and Waaland , JR . 2003 . Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera . Eur. J. Phycol. , 38 : 277 – 294 .
- Hernandez , I , Peralta , G , Perez-Llorens , JL and Vergara , JJ . 1997 . Biomass and dynamics of growth of Ulva species in Palmones River estuary . J. Phycol. , 33 : 764 – 772 .
- Horner , RA , Garrison , DL and Plumley , FG . 1997 . Harmful algal blooms and red tide problems on the U. S. west coast . Limnol. Oceanogr. , 42 : 1076 – 1088 .
- Inderjit and Dakshini , KMM . 1994 . Algal allelopathy . Bot. Rev. , 60 : 182 – 196 .
- Jeong , JH , Jin , HJ , Sohn , CH , Suh , KH and Hong , YK . 2000 . Algicidal activity of the seaweed Corallina pilulifera against red tide microalgae . J. Appl. Phycol. , 12 : 37 – 43 .
- Keating , KI . 1977 . Allelopathic influence on blue-green bloom sequence in a eutrophic lake . Science , 196 : 885 – 887 .
- Keating , KI . 1978 . Blue-green algal inhibition of diatom growth: transition from mesotrophic to eutrophic community structure . Science , 199 : 971 – 973 .
- Kim , HG . 1997 . Recent harmful algal blooms and mitigation strategies in Korea . Ocean Res. , 19 : 185 – 192 .
- Körner , S and Nicklisch , A . 2002 . Allelopathic growth inhibition of selected phytoplankton species by submerged macrophytes . J. Phycol. , 38 : 862 – 871 .
- Lotze , HK and Schramm , W . 2000 . Ecophysiological traits explain species dominance patterns in macroalgal blooms . J. Phycol. , 36 : 287 – 295 .
- Molisch H 1937 Der Einfluss einer Pflanze auf die andere-Allelopathie, Fischer Jena
- Nakai , S , Hosomi , M , Okada , M and Murakami , A . 1996 . Control of algal growth by macrophytes and macrophyte-extracted bioactive compounds . Wat. Sci. Tech. , 34 : 227 – 235 .
- Nakai , S , Inoue , Y , Hosomi , M and Murakami , A . 1999 . Growth inhibition of blue-green algae by allelopathic effects of macrophytes . Wat. Sci. Tech. , 39 : 47 – 53 .
- Nakai , S , Inoue , Y , Hosomi , M and Murakami , A . 2000 . Myriophyllum spicatum-released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa . Water Res. , 34 : 3026 – 3032 .
- Nelson , TA and Lee , A . 2001 . A manipulative experiment demonstrates that blooms of macroalga Ulvaria obscura can reduce eelgrass shoot density . Aquat. Bot. , 71 : 149 – 154 .
- Nelson , TA , Lee , D , Smith , BC and Prins , R . 2002 . Are ‘green tides’ harmful algal blooms? allelopathic properties of extracts from Ulva fenestrata and Ulvaria obscura . J. Phycol. , 38 ( Suppl. ) : S28 – S29 .
- Qi , Y , Zhang , Z , Hong , Y , Lu , S , Zhu , C and Li , Y . 1993 . Occurrence of red tides on the coasts of China . Toxic Phytoplankton Blooms Sea , 3 : 43 – 46 .
- Raffaelli , D , Raven , J and Poole , L . 1998 . Ecological impact of green macroalgal blooms . Oceanogr.Mar. Biol. Annu. Rev. , 36 : 97 – 125 .
- Schagerl , M , Unterrieder , I and Angeler , DG . 2002 . Allelopathy among cyanoprokaryota and other algae originating from lake Neusiedlersee (Austria) . Internat. Rev. Hydrobiol , 87 : 365 – 374 .
- Schmidt , LE and Hansen , PJ . 2001 . Allelopathy in the prymnesiophyte Chrysochromulina polylepis: effect of cell concentration, growth phase and pH . Mar. Ecol., Prog. Ser. , 216 : 67 – 81 .
- Smayda , TJ . 1997 . Harmful algal blooms: their ecophysiology and general relevance to phytoplankton blooms in the sea . Limnol. Oceanogr. , 42 : 1137 – 1153 .
- Sukenik , A , Eshkol , R , Livne , A , Hadas , O , Rom , M , Tchernov , D , Vardi , A and Kaplan , A . 2002 . Inhibition of growth and photosynthesis of the dinoflagellate Peridinium gatunense by Microcystis sp. (cyanobacteria): a novel allelopathic mechanism . Limnol. Oceanogr. , 47 : 1656 – 1663 .
- Sun , W , Yu , Z and Yu , S . 1989 . The harness of an eutrophic water body by water-hyacinth . Acta Scientiae Circumstantiae , 9 : 188 – 195 .
- Szczepanski , AJ . 1977 . Allelopathy as a means of biological control of water weeds . Aquat. Bot. , 3 : 193 – 197 .
- Taylor , R . 1999 . The green tide threat in the UK–a brief overview with particular reference to Langstone harbour, south coast of England and the Ythan estuary, east coast of Scotland . Bot. J. Scotl. , 51 : 195 – 295 .
- Taylor , R , Fletcher , RL and Raven , JA . 2001 . Preliminary studies on the growth of selected ‘green tide’ algae in laboratory culture: effects of irradiance, temperature, salinity and nutrients on growth rate . Bot. Marina , 44 : 327 – 336 .
- Valiela , I , McClelland , J , Hauxwell , J , Behr , PJ , Hersh , D and Foreman , K . 1997 . Macroalgal blooms in shallow estuaries: controls and ecophysiological and ecosystem consequences . Limnol. Oceanogr. , 42 : 1105 – 1118 .
- Van Alstyne , KL . 2001 . Are bloom-forming green algae chemically defended? . J. Phycol. , 37 ( Suppl. ) : S50
- Van Alstyne , KL , Wolfe , GV , Freidenburg , TL , Neill , A and Hicken , C . 2001 . Activated defense systems in marine macroalgae: evidence for an ecological role for DMSP cleavage . Mar. Ecol. Prog. Ser. , 213 : 53 – 65 .
- Van Donk , E and Van de Bund , WJ . 2002 . Impact of submerged macrophytes including charophytes on phyto- and zooplankton communities: allelopathy versus other mechanisms . Aquat. Bot. , 72 : 261 – 274 .
- Zhu , M , Li , R , Mu , X and Ji , R . 1997 . Harmful algal blooms in China Seas . Ocean Res. , 19 : 173 – 184 .