Abstract
The physiology (growth rate, biochemical composition and flotation rate) of the toxic cyanobacterium Anabaena circinalis was studied in relation to irradiance and mixing regime in two small-scale (250 ml or 1 l) batch culture experiments and one medium scale (1 m high, 4.4 l), semi-continuous microcosm experiment. In batch cultures, A. circinalis had a relatively high irradiance requirement as indicated by its compensation irradiance, E c (13 ± 2 μmol m−2 s−1). The growth efficiency (αg) of A. circinalis, estimated as the initial slope of the growth-irradiance curve , was in the mid to high range depending on the model applied (Monod: 0.33 ± 0.19 m2 mol−1, exponential: 0.43 ± 0.15 m2 mol−1). The flotation rate of the A. circinalis population was 0.69 ± 0.11 m d−1 at 100 μmol m−2 s−1 and decreased at higher irradiance to be negative at irradiances over 135 μmol m−2 s−1. In a microcosm experiment, different mixing intervals were tested (10 min (MIXED) and 48 h (CALM)) across three irradiance treatments. The irradiance treatments differed in both their degree of vertical light attenuation and mean integrated irradiance. The mixing regime had no effect on growth rate in the high irradiance (HE) and medium irradiance (ME) treatments (mean integrated irradiances 60 and 15 μmol m−2 s−1, respectively). However, an increase in A. circinalis flotation rate in the lowest irradiance treatment (LE; mean integrated irradiance 7 μmol m−2 s−1) resulted in a 44% higher growth rate in the CALM microcosm through access to greater irradiance. This suggests that short stable periods (e.g. diurnal stratification) in highly light limited treatments will assist the growth of A. circinalis due to its ability to gain ready access to light in the euphotic zone.
Introduction
Photoacclimation by phytoplankton provides a means to make efficient use of periodically limiting irradiance (Lewis et al., Citation1984 a,Citation b ; Falkowski et al., Citation1985; Tilzer, Citation1987) and a mechanism to limit photoinhibitory damage at high irradiances (Raven, Citation1984; Geider et al., Citation1998). Photoacclimation responses can be modelled and used to predict growth and behaviour in nature (Cullen, Citation1990). Such models provide an understanding of the dynamics of phytoplankton growth and bloom formation, that has particular significance in its application to noxious bloom-forming cyanobacteria. Photoacclimation responses such as changes in the content and relative composition of pigments (Raps et al., Citation1983; Post et al., Citation1984; Wyman & Fay, Citation1986), the chl a : C ratio (Raps et al., Citation1983; Falkowski et al., Citation1985; Langdon, Citation1987; Cullen & Lewis, Citation1988) and the general biochemical composition (Geider et al., Citation1985; Thompson, Citation1999) are ubiquitous amongst phytoplankton. Many cyanobacteria also respond to changes in irradiance and recent light history by altering buoyancy (e.g. Kromkamp & Mur, Citation1984; Kromkamp & Walsby, Citation1990; Ibelings et al., Citation1991; Kinsman et al., Citation1991; Visser et al., Citation1997).
Fluctuations in cyanobacterial buoyancy may be due to either the collapse of gas vesicles under high cellular turgor pressures (Oliver & Walsby, Citation1984), the dilution of gas vesicles as a result of growth (Konopka et al., Citation1987), the accumulation of carbohydrate to create ballast (Konopka et al., Citation1987; Kinsman et al., Citation1991) or a combination of these. The process considered most often to influence buoyancy in nature is the carbohydrate ballast mechanism (Kinsman et al., Citation1991; Walsby, Citation1994), in which exposure to high irradiance produces relatively heavy carbohydrate through photosynthesis, counteracting the buoyancy of gas vesicles.
Buoyancy may provide cyanobacteria a substantial growth advantage, particularly in turbid conditions, by allowing access to light in surface waters during periods of thermal stratification (Humphries & Lyne, Citation1988; Sherman & Webster, Citation1994). The turbidity of Australian rivers, lakes and impoundments is typically high to very high (Kirk, Citation1994), often caused by large quantities of particulate clays (Webster et al., Citation2000). Irradiance available to phytoplankton varies greatly in many Australian waters in response to the dramatic fluctuations in light attenuation coefficients associated with changes in river flow conditions (Oliver, Citation1990; Hötzel & Croome, Citation1996). The initiation of Anabaena circinalis Rabenhorst blooms in Australian river weir-pools has generally been associated with the establishment of persistent (approximately 14 days) thermal stratification (Sherman et al., Citation1998). In addition, recent work indicates that the buoyancy of A. circinalis provides it with a significant photosynthetic advantage in a diurnally mixed regime relative to a mixed regime in highly turbid conditions in the Darling River, Australia (Mitrovic et al., Citation2001).
Despite theoretical (Humphries & Lyne, Citation1988) and field (Walsby et al., Citation1997; Mitrovic et al., Citation2001) evidence that cyanobacteria gain a photosynthetic advantage from buoyancy, there has never, to the best of our knowledge, been an experimental demonstration of an increase in growth due to buoyancy in A. circinalis. In the present experiments, using a cultured strain of A. circinalis, we aimed to determine the effects of changes in light availability on its growth, buoyancy and biochemistry. The study was undertaken with flask-scale experiments to define relationships between irradiance, growth, buoyancy and composition in A. circinalis. A microcosm experiment was also undertaken in one of the first attempts to test the interaction of two major factors (irradiance and mixing) simultaneously in a realistic fashion. Three vertically attenuated irradiance treatments were established sequentially with time, to simulate a range of light conditions frequently encountered in Australian rivers, weir pools and reservoirs. Two mixing treatments were maintained for the duration of the experiment at either 10 min (MIXED) or 48 h (CALM) intervals. We tested the hypothesis that growth of A. circinalis would be significantly higher with short, regular periods of stability (CALM treatment) than with continuous mixing (MIXED treatment). In addition we tested the hypothesis that increased light limitation would provide a greater relative growth advantage in the CALM treatment due to the greater buoyancy of cells at low irradiance.
Materials and methods
General
A filamentous, non-colonial strain of the cyanobacterium Anabaena circinalis (ACMB13) was obtained from the CSIRO Collection of Living Microalgae (www.marine.csiro.au/microalgae). The strain was originally isolated in 1993 by W. Van Dok from Mt. Bold Reservoir, South Australia. All inoculum and experimental cultures were grown at 20°C under a 16 : 8 h L : D light cycle with cool white fluorescent tubes (Sylvania).
Irradiance experiments
Two irradiance experiments used inocula of A. circinalis acclimated to experimental conditions for two transfers. The volume of inoculum was determined so that the initial cell concentration was about 3 × 104 cells ml−1. For experiment 1 (growth-irradiance experiment), batch cultures were grown in 250-ml Erlenmeyer side-arm flasks at irradiances in the range 18–450 μmol m−2 s−1, and growth rate and biochemical composition determined. Three or four replicates were grown at each of seven irradiances. Cultures were grown in modified WC medium (McCausland et al., Citation2001) with an additional 5 mM HEPES to aid with pH control (Smith & Foy, Citation1974), and were mixed daily.
In experiment 2 (flotation-irradiance experiment), cultures were grown in triplicate in 500 ml of modified WC medium in 1-l Erlenmeyer flasks at 100, 135, 150, and 200 μmol m−2 s−1 to determine the relationship between flotation rate and irradiance.
Microcosm experiment: irradiance and mixing
In experiment 3 (microcosm experiment), 420 ml of A. circinalis inoculum culture was added to each of six transparent acrylic tubular microcosms (1 m high × 0.075 m diam.; capacity 4.4 l), and filled to a volume of 4.2 l with additional WC medium. This experiment was conducted under clean laboratory, but non-axenic conditions. The microcosms were illuminated with halogen lamps with dichroic reflectors (50 W General Electric Multi-Mirror Lamp), with a 16 : 8 h L : D cycle. Three irradiance treatments were established sequentially over time, High Irradiance (HE) → Medium Irradiance (ME) → Low Irradiance (LE), to simulate a range of conditions experienced in nature (, ). Vertical irradiance profiles in the microcosms were measured with a Biospherical Instruments QSL-100 PAR meter with a 4π collector ().
Fig. 1. Vertical irradiance distribution in microcosms under HE (square), ME (diamond) and LE (circle) conditions. The depressed irradiance at the surface of the HE and ME conditions is due to some shading from the top edge of the microcosm.
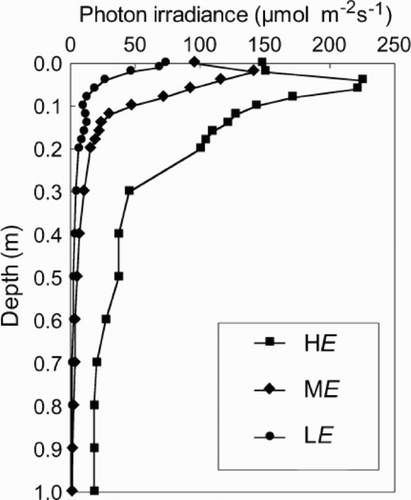
Table 1 . Summary of light conditions inside the microcosms in each of the three irradiance treatments. Data are the mean irradiance through the column depth, and the vertical light attenuation coefficient (K d) determined over the first 0.3 m and the whole column (1 m)
Irradiance treatments were established by adding black plastic sheeting to the lower region (90–99%) of the microcosms and changing the angle and distance of the light source. Shading from the top edge of the microcosm, caused by the high angle of the light source, depressed irradiance in the top 20 mm of the water column of the HE and ME treatments (). However, from 20 mm depth, there was an approximately exponential attenuation of light. Two mixing treatments of 10 min (MIXED treatment) and 48 h (CALM treatment), each with three replicates, were maintained for the duration of the experiment. The mixing apparatus consisted of an electronically controlled winch system that lowered and raised perforated stainless steel plungers (70 mm diameter) through the depth of the microcosms at the prescribed mixing intervals (McCausland et al., Citation2001).
The microcosm cultures were maintained semi-continuously with the addition of modified WC medium (McCausland et al., Citation2001) ensuring nutrient–replete conditions. The first sampling followed the establishment of a steady-state in the HE conditions. The volume removed at each sampling was calculated to return the cell concentration to 3 × 104 cells ml−1. Control of pH was accomplished using low cell concentrations and ensuring that the exchange medium had a pH of ∼6.8, by bubbling for a few seconds with 0.2 μm filtered CO2. The pH of all cultures was ∼7.3 following medium replenishment, and rose to 7.7–8.7 at the time of sampling in the ME and LE conditions, and to 8.9–9.5 after 48 h in HE.
Growth determination and curve fitting
In experiment 1, growth rate was estimated from in vivo chlorophyll a (chl a) fluorescence, measured with a Turner Model 10 fluorometer at the same time each day on the flask side-arm tubes. Growth rates were calculated as regressions of loge chl a fluorescence vs. time for each culture. Growth irradiance data were fitted to exponential (after Geider, Citation1987)
In the microcosm experiment, specific growth rates (d−1) were calculated for the period between sampling (Guillard, Citation1973) using cell concentrations at the start and end of each period. The period between sampling depended on the irradiance treatment (), which was timed to approximate similar generation times for each irradiance treatment and to coincide with the 48 h mixing regime in the CALM treatment.
Biochemical analyses
Cultures were sampled in logarithmic growth phase (Experiment 1) or immediately after mixing in the microcosms (Experiment 3). Sub-samples were fixed in Lugol's solution (Throndsen, Citation1978), and counted in Sedgwick–Rafter chambers. Prior to counting, trichomes were broken into smaller units by sonication (McCausland et al., Citation2001). For chl a determination, sub-samples (15–265 ml) were filtered onto 25 mm glass-fibre filters (Whatman GF/C) and stored at − 20°C for 2 months prior to analysis. Chlorophyll a was extracted from filters in 90% acetone and determined spectrophotometrically (Jeffrey & Humphrey, Citation1975). Sub-samples (4–19 ml) for the determination of particulate organic carbon (POC) were filtered onto pre-combusted 13 mm glass-fibre filters (Gelman A/E), dried at 50°C for 48 h and stored for 1–2 months under vacuum prior to analysis. POC analyses were performed on a Carlo Erba Elemental Analyser (EA1108) at the Central Science Laboratory, University of Tasmania. Carbohydrate analyses were performed only in the microcosm experiment. Sub-samples (60–200 ml) were filtered onto 25 mm glass-fibre filters (Whatman GF/C) and stored at − 20°C. These were later hydrolysed with 3.9 ml of 0.5 M H2SO4 at 100°C for 4 h in polypropylene centrifuge tubes. Carbohydrate concentrations in the hydrolysed extracts were estimated using the phenol-sulphuric acid method (Dubois et al., Citation1956) against a glucose standard curve.
Flotation rate and biomass distribution
For experiments 2 and 3, flotation rate was measured in triplicate on log-phase batch (experiment 2) or post-mixing microcosm (experiment 3) cultures in water-jacketed, temperature-controlled (20°C) SETCOL columns (vol. 370 ml) (Bienfang, Citation1981). We altered the method (Waite et al., Citation1992) to account for additional growth during the flotation rate measurement period. For both experiments, flotation rate was determined on samples added to the SETCOL columns at 12:00 h. The culture material was allowed to stand undisturbed for 3 to 4 h, allowing cells time to rise or sink. The top, middle and bottom sections of the SETCOL columns were then individually sampled (19–300 ml), and filtered on to 25 mm Whatman GF/C filters. Flotation rates of cells in experiment 2 were based on dry weight measurements (Bienfang, Citation1981; McCausland et al., Citation2001), however in experiment 3, chlorophyll a was used as the measure of biomass. In experiment 3, filters were stored at − 20°C for 2–3 months before being analysed fluorometrically for chl a. Extractions were made in 90% acetone and then determined on a calibrated Turner Model 10 fluorometer. The measurement of chlorophyll a by fluorometry is significantly more sensitive (Jeffrey & Welschmeyer, Citation1997) than dry weight, and provided accurate determination of flotation rate where biomass was low in the ME and LE microcosm treatments.
Changes in vertical biomass distribution within the microcosms were determined from samples (21–92 ml) collected in the CALM microcosms, just prior to the 48 h mixing, at three depths (0.02, 0.50, 0.95 m) in both the HE and ME treatments. The samples were taken at Day 2 (HE conditions) and Day 20 (ME conditions), 8 days following the reduction in irradiance. Samples were removed from each depth using a 3 mm diameter silicon tube and syringe, and filtered onto 25 mm Whatman GF/C filters. These were stored at − 20°C for 1 month prior to analysis. Chlorophyll a was used as the biomass measure, extracted in 90% acetone and measured on a calibrated Turner Model 10 fluorometer.
Results
Irradiance experiments
Growth rate
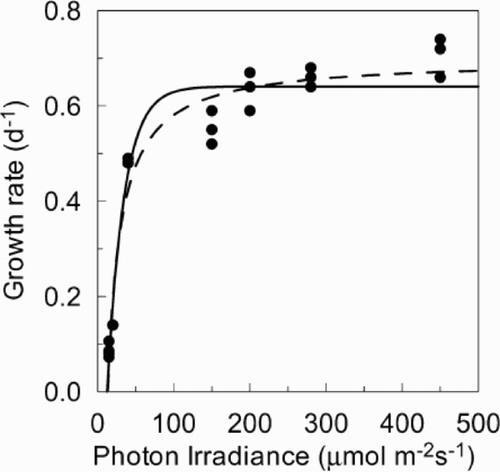
In experiment 1, the exponential and Monod models were identical in their goodness-of-fit (; r 2 = 0.96 for both). Using the exponential model we estimated the following parameters (± 95% confidence interval): E k for growth = 22 ± 7 μmol m−2 s−1, μmax = 0.64 ±0.03 d−1 and E c = 13 ± 2 μmol m−2 s−1. Using the Monod model these parameters were: E k for growth = 31 ± 8 μmol m−2 s−1, μmax = 0.70 ±0.04 d−1 and E c = 13 ± 2 μmol m−2 s−1. The initial slope of the curve (αg = μmax/E k, ‘growth efficiency’), corrected for the 16 h photoperiod, was 0.44 ± 0.15 m2 mol photons−1 for the exponential curve and 0.34 ± 0.19 m2 mol photons−1 for the Monod curve. The estimates made by each model of these parameters were not significantly different from each other.
Chorophyll a and carbon quotas
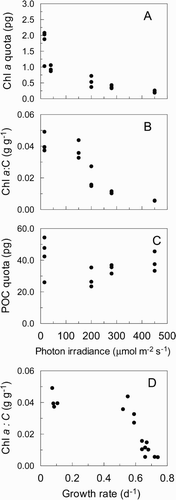
The contents per cell (quota) of chl a in experiment 1 were high at low irradiances, and appeared to decrease exponentially with irradiance (). The chl a : C ratio () appeared to differ from the chl a quota in its relationship to irradiance, in having high and relatively steady values up to 150 μmol m−2 s−1, followed by an exponential decline at irradiances > 150 μmol m−2 s−1. The carbon cell quota showed a three-fold variation, with no pattern relating to changing irradiance ().
The relationship of the chl a : C ratio with growth () follows the same pattern as in many phytoplankton (Langdon, Citation1988). Over most of the growth limiting irradiance, the relationship remains steady until at approximately 80% μmax, (0.52 or 0.64 d−1 depending on the model applied) there is an asymptotic decrease in chl a : C.
Flotation rate
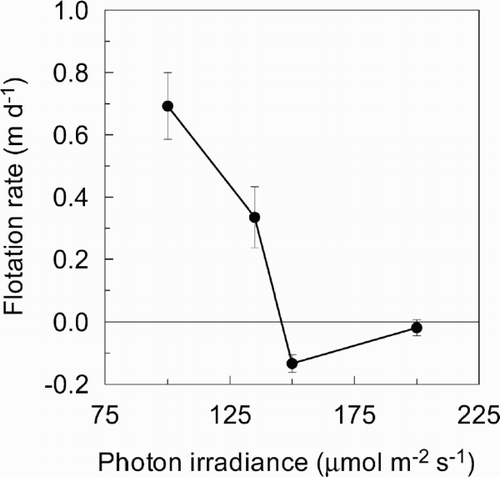
The net flotation rate of A. circinalis in experiment 2 decreased with increased irradiance (). A mean flotation rate of 0.69 m d−1 at 100 μmol m−2 s−1 decreased to a slightly negative flotation rate of − 0.13 m d−1 at 150 μmol m−2 s−1. However, at 200 μmol m−2 s−1, the flotation rate was not significantly different from zero.
Microcosm experiment
Physiological relationships with irradiance and mixing
The use of fixed effects 2-way ANOVAs where irradiance and mixing were the factors being analysed indicated that growth rate (p < 0.001), flotation rate (p = 0.02), carbohydrate quota (p < 0.001) and the chl a : carbohydrate ratio (p < 0.001) differed significantly with irradiance (HE, ME, LE). However none of the measured response factors differed significantly with mixing (CALM, MIXED). There was no significant interaction between mixing and irradiance.
Growth rate reduced substantially in the ME and LE treatments when the irradiance was reduced from HE. (, ). Flotation rate increased by around 200% following the reduction in irradiance (HE to ME). Evidence of this change in flotation rate was observed 17 h after the reduction in irradiance, as a layer of cells on the surface of the water column in the CALM treatment, where previously there had been none. This obvious layer of cells was constantly present in the CALM mixing regime in the ME and LE treatments. This change in the vertical distribution of biomass was confirmed by the distribution of chl a (), which at 2 cm depth (standardized to cell concentration) was 17 times greater in the ME treatment on day 20 (411 μg l−1) than in the HE treatment on day 2 (24 μg l−1). Carbohydrate cell quotas averaged 12.0 pg in both mixing treatments at HE and fell 40% following the transition to ME. Carbohydrate quotas in ME and LE treatments were similar, and showed no further decline with the reduction in irradiance ().
Fig. 5. Growth rates of Anabaena circinalis in microcosms under 10 min (MIXED) and 48 h (CALM) mixing regimes as light was sequentially reduced (HE→ME→LE). The L : D cycle was 16 : 8 h and temperature 20°C. Data points are means of three replicate cultures, and error bars are ± 1 S.D.
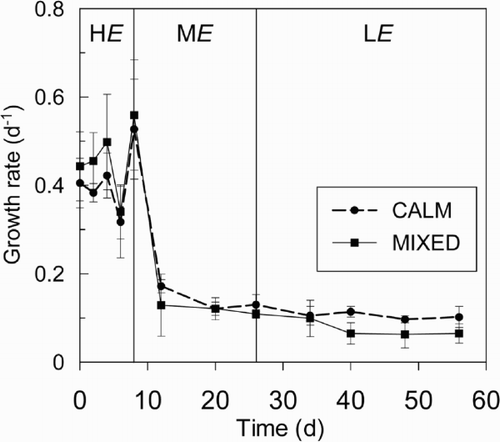
Fig. 6. Physiological measurements of Anabaena circinalis from the microcosm experiment for three irradiance treatments. (A) chlorophyll a quota (n: HE = 12, ME = 6, LE = 9), (B) carbon quota (n: HE = 9, ME = 6, LE = 9) (C) chl a : carbon ratio (n: HE = 9, ME = 6, LE = 9), (D) carbohydrate quota (n: HE = 12, ME = 6, LE = 3), (E) chl a : carbohydrate ratio (n: HE = 12, ME = 6, LE = 3), and (F) flotation rate (n = 3). Individual t-test comparisons of mixing regimes (MIXED and CALM) were performed for each irradiance treatment, and significant differences indicated with an asterisk. Photoperiod and temperature were 16 : 8 h L : D and 20°C, respectively. Error bars are ± 1 S.D.
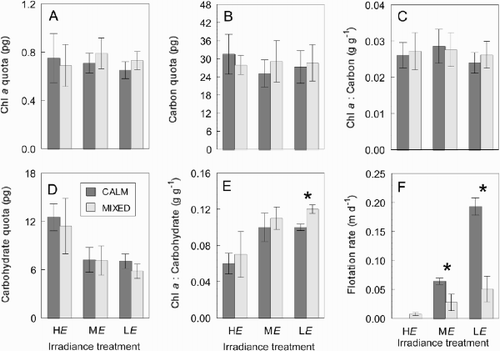
Fig. 7. Depth distribution of chlorophyll a in microcosms of Anabaena circinalis grown under (A) HE conditions on day 2 and (B) ME conditions on day 20. Data points are means of thee replicate cultures, and error bars are ± 1 S.D.
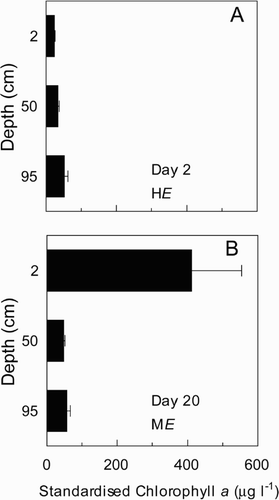
Though ANOVA indicated no significant differences in the cell physiology as a result of mixing across the full range of irradiances, analysis within individual irradiance treatments using Student's t-test, revealed some significant differences. Growth rates were similar for CALM and MIXED regimes in the HE and ME treatments (, ). However in the LE treatment, the mean growth rate was 44% greater (p = 0.006) in the CALM treatment (, ). Flotation rate was also significantly higher under the CALM mixing regime in both the ME (p = 0.05) and LE (p = 0.003) treatments (). In the LE treatment, the dfference in flotation rate was especially marked; the rate was approximately four times greater in the CALM than the MIXED regime. Flotation rates in the HE treatment could not be compared between mixing regimes, as it was only determined for the MIXED treatment. The only significant difference in cell composition was observed in the LE treatment for the Chl a : carbohydrate ratio (p = 0.05), which was 20% higher in the MIXED regime.
Discussion
Growth–irradiance relationship
The growth–irradiance relationship is fundamental to estimate phytoplankton growth in natural systems (Cullen, Citation1990) and can be used to compare the competitive ability of species across a range of light conditions (Langdon, Citation1988). The type of model used to describe this relationship may provide substantially different predictions (Bannister, Citation1990). We have applied both the exponential and Monod models to our data, with similar results. Previous work with the diatom Thalassiosira pseudonana (Thompson, Citation1999) and several cyanobacteria (Foy et al., Citation1976) indicated that longer days provide more abrupt changes in growth-irradiance curves, better suited to description by an exponential model. A daylength of around 16 h appeared to be transitional between the appropriate use of either the Monod or the more abrupt exponential model for T. pseudonana (Thompson, Citation1999). The similar goodness-of-fit of both curves to our data for A. circinalis grown in 16 : 8 h L : D suggests that this is also a transitional point for this species.
The compensation irradiance (E c) and growth efficiency (αg) together define the capacity of a species to grow in light-limited conditions. The high value for E c of 13 μmol m−2 s−1 derived by both models appears to be accurate, as attempts to grow cultures at 11 μmol m−2 s−1 proved unsuccessful. By comparison, the compensation irradiance of the marine diatom Phaeodactylum tricornutum was estimated to be twenty times less at 0.5 μmol m−2 s−1 (Geider et al., Citation1985). The high E c of A. circinalis may be due to very high energy costs of N2 fixation, requiring production of enzymes such as nitrogenase (Zevenboom et al., Citation1981; Paerl, Citation1990; De Nobel et al., Citation1997). Although our experiments were run with excess NO3, with no apparent requirement to fix N2, it is possible that the mere ability to fix N2 requires an energy expense. This has been demonstrated previously when, under NO3-replete conditions, the irradiance requirements of the heterocystous cyanobacterium Aphanizomenon flos-aquae were higher than those of a non-heterocystous mutant of the same species, incapable of N2 fixation (Zevenboom et al., Citation1981). Our data indicate that A. circinalis is not a cyanobacterium that requires only low ambient light conditions to out-compete other species (Zevenboom, Citation1986). Instead, the high E c suggests that it is necessary for A. circinalis to gain access to high light and utilise its buoyancy to competitive advantage, particularly in conditions with high vertical light attenuation, typical of many Australian waters.
The growth efficiency (αg) calculated for A. circinalis in this study (Monod, 0.34 ± 0.19 m2 mol−1; exponential, 0.44 ± 0.15 m2 mol−1) had wide confidence limits because of the scarcity of data at irradiances < 50 μmol m−2 s−1. Despite this, it is clear that, relative to other species, the growth efficiency of A. circinalis is in the mid to high range (∼ 0.40 m2 mol− 1 = 50 × 10−3 div d−1 μmol m−2 s−1; cf. Langdon, Citation1988), and is higher than that of other heterocystous freshwater cyanobacteria (0.26–0.32 m2 mol− 1) estimated from growth-irradiance data (Raps et al., Citation1983; De Nobel et al., Citation1998). The dominance of A. circinalis in stable and often turbid waters is aided by its buoyancy (Humphries & Lyne, Citation1988; Walsby et al., Citation1997). Though this species is not suited to growth at low irradiance, as indicated by its E c, it appears that when exposed to irradiance conditions suitable for growth, its high αg allows it to take full advantage of these conditions.
Chlorophyll a, carbon and the chl a : C ratio
The chl a : C ratio of A. circinalis was relatively constant at growth limiting irradiances, and decreased exponentially at irradiances > 150 μmol m−2 s−1, slightly greater than the point of saturation for growth (). The different exponential relationship of chl a quota across all irradiances may be an experimental artefact. We were unable to determine the chl a or C quotas at 150 μmol m−2 s−1 due to the unfortunate loss of samples for cell counts. If it is assumed that the relatively stable C quota remained so at 150 μmol m−2 s−1 (), then the real trend in chl a quota was similar to that for chl a : C, remaining steady up to 150 μmol m−2 s−1, followed by an exponential decrease. This differs from the more commonly observed pattern in phytoplankton of an exponential decrease in the chl a : C ratio and chl a quota over the full range of irradiances (Falkowski et al., Citation1985; Geider et al., Citation1985; Post et al., Citation1985; Cullen & Lewis, Citation1988; Sakshung et al., Citation1989; Thompson, Citation1999), with the exception of very low irradiances at which cell bleaching may occur (Falkowski, Citation1980; Thompson et al., Citation1989).
There have been some indications of a similar stepped pattern in the chl a quota and chl a : C ratio in other species. For instance, in Thalassiosira pseudonana, a maximum quota was observed at an irradiance corresponding to the point of light limitation, below which there was little change (Thompson et al., Citation1989). Similar results for chl a quotas were also presented for Synechococcus sp. (Kana & Glibert, Citation1987) and several other cyanobacteria (Utkilen et al., Citation1983; Wyman & Fay, Citation1986). This plateau in chl a quota may result from the inability to allocate energy resources to chl a synthesis when the energy input (irradiance) is low (Wyman & Fay, Citation1986). High energy requirements for metabolic maintenance in A. circinalis, as suggested earlier by the high E c, may contribute to this pattern in chl a : C ratio (and by inference in the chl a quota). It is also possible that there is a maximum chl a quota, as previously suggested for Synechococcus which was proposed to be genotypically defined by the maximum attainable chl a content of thylakoids and area of the photosynthetic membrane (Utkilen et al., Citation1983; Wyman & Fay, Citation1986).
Buoyancy
In experiment 2, the decrease from net positive to net negative buoyancy of A. circinalis over a relatively small irradiance range (135–150 μmol m−2 s−1) occurs just above growth saturating irradiance. This lends support to the argument that buoyancy regulation provides an advantage in gaining access to light, but also acts as a feedback mechanism to provide protection from light stress (Reynolds, Citation1984). A loss of buoyancy in natural stratified conditions once growth is maximal would be potentially advantageous since it avoids damage by light-induced photo-oxidation at high surface irradiances.
Flotation rates in natural populations of non-colonial Anabaena spp. vary considerably from 0.11 m d−1 (Walsby et al., Citation1987) to 9.4 m d−1 (Brookes et al., Citation1999). A field experiment on a naturally occurring Australian population of A. circinalis indicated that a total daily irradiance > 6 mol m−2 d−1 initiated a decline in buoyancy (Brookes et al., Citation1999). Since this corresponds to 104 μmol m−2 s−1 in a 16 h photoperiod, there is a remarkable degree of agreement between laboratory and field results.
Microcosm experiment
Results from the microcosm experiment (experiment 3) confirmed the hypothesis that a calm, stable water column favours the growth of A. circinalis under conditions of high light attenuation. The significantly higher growth rate observed under the CALM regime in the LE treatment is evidence of this (, ). The high vertical light attenuation in the LE treatment (, ) and the higher flotation rate at LE provided the population in the CALM treatment with a higher irradiance than the MIXED treatment (McCausland et al., Citation2001), and this was reflected in the higher growth rate. Under the HE and ME conditions, the difference in irradiance between MIXED and CALM treatments was probably insufficient to produce a significant difference in growth rate due to the relatively low flotation rate of A. circinalis (McCausland et al., Citation2001).
The CALM LE cells had the tendency for higher mean carbohydrate quotas and lower mean chl a quotas than in the MIXED regime. However, it was only the ratio of these two (chl a : carbohydrate) at LE that showed a significant difference between mixing regimes (). The chl a : carbohydrate ratio therefore appears to be a sensitive indicator of light history in this species. It is more common to use the chl a : C ratio as an indicator of light acclimation, but Cullen & Lewis (Citation1988) previously demonstrated that a higher chl a : carbohydrate ratio was also a good and slightly more sensitive indicator of photoacclimation in the marine diatom Thalassiosira pseudonana. The difference in chl a : carbohydrate between mixing treatments in the LE treatment confirms that the CALM treatment cells were being exposed to higher irradiance, due to the buoyancy-influenced concentration of cells towards the surface. The high degree of light-limitation at LE appears to have caused the increase in flotation rate, and subsequently a higher growth rate in the CALM regime. The cause for the increase in flotation rate is discussed below.
The ME treatment was also a highly light-limited, however there was less of an increase in flotation rate and subsequently no growth advantage observed under the CALM regime. Here, it seems the lower flotation rate of A. circinalis in the ME treatment ensured that insufficient cells were able to concentrate a significant proportion of the population in the upper water column over the 48 h period of stability. With other factors being equal, a higher flotation rate than observed in the ME treatment appears essential for A. circinalis to gain a significant growth advantage in the short-term calm conditions, relative to mixed conditions. Mitrovic et al. (Citation2001) demonstrated that a flotation rate of 3.6 m d−1 in A. circinalis in a shallow (2.5 m), diurnally mixed, extremely light limited treatment in the Darling River, Australia, provided a five-fold photosynthetic advantage relative to a continuously mixed water column. This significant photosynthetic advantage is substantially higher than the observed 44% increase in growth rate observed in the LE treatment in this experiment and is probably due to the substantially higher flotation rate (3.6 m d−1 cf. 0.19 m d−1). Though short periods of stability (e.g. during diurnal stratification) do not appear conducive to the occurrence of blooms of A. circinalis (Bormans et al., Citation1997), both our results and those of Mitrovic et al. (Citation2001) demonstrate that short-term calm conditions will increase photosynthetic and growth rates and facilitate the transition to dominance during subsequent periods of sustained stability of 10–14 days.
It appears that trichome aggregation played a significant part in the greater buoyancy of cells in the CALM treatments. The greater buoyancy as measured at the end of the 48 h period cannot be attributed to lower carbohydrate quotas, which were very similar between mixing regimes. Instead, the higher flotation rate in the CALM treatment under ME and especially LE conditions was probably due mostly to trichome aggregation (Brookes et al., Citation1999). It is likely that as the trichomes rose toward the surface of the CALM microcosms they formed larger aggregations (Alldredge & Gotschalk, Citation1989). In contrast, regular turbulence within the MIXED treatment probably caused the lower flotation rates by preventing trichome aggregation (Riebesell, Citation1992). As CALM cultures had been mixed only once prior to the SETCOL flotation measurement, it is most likely that trichome aggregations remained intact. We have no microscopic observations to confirm this, but other known causes of buoyancy regulation are unable to explain our results. The collapse of gas vesicles (Walsby, Citation1994), the dilution of gas vesicles as a consequence of high growth rates (Konopka et al., Citation1987), decreases in gas vesicle production at high irradiance (Utkilen et al., Citation1985) or a dilution of gas vesicles by increasing cell volume (Utkilen et al., Citation1985), all work to decrease buoyancy and flotation rates at higher irradiance. It is assumed that water column stability in combination with buoyancy in CALM treatment provided a higher irradiance to cells. Therefore, if any of these mechanisms were operating, we should have seen a decrease in buoyancy in the CALM treatment, rather than the observed increase.
The influence of carbohydrate ballast mechanism on buoyancy and the flotation rate is seen when comparing the irradiance treatments in MIXED conditions. As discussed, the aggregation of trichomes in these treatments was unlikely, so that any differences in flotation rate were the result of differences in carbohydrate ballast. The carbohydrate quota had only a slight negative relationship with flotation rate in the MIXED cells. A two-fold decrease in carbohydrate between the HE and LE treatments resulted in a mean gain in flotation rate of only 0.03 m d−1. It is clear from this that in A. circinalis carbohydrate ballast has only a minor influence on flotation rate. The advantage of aggregation to increase the flotation rate, as described by the Stokes equation (Oliver & Ganf, Citation2000), therefore appears essential for A. circinalis to maximise the competitive advantage of its buoyancy.
It may be speculated that the lower flotation rate in the CALM ME relative to CALM LE cells is due to the lower chance of forming trichome aggregations. It is first worth comparing the carbohydrate quotas of MIXED regime cells at LE and ME. The values for the MIXED treatments show the composition of cells at the mean water column irradiance, and the mean carbohydrate quotas were 23% higher (not significant) at ME than at LE. The carbohydrate quotas in the CALM treatments were measured at the end of the 48 h calm period, and do not reveal how the quotas may have changed over the CALM period in response to changes in vertical distribution and irradiance experienced by the population (Kromkamp & Mur, Citation1984). Therefore, it is probable that our carbohydrate data over-estimate the carbohydrate content, and do not clearly indicate how this influenced buoyancy. It is possible that, immediately after mixing, the higher irradiance received by cells in ME was responsible for production of more carbohydrate than in LE, as was seen in the MIXED treatments. This in turn could have caused a lower intrinsic flotation rate in ME cells. More importantly, the lower intrinsic flotation rate could have reduced the potential for a higher flotation rate, by reducing the chance of trichome aggregation.
The ability of A. circinalis to grow at all in the MIXED treatment at LE is surprising considering that the mean irradiance is lower than E c determined in experiment 1. This suggests that photoacclimation occurred as a result of extended periods at low irradiance (Cullen & Lewis, Citation1988). A. circinalis cells grown under low irradiance conditions for a substantial period appear to have decreased their E c, apparently optimising their photosynthetic efficiency. Prior to the shift to LE, the MIXED cell population was at a mean irradiance of 15 μmol m−2 s−1 for a period of 18 days in the ME treatment. This gradual introduction to LE conditions may have allowed it to successfully adapt to these conditions. Despite there being no increase in chl a at LE as a sign of photoacclimation, this is consistent with our results from experiment 1. Anabaena circinalis maintains a steady chl a content up to irradiances of around 150 μmol m−2 s−1, as discussed earlier. Despite the ability of this species to acclimate to reduced irradiance, this is likely to be insignificant in determining its capacity to dominate the phytoplankton community, because of its very low growth rate.
In summary, our results confirm that A. circinalis is a cyanobacterium that requires high irradiance to grow and its light-mediated buoyancy allows it to gain access to such conditions. As such, it requires water column stability to dominate in highly vertically attenuated irradiance conditions. Periods of intermittent stability of the order tested in these experiments (48 h) provide this species with a significant increase in growth relative to mixed conditions, and may facilitate species dominance at a later period of sustained stability.
Acknowledgements
We thank Ian Jameson, two anonymous reviewers and Prof. Matthew Dring for providing comments that substantially improved the manuscript. MM is grateful to CSIRO Marine Research Laboratories, Hobart, where this work was undertaken and to University of Tasmania, School of Aquaculture, Launceston. This work was funded by Land and Water Resources Research and Development Corporation NEMP grant CSF-1.
References
References
- Alldredge , AL and Gotschalk , CC . 1989 . Direct observations of the mass flocculation of diatom blooms: characteristics, settling velocities and formation of diatom aggregates . Deep Sea Res. , 36 : 159 – 171 .
- Bannister , TT . 1990 . Comparison of Kiefer-Mitchell and Bannister-Laws algal models . Limnol. Oceanogr. , 35 : 972 – 979 .
- Bienfang , PK . 1981 . SETCOL–A technologically simple and reliable method for measuring phytoplankton sinking rates . Can. J. Fish. Aquat. Sci. , 38 : 1289 – 1294 .
- Bormans , M , Maier , H , Burch , M and Baker , P . 1997 . Temperature stratification in the lower River Murray, Australia: Implication for cyanobacterial bloom development . Mar. Freshwat. Res. , 48 : 647 – 654 .
- Brookes , JD , Ganf , GG , Green , D and Whittington , J . 1999 . The influence of light and nutrients on buoyancy, filament aggregation and flotation of Anabaena circinalis . J. Plankton Res. , 21 : 327 – 341 .
- Cullen , JJ . 1990 . On models of growth and photosynthesis in phytoplankton . Deep Sea Res. , 37 : 667 – 683 .
- Cullen , JJ and Lewis , MR . 1988 . The kinetics of algal photoadaptation in the context of vertical mixing . J. Plankton Res. , 15 : 1039 – 1063 .
- De Nobel , WT , Matthijs , CP , von Elert , E and Mur , LR . 1998 . Comparison of the light-limited growth of the nitrogen fixing cyanobacteria Anabaena and Aphanizomenon . New Phytol. , 138 : 579 – 587 .
- De Nobel , WT , Snoep , JL , Westerhoff , HV and Mur , LR . 1997 . Interaction of nitrogen fixation and phosphorus limitation in Aphanizomenon flos-aquae (Cyanophyceae) . J. Phycol. , 33 : 794 – 799 .
- Dubois , M , Gillies , KA , Hamilton , JK , Rebers , PA and Smith , F . 1956 . Colorimetric method for the determination of sugars and related substances . Anal. Chem. , 28 : 350 – 356 .
- Falkowski PG 1980 Light-shade adaptation in marine phytoplankton In: Primary Productivity in the Sea (Falkowski, P.G., editor) 99 119 Plenum Press New York
- Falkowski , PG , Dubinsky , Z and Wyman , K . 1985 . Growth-irradiance relationships of phytoplankton . Limnol. Oceanogr , 30 : 311 – 321 .
- Foy , RH , Gibson , CE and Smith , RV . 1976 . The influence of daylength, light intensity and temperature on the growth rates of planktonic blue-green algae . Br. Phycol. J. , 11 : 151 – 163 .
- Geider , RJ . 1987 . Light and temperature dependence of the carbon to chlorophyll a ratio in microalgae and cyanobacteria: implications for physiology and growth of phytoplankton . New Phytol. , 106 : 1 – 34 .
- Geider , RJ , Osborne , BA and Raven , JA . 1985 . Light dependence of growth and photosynthesis in Phaeodactylum tricornutum (Bacillariophyceae) . J. Phycol. , 21 : 609 – 619 .
- Geider , RJ , Macintyre , HL and Kana , TM . 1998 . A dynamic regulatory model of phytoplankton acclimation to light, nutrients and temperature . Limnol. Oceanogr. , 43 : 679 – 694 .
- Guillard RRL 1973 Division rates, In: Handbook of Phycological Methods: Culture Methods and Growth Measurements (Stein, J.R., editor) 289 311 Cambridge University Press Cambridge
- Hötzel , G and Croome , R . 1996 . Population dynamics of Aulacoseira granulata (Ehr.) Simonson (Bacillariophyceae, Centrales), the dominant alga in the Murray River, Australia . Arch. Hydrobiol. , 136 : 191 – 215 .
- Humphries , SE and Lyne , VD . 1988 . Cyanophyte blooms: The role of cell buoyancy . Limnol. Oceanogr. , 33 : 79 – 91 .
- Ibelings , BW , Mur , LR , Kinsman , R and Walsby , AE . 1991 . Microcystis changes its buyoancy in response to the average irradiance in the surface mixed layer . Arch. Hydrobiol. , 120 : 385 – 401 .
- Jeffrey , SW and Humphrey , GF . 1975 . New spectrophotometric equations for determining chlorophylls a, b, c 1 and c 2 in higher plants, algae, and natural phytoplankton . Biochem. Physiol. Planzen. , 167 : 191 – 194 .
- Jeffrey SW Welschmeyer NA 1997 Spectrophotometric and fluorometric equations in common use in oceanography In: Phytoplankton Pigments in Oceanography (Jeffrey, S.W., Mantoura, R.F.C. & Wright, S.W., editors) 597 615 UNESCO Paris
- Kana , TM and Glibert , PM . 1987 . Effect of irradiances up to 2000 μE m−2 s−1 on marine Synechococcus WH7803-I. Growth, pigmentation, and cell composition . Deep Sea Res. , 34 : 479 – 495 .
- Kinsman , R , Ibelings , BW and Walsby , AE . 1991 . Gas vesicle collapse by turgor pressure and its role in buoyancy regulation by Anabaena flos-aquae . J. Gen. Microbiol. , 137 : 1171 – 1178 .
- Kirk JTO 1994 Light and Photosynthesis in Aquatic Ecosystems 2nd edition Cambridge University Press Cambridge
- Konopka , A , Kromkamp , JC and Mur , LR . 1987 . Regulation of gas vesicle content and buoyancy in light- or phosphate-limited cultures of Aphanizomenon flos-aquae (Cyanophyta) . J. Phycol , 23 : 70 – 78 .
- Kromkamp , J and Walsby , AE . 1990 . A computer model of buoyancy and vertical migration in cyanobacteria . J. Plankton Res. , 12 : 161 – 183 .
- Kromkamp , JC and Mur , LR . 1984 . Buoyant density changes in the cyanobacterium Microcystis aeruginosa due to changes in the cellular carbohydrate content . FEMS Microbiol. Lett. , 25 : 105 – 109 .
- Langdon , C . 1987 . On the causes of interspecific differences in the growth-irradiance relationship for phytoplankton. Part I. A comparative study of the growth-irradiance relationship of three marine phytoplankton species: Skeletonema costatum, Olisthodiscus luteus and Gonyaulax tamarensis . J. Plankton Res. , 9 : 459 – 482 .
- Langdon , C . 1988 . On the causes of interspecific differences in the growth-irradiance relationship for phytoplankton. II. A general review . J. Plankton Res. , 10 : 1291 – 1312 .
- Lewis , MR , Cullen , JJ and Platt , T . 1984a . Relationships between vertical mixing and photoadaptation of phytoplankton: similarity criteria . Mar. Ecol. Prog. Ser. , 15 : 141 – 149 .
- Lewis , MR , Horne , EPW , Cullen , JJ , Oakey , NS and Platt , T . 1984b . Turbulent motions may control phytoplankton photosynthesis in the upper ocean . Nature , 311 : 49 – 50 .
- McCausland , MA , Thompson , PA and Blackburn , SI . 2001 . The effect of changes in light availability, caused by mixing, on the growth of Anabaena circinalis (Nostocales, Cyanobacteria) and Aulacoseira sp. (Centrales, Bacillariophyceae) . Phycologia , 40 : 530 – 541 .
- Mitrovic , SM , Bowling , LC and Buckney , RT . 2001 . Vertical disentrainment of Anabaena circinalis in the turbid, freshwater Darling River, Australia: quantifying potential benefits from buoyancy . J. Plankton Res. , 23 : 47 – 55 .
- Monod , J . 1949 . The growth of bacterial cultures . Ann. Rev. Microb. , 3 : 371 – 394 .
- Oliver , RL . 1990 . Optical properties of waters in the Murray-Darling Basin, South-eastern Australia . Aust. J. Mar. Freshwat. Res. , 41 : 581 – 601 .
- Oliver RL Ganf GG 2000 Freshwater blooms In: The Ecology of Cyanobacteria (Whitton, B.A. & Potts, M, editors) 149 194 Kluwer Academic Publishers
- Oliver , RL and Walsby , AE . 1984 . Direct evidence for the role of light-mediated gas vesicle collapse in the buoyancy regulation of Anabaena flos-aquae (cyanobacteria) . Limnol. Oceanogr. , 29 : 879 – 886 .
- Paerl , HW . 1990 . Physiological ecology and regulation of N2 fixation in natural waters . Adv. Microb. Ecol. , 11 : 305 – 344 .
- Post , AF , de Wit , R and Muur , LR . 1985 . Interactions between temperature and light intensity on growth and photosynthesis of the cyanobacterium Oscillatoria agardhii . J. Plankton Res. , 7 : 487 – 495 .
- Post , AF , Dubinsky , Z , Wyman , K and Falkowski , PG . 1984 . Kinetics of light-intensity adaptation in a marine planktonic diatom . Mar. Biol. , 83 : 231 – 238 .
- Raps , S , Wyman , K , Siegelman , HW and Falkowski , PG . 1983 . Adaptation of the cyanobacterium Microcystis aeruginosa to light intensity . Plant Physiol. , 72 : 829 – 832 .
- Raven , JA . 1984 . A cost-benefit analysis of photon absorption by photosynthetic unicells . New Phytol. , 98 : 593 – 625 .
- Reynolds CS 1984 The Ecology of Freshwater Phytoplankton, Cambridge University Press Cambridge
- Riebesell , U . 1992 . The formation of large marine snow and its sustained residence in surface waters . Limnol. Oceanogr. , 37 : 63 – 76 .
- Rother , JA and Fay , P . 1979 . Blue-green algal growth and sporulation in response to simulated surface bloom conditions . Br. Phycol. J. , 14 : 59 – 68 .
- Sakshung , E , Andersen , K and Kiefer , DA . 1989 . A steady-state description of growth and light absorption in the marine planktonic diatom Skeletonema costatum . Limnol. Oceanogr. , 34 : 198 – 205 .
- Sherman , BS and Webster , IT . 1994 . A model for the light-limited growth of buoyant phytoplankton in a shallow, turbid waterbody . Aust. J. Mar. Freshwat. Res. , 45 : 847 – 862 .
- Sherman , BS , Webster , IT , Jones , GJ and Oliver , RL . 1998 . Transitions between Aulacoseira and Anabaena dominance in a turbid river weir pool . Limnol. Oceanogr. , 43 : 1902 – 1915 .
- Smith , RV and Foy , RH . 1974 . Improved hydrogen ion buffering of media for the culture of freshwater algae . Br. Phycol. J. , 9 : 239 – 245 .
- Thompson , PA . 1999 . The response of growth and biochemical composition to variations in daylength, temperature, and irradiance in the marine diatom Thalassiosira pseudonana (Bacillariophyceae) . J. Phycol. , 35 : 1215 – 1223 .
- Thompson , PA , Levasseur , ME and Harrison , PJ . 1989 . Light-limited growth on ammonium vs. nitrate: What is the advantage for marine phytoplankton? . Limnol. Oceanogr. , 34 : 1014 – 1024 .
- Throndsen J 1978 Preservation and storage In: Phytoplankton Manua (Sournia, A., editor) 69 74 UNESCO Paris
- Tilzer , MM . 1987 . Light-dependence of photosynthesis and growth in cyanobacteria: implications for their dominance in eutrophic lakes . N.Z. J. Mar. Freshwat. Res. , 21 : 401 – 412 .
- Utkilen , HC , Briseid , T and Erikson , B . 1983 . Variation in photosynthetic membrane and pigment content with light intensity Anacystis nidulans grown in continuous culture . J. Gen. Microbiol. , 129 : 1717 – 1720 .
- Utkilen , HC , Oliver , RL and Walsby , AE . 1985 . Buoyancy regulation in a red Oscillatoria unable to collapse gas vacuoles by turgor pressure . Arch. Hydrobiol. , 102 : 319 – 329 .
- Visser , PM , Passarge , J and Mur , LR . 1997 . Modelling vertical migration of the cyanobacterium Microcystis . Hydrobiologia , 349 : 99 – 109 .
- Waite , AM , Thompson , PA and Harrison , PJ . 1992 . Does energy control sinking rates of marine diatoms? . Limnol. Oceanogr. , 37 : 468 – 477 .
- Walsby , AE . 1994 . Gas vesicles . Microbiol. Rev. , 58 : 94 – 144 .
- Walsby , AE , Reynolds , CS , Oliver , RL , Kromkamp , J and Gibbs , MM . 1987 . The role of buoyancy in the distribution of Anabaena sp. in Lake Rotongaio . N.Z. J. Mar. Freshwat. Res. , 21 : 525 – 526 .
- Walsby , AE , Hayes , PK , Boje , R and Stal , LJ . 1997 . The selective advantage of buoyancy provided by gas vesicles for planktonic cyanobacteria in the Baltic Sea . New Phytol. , 136 : 407 – 417 .
- Webster , IT , Sherman , BS , Bormans , M and Jones , G . 2000 . Management strategies for cyanobacterial blooms in an impounded lowland river . Regul. Riv. Res. Manag. , 16 : 513 – 525 .
- Wyman , M and Fay , P . 1986 . Underwater light climate and the growth and pigmentation of planktonic blue-green algae (Cyanobacteria) I. The influence of light quantity . Proc. R. Soc. Lond. B , 227 : 367 – 380 .
- Zevenboom , W . 1986 . Ecophysiology of nutrient uptake, photosynthesis and growth . Can. Bull. Fish. Aquat. Sci. , 214 : 391 – 422 .
- Zevenboom , W , Van der Does , J , Bruning , K and Mur , LR . 1981 . A non-hetrocystous mutant of Aphanizomenon flos-aquae, selected by competition in light limited continuous culture . FEMS Microbiol. Lett. , 10 : 11 – 16 .