Abstract
Grateloupia is one of the most taxonomically complex genera of the Cryptonemiales. Of four species reported to occur in Ireland and Britain, only G. filicina (J.V. Lamouroux) C. Agardh and G. dichotoma J. Agardh are considered as native, with Grateloupia filicina var. luxurians A. Gepp & E.S. Gepp and G. doryphora (Montagne) Howe thought to be recent introductions. Sequence data from the plastid-encoded rbcL gene have been used to assist in clarifying the taxonomic position of these and other members of the Halymeniaceae from Ireland, Britain and neighbouring coasts. Molecular and morphological evidence indicate that the introduced alga G. filicina var. luxurians is only distantly related to the type species, G. filicina. It is here raised to species status as G. luxurians (A. Gepp & E.S. Gepp) R.J. Wilkes, L.M. McIvor & Guiry, stat. nov. A further species, Grateloupia minima P.L. Crouan & H.M. Crouan, has also been reported in the north-eastern Atlantic, but is currently considered as a seasonal or juvenile form of G. filicina. Based on morphological evidence and sequence data, G. minima is distinct from G. filicina, and is hereby reinstated. Although rarely reported, the closely related taxon, Dermocorynus montagnei P. L. Crouan & H. M. Crouan was also included in this study. Molecular and morphological data place Dermocorynus in a clade of Grateloupia species and we therefore propose that Dermocorynus be placed in synonymy with Grateloupia. The relationship between morphology and phylogeny within Grateloupia is discussed in the light of these results.
Introduction
Grateloupia (Cryptonemiales, Rhodophyta) is the largest genus in the Halymeniaceae, presently comprising some 53 species (Guiry & Nic Dhonncha, Citation2003), and is one of the most taxonomically complex of the Cryptonemiales. Although four species or subspecies are reported to occur in Britain and Ireland (G. filicina (J.V. Lamouroux) C. Agardh, G. dichotoma J. Agardh, G. filicina var. luxurians A. Gepp & E.S. Gepp and G. turuturu Yamada), only G. filicina and G. dichotoma are considered to be native (Hardy & Guiry, Citation2003). Grateloupia filicina var. luxurians, for which the type locality is New South Wales, Australia (Silva et al., Citation1996), was first recorded from the south coast of England in 1968. It is thought to have been present in England since at least the late 1940s (Farnham & Irvine, Citation1968), and is now also established in Brittany (Cabioch et al., Citation1997). The other introduced species, Grateloupia turuturu (Cabioch et al., Citation1997; Simon et al., Citation2001), is also reported to be invasive in the north-western Atlantic (Villalard-Bohnsack & Harlin, Citation1997). Originally described from Japan, Atlantic populations of G. turuturu were initially misidentified as G. doryphora (Montagne) Howe; however, a subsequent morphological and molecular investigation has shown these populations to be G. turuturu (Gavio & Fredericq, Citation2002).
Grateloupia minima P.L. Crouan & H.M. Crouan is the only other species of Grateloupia reported from north-east Atlantic coasts (Newton, Citation1931; Irvine, Citation1983). It was described as a small species consisting of simple fronds a few millimetres in length that arise from an epilithic crust, and has only has been reported to occur at extreme low water (Crouan & Crouan, Citation1860). Grateloupia minima was subsequently placed in synonymy with G. filicina (Dixon, Citation1958; Cabioch & Giraud, Citation1982) on the grounds that it represented a seasonal or juvenile form. Subsequent analyses have, however, shown that some of the numerous formae and varieties of G. filicina represent distinct species (Wang et al., Citation2000; Kawaguchi et al., Citation2001), raising doubts about the taxonomic status of G. minima.
On north-eastern Atlantic coasts, an alga that closely resembles the genus Grateloupia morphologically is Dermocorynus montagnei, the type species of its genus. This species, superficially similar to Grateloupia minima, consists of an expanded crust that attaches strongly to small mobile pebbles. These crusts bear small reproductive papillae, which may be either tetrasporangial or gametangial. The vegetative and reproductive morphology of D. montagnei closely resembles that of Grateloupia (Chiang, Citation1970; Guiry & Maggs, Citation1982), and its life history and spore germination patterns resemble those of G. filicina (Guiry & Maggs, Citation1982). The generic attribution of this species is considered doubtful (Guiry & Maggs, Citation1982; Wang et al., Citation2001; Kawaguchi et al., Citation2002).
The present study aims to clarify the taxonomic status of these members of the Halymeniaceae from Ireland, Britain and neighbouring coasts, using rbcL sequence data. We also discuss the phylogenetic utility of morphological features, previously considered characteristic within the family, in the light of the molecular evidence.
Materials and methods
Fresh and silica-gel-dried samples were collected from a number of sites in Ireland, Britain, France and Italy (). Samples of Dermocorynus montagnei from Flannery Bridge, Kilkerrin, Co. Galway, described in detail by Guiry & Maggs (Citation1982) were available only as herbarium-preserved material. Repeated searches of the site failed to reveal populations, probably due to environmental damage caused by the building of a new road bridge in the 1990s. Although not included in the molecular investigations, putative Grateloupia minima was collected in August 2003 from Pointe de Diable, Rade de Brest, Brittany, France, less than 30 km from the type locality.
Table 1 . Collection data, GenBank accession numbers and GALW herbarium accession numbers for field collections and GenBank samples of Grateloupia and other Halymeniaceae used in the molecular analyses
DNA was extracted from fresh, herbarium and silica gel-dried material using a CTAB extraction method modified after Doyle & Doyle (Citation1987) and Serrão et al. (Citation1999). The rbcL gene was PCR amplified using either a single primer pair (F8 & R1381) or a set of three pairs (F8 & R646; F481 & R1150; F765 & R1381) (Wang et al. Citation2000). The reaction mix consisted of 2.5 μl of 10 × PCR buffer, 1 mM MgCl2, 200 μM dNTPs, 0.2 μM primers and 0.6 units of Taq (Sigma UK Ltd or Biogene UK Ltd) in each 25 μl reaction. Reaction profiles were an initial denaturation of 93°C for 1 min followed by 35 cycles of 94°C for 30 s, 55°C for 30 s and 72°C for 45 s with a final extension at 72°C for 5 min. PCR reactions were run on a Hybaid OMN-E or Hybaid PCR-express thermocycler.
PCR products were agarose gel-purified using the Roche High-Pure PCR product purification kit according to the manufacturer's instructions. Purified products were sent for commercial sequencing (MWG, Biotech, UK, Ltd. or Lark Technologies UK, Ltd). Voucher specimens of all collected materials were prepared and are stored in the NUI, Galway Phycological Herbarium (GALW).
Sequences were aligned by eye using Genedoc 2.6.002 (Nicholas & Nicholas, Citation1997) and the data were analysed for maximum parsimony (MP) and neighbour joining (NJ), using a Kimura 2-parameter distance matrix as input, and for maximum likelihood (ML) using PAUP* 4b10 (Swofford, Citation2002). MP analysis was performed with 50 random sequence additions. Modeltest (Posada & Crandall, Citation1998) was used to determine the correct parameters for the ML analyses, and specified a General Time Reversible model with a gamma distribution and proportion of invariable sites (GTR+I+G). The rate matrix was specified as [A−C] = 0.8809, [A−G] = 4.8208, [A−T] = 0.9047, [C−G] = 0.9535, [C−T] = 10.8807 and [G−T] = 1.0000, with the base frequencies at A = 0.3239, C = 0.1442, G = 0.1938, and T = 0.3381. The proportion of invariable sites was set at 0.5597, with a gamma distribution of 0.9981. The robustness of each analysis was tested by bootstrapping the dataset 1000 times for MP and NJ, and 100 times for ML.
Results
The amount of sequence data generated from the rbcL gene ranged from 668 to 1253 bp for samples included in this analysis. Previously published sequences were obtained from Genbank () and added to the dataset to give a 1253-bp sequence alignment for analyses. No insertions or deletions were found, making the alignment unambiguous. The alignment contained 293 parsimony-informative sites with 855 invariable positions. Trees were rooted with Sebdenia monardiana (Montagne) Berthold, which is the generitype of the name-bringing genus of the family Sebdeniaceae, a sister group to the Halymeniaceae (Saunders & Kraft, Citation1996).
All analyses resulted in mostly identical trees with only minor difference within sub-clades. The maximum-likelihood tree with bootstrap values from each analysis overlaid on the branches is shown in . A clade containing Aeodes nitidissima J. Agardh, Pachymenia carnosa (J. Agardh) J. Agardh and P. cornea (Kützing) Chiang was placed basally in all analyses with 100% bootstrap support. Halymenia floresii (Clemente y Rubio) C. Agardh and H. dilatata Zanardini formed another well-supported group (BP = 100%) and the remaining species of Grateloupia were resolved in a clade with moderate to high bootstrap support (BP = 88−100%).
Fig. 1. Maximum Likelihood tree for Grateloupia and other Halymeniaceae, with bootstrap values for Maximum Parsimony (1000), Neighbour Joining (1000) and Maximum Likelihood (100) analyses overlaid on the branches.
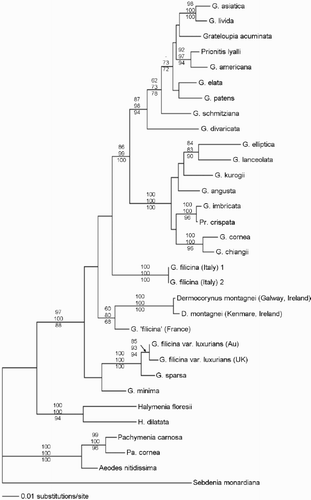
Within the large Grateloupia clade, G. filicina var. luxurians from Britain and the sample from the type locality were placed together with strong bootstrap support (85–94%) and, while only 1069 bp of the Australian isolate were successfully sequenced, rbcL sequence divergence between the two samples was low at only 0.5%, constituting only 5 base pairs. Grateloupia minima was placed weakly in a group with G. filicina var. luxurians and G. sparsa (Okamura) Chiang. Both Dermocorynus samples were placed together with 100% support in each analysis. The two sequences differed by only 2 out of the 867 bp (0.2%) successfully sequenced from the herbarium material. Attempts to obtain full sequences from incompletely sequenced samples failed, probably due to storage and preservation conditions and to the age of the herbarium sample of Dermocorynus. The only other species represented by more than one isolate was G. filicina from Italy. All samples were collected from the same general location and their rbcL sequences were identical. The remaining samples, all of Pacific origin, were placed separately from the other samples in two well-supported sub-clades.
To assess the significance of missing data, a second analysis was undertaken using a truncated dataset. The resultant trees (not shown) had the same topology but had lower bootstrap support for the arrangements. Similar results using partial rbcL sequences have been described by previous authors (Freshwater et al., Citation1995).
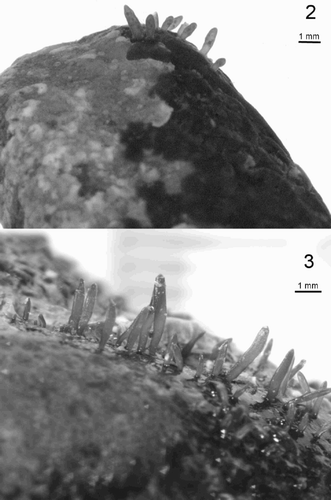
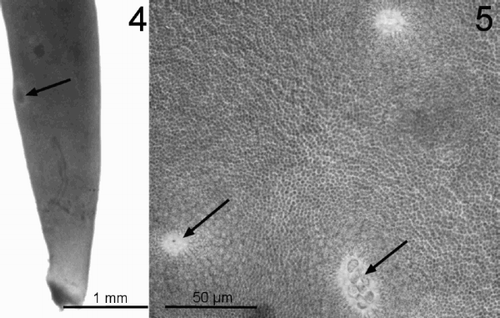
The minute Grateloupia thallus from Ireland and France included in our investigation matches previously published descriptions of Grateloupia minima (Crouan & Crouan, Citation1860, Citation1867; Irvine, Citation1983). Morphological examinations of G. minima collected at extreme low water on loose stones in France agreed very closely with those of previous investigations (Crouan & Crouan, Citation1860; Newton, Citation1931). Superficially similar to those of Dermocorynus montagnei (), the thalli are short, simple, rarely branched, up to 10 mm in length, arising from a purple-violet crust. The erect axes of this species are compressed, lanceolate and occasionally incurved (). The thalli were morphologically almost identical to the Irish samples used in the molecular examination but the French samples collected in August 2003 were reproductive (, ).
Figs 2–3. Fig. 2. Dermocorynus montagnei from Kenmare Bay, Co. Kerry, Ireland. Fig. 3 Grateloupia minima from Finavarra, Co. Clare, Ireland.
Figs 4–5. Reproductive isolate of Grateloupia minima from Pointe de Diable, Brittany, France. Arrows highlight reproductive organs.
Morphological differences between Grateloupia filicina and G. filicina var. luxurians (as G. luxurians) are summarised in . The primary distinguishing features are the larger more luxuriant habit, common facial proliferations and more mucilaginous texture of mature var. luxurians thalli compared to G. filicina (Gepp & Gepp, Citation1906; Irvine, Citation1983). In addition, the cystocarps of G. filicina are generally confined to the lateral pinnae while those of G. filicina var. luxurians are present on both the pinnae and main axes of the thalli. Grateloupia filicina var. luxurians is a larger, more foliose seaweed with fringes of ramuli growing from the margins as well as from the laminar surfaces of the thalli, whereas the branch-like proliferations seen in G. filicina arise solely from the margins of the main axes of the thalli (Gepp & Gepp, Citation1906; Farnham & Irvine, Citation1968).
Table 2 . Morphological features of Grateloupia and Dermocorynus
Discussion
Grateloupia filicina var. luxurians was present on the south coast of England prior to 1947, to judge from misidentified herbarium specimens (Farnham & Irvine, Citation1968; Farnham, Citation1980) although this entity is too large and distinctive to have been overlooked if the introduction had been prior to this. First described from Sydney, Australia (Gepp & Gepp, Citation1906), this variety of G. filicina occurs only in Australia and the Mediterranean (Verlaque, Citation2001). These large, alternately branched and luxuriant thalli can reach lengths of up to 1000 mm in Britain (Irvine & Farnham, Citation1983), and over 400 mm along coasts in Brittany (Cabioch et al., Citation1997), in contrast to populations in Australia, which only reach 220 mm (Gepp & Gepp, Citation1906; Womersley & Lewis, Citation1994). Otherwise, the overall morphology of the British and Australian plants is in close agreement (Farnham, Citation1980). The rbcL sequence data from this study confirm that the UK populations are the same species as found in Australia, with sequence divergences well within the interspecific values observed within Grateloupia (e.g. Wang et al., Citation2000; Gavio & Fredericq, Citation2002). These results, however, indicate that G. filicina var. luxurians represents a distinct species from G. filicina. Although the morphology of G. filicina var. luxurians is known to vary (Cabioch et al., Citation1997), it shows sufficient vegetative morphological differences from G. filicina to warrant considering it as a distinct species. We therefore propose to raise Grateloupia filicina var. luxurians to the rank of species as follows:
Grateloupia luxurians (A. Gepp & E.S. Gepp) R.J. Wilkes, L.M. McIvor & Guiry stat. nov.
Basionym: Grateloupia filicina var. luxurians. Gepp, A. and Gepp, E.S. Some marine algae from New South Wales. Journal of Botany, 44: 259–260, 1906.
Dermocorynus and Grateloupia are considered as two closely related yet distinct genera within the Halymeniaceae (Guiry & Maggs, Citation1982). Dermocorynus has a unique and highly specialised habitat compared to other Halymeniaceae (Guiry & Maggs, Citation1982). It occurs as an epilithic crust on subtidal, motile pebbles () or detached maërl fragments, from which erect axes arise. Previous morphological studies have, however, shown that the reproductive and vegetative morphologies of Dermocorynus montagnei are almost identical to those of Grateloupia (Chiang, Citation1970; Guiry & Maggs, Citation1982). Our analyses of rbcL sequence data agree with this morphological evidence and suggest that, rather than being a member of a separate genus, D. montagnei is more correctly placed within the genus Grateloupia. On the basis of this molecular and morphological evidence, we propose that the genus Dermocorynus be placed in synonymy with Grateloupia by transferring the type species D. montagnei to Grateloupia as follows:
Grateloupia montagnei (P.L. Crouan & H.M. Crouan) R.J. Wilkes, L.M. McIvor & Guiry, comb. nov.
Basionym: Dermocorynus montagnei. P.L. Crouan & H.M. Crouan. Note sur quelques algues marines nouvelles de la rade de Brest. Annales des Sciences Naturelles; Botanique, 9: 69–70, pl. 3: Figs 1a, b, c, d, 1858.
The minute Grateloupia thallus from Ireland and France included in our analyses matches previously published descriptions of Grateloupia minima (Crouan & Crouan, Citation1860, Citation1867; Irvine, Citation1983), and grouped consistently within the genus Grateloupia. Grateloupia minima was first described on the basis of its habitat and morphology (Crouan & Crouan, Citation1860) but later examinations have firmly placed G. minima in synonymy with G. filicina (Dixon, Citation1958; Cabioch & Giraud, Citation1982; Irvine, Citation1983) as a seasonal or juvenile form. However, the minute Grateloupia groups separately from all G. filicina samples included in our analyses, including the Italian G. filicina isolate used by recent authors as typical of the species (Kawaguchi et al., Citation2001). The presence of reproductive structures on the French sample provides support for the hypothesis that G. minima represents a separate entity and not merely a juvenile form of G. filicina. We therefore recommend the reinstatement of the name Grateloupia minima for such plants.
Taxonomy within Grateloupia and closely related genera remains difficult and the need to establish more reliable criteria has long been recognised (e.g. Kraft, Citation1977). The use of modern molecular techniques shows that many morphological characters traditionally used in taxonomic investigations of this genus are of little use in elucidating the true relationships (Wang et al., Citation2001). The present study highlights several examples where vegetative characteristics give a misleading interpretation of the taxonomic position. The two species with diminutive morphologies, Grateloupia montagnei and G. minima, examined in these investigations have been the subject of much taxonomic debate in the past, being considered at various times as distinct species, separate genera and juvenile forms of other species (Chiang, Citation1970; Guiry & Maggs, Citation1982). Our analyses indicate that either this reduced habit has evolved independently more than once or the reduced erect axes are a phenotypic adaptation to living in high-energy environments.
Reliance on purely external morphological criteria has led to considerable confusion with regard to the generitype, G. filicina. This species has been reported from localities worldwide and includes thalli with widely varying morphologies (Kawaguchi et al., Citation2002). Recent studies have shown that many of these populations are in fact taxonomically distinct entities and that the species requires more detailed investigation to determine its true distribution (Wang et al., Citation2000; Kawaguchi et al., Citation2001; Wang et al., Citation2001). Although initially identified as G. filicina, the sample from France used in these investigations is clearly not the same species as the other G. filicina isolates included and its true taxonomic position needs to be addressed. Grateloupia filicina was first described in 1813 from Trieste in the Adriatic (Lamouroux, Citation1813); however, the samples included in the present study are from the western coasts of Italy in the Tyrrhenian Sea. Material from Trieste was not available for analysis in this study so the relationships between the isolates included and the type material is unknown. It is not possible to say whether the western Italian G. filicina populations are a long-established extension of the range of the species from its type locality in the Adriatic or if they are a more recent introduction. Grateloupia filicina may be one of the few species of Grateloupia with a truly cosmopolitan distribution. Geographically distinct populations may comprise a single species but this appears unlikely, since it has been previously demonstrated that geographically isolated populations of G. asiatica and G. catenata represent distinct molecular and morphological species (Kawaguchi et al., Citation2001; Wang et al., Citation2001).
The elevation of G. luxurians to the rank of species, the placing of Dermocorynus in synonymy with Grateloupia and the reinstatement of Grateloupia minima in this investigation are indications of the extent to which morphological variation within the genus has been misinterpreted. It is clear both from these and other recent investigations that the taxonomic status of members of Grateloupia and related genera requires detailed investigation to clarify the position of species within this large and diverse group of red algae. The numerous taxonomic anomalies shown by recent molecular investigations have highlighted the need for more robust characters for species delineation among these seaweeds. The use of vegetative morphological characters is being shown to be of little taxonomic utility within the Halymeniales and in other red algal families such as the Hildenbrandiaceae (Sherwood & Sheath, Citation2000) and the Rhodomelaceae (McIvor et al., Citation2002).
Acknowledgements
We thank Drs Robert Fletcher, Maurizio Gargiulo and Marina Morabito for providing samples, and Drs Gerald T. Kraft and Erwan Ar Gall for assistance in field collections. This research was supported by the Irish Higher Education Authority's Programme for Research in Third Level Institutions, Cycle 2, to the Environmental Change Institute, NUI, Galway.
References
References
- Cabioch , J and Giraud , G . 1982 . La structure hildenbrandioïde, strategie adaptive chez les Floridées . Phycologia , 21 : 307 – 315 .
- Cabioch , J , Castric-Fey , A , L’hardy-Halos , M-T and Rio , A . 1997 . Grateloupia doryphora et Grateloupia filicina var. luxurians (Rhodophyta, Halymeniaceae) sur les côtes de Bretagne (France) . Cryptogamie Algol. , 18 : 117 – 137 .
- Chiang , Y-M . 1970 . Morphological studies of red algae of the family Cryptonemiaceae . Univ. Calif. Publ. Bot. , 58 : 1 – 83 .
- Crouan , PL and Crouan , HM . 1858 . Note sur quelques algues marines nouvelles de la rade de Brest . Ann. Sci. Nat. Bot., Ser. 4 , 9 : 69 – 70 .
- Crouan , PL and Crouan , HM . 1860 . Liste des algues marines découvertes dans le Finistère depuis la publication des algues de ce département en 1852 . Bull. Soc. Bot. France , 7 : 367 – 373 .
- Crouan PL Crouan HM 1867 Florule du Finistère. Contenant les descriptions de 360 espèces nouvelles de Sporogames, de nombreuses observations et une synonymie des plantes cellulaires et vasculaires, Klincksieck; Lefournier Paris & Brest
- Dixon , PS . 1958 . The morphology, ecology and taxonomy of certain Florideae . Br. Phycol. Bull , 6 : 32 – 33 .
- Doyle , JJ and Doyle , JL . 1987 . A rapid DNA isolation procedure for small quantities of fresh leaf tissue . Phytochem. Bull. , 19 : 11 – 15 .
- Farnham , WF and Irvine , LM . 1968 . Occurrence of unusually large plants of Grateloupia in the vicinity of Portsmouth . Nature , 219 : 744 – 746 .
- Farnham WF 1980 Studies on aliens in the marine flora of southern England In The Shore Environment Vol. 2: Ecosystems (Price, J.H., Irvine, D.E.G. & Farnham, W.F., editors) 875 914 Academic Press London
- Fredericq , S , Hommersand , M and Freshwater , DW . 1996 . The molecular systematics of some agar- and carrageenan-containing marine red algae based on rbcL sequence analysis . Hydrobiologia , 326/327 : 125 – 135 .
- Freshwater , DW , Fredericq , S and Hommersand , MH . 1995 . A molecular phylogeny of the Gelidiales (Rhodophyta) based on analysis of plastid rbcL nucleotide sequences . J. Phycol , 31 : 616 – 632 .
- Gavio , B and Fredericq , S . 2002 . Grateloupia turuturu (Halymeniaceae, Rhodophyta) is the correct name of the non-native species in the Atlantic known as Grateloupia doryphora . Eur. J. Phycol. , 37 : 349 – 360 .
- Gepp , A and Gepp , ES . 1906 . Some marine algae from New South Wales . J. Bot. , 44 : 259 – 261 .
- Guiry , MD and Maggs , CA . 1982 . The morphology and life history of Dermocorynus montagnei Crouan frat (Halymeniaceae; Rhodophyta) from Ireland. . Br. Phycol. J. , 17 : 215 – 228 .
- Guiry MD Nic Dhonncha E 2003 AlgaeBase, World Wide Web electronic publication www.algaebase. org: accessed on 1-9-2003
- Hardy FG Guiry MD 2003 A Check-list and Atlas of the Seaweeds of Britain and Ireland, British Phycological Society London
- Hommersand MH Fredericq S 2003 Biogeography of the marine red algae of the South African West Coast: a molecular approach In Proceedings of the 17th International Seaweed Symposium (Chapman, A.R.O., Anderson, R.J., Vreeland, V.J. & Davison, I.R., editors) 325 336 Oxford University Press Cape Town
- Irvine LM Farnham WF 1983 Halymeniaceae In Seaweeds of the British Isles. Volume 1. Rhodophyta, Part 2A Cryptonemiales (sensu stricto), Palmariales, Rhodymeniales (Irvine, L.M., editor) 17 51 British Museum (Natural History), London
- Irvine LM 1983 Seaweeds of the British Isles Volume 1. Rhodophyta, Part 2A: Cryptonemiales (sensu stricto) Palmariales, Rhodymeniales, British Museum (Natural History) London
- Kawaguchi , S , Wang , HW , Horiguchi , T , Sartoni , G and Masuda , M . 2001 . A comparative study of the red alga Grateloupia filicina (Halymeniaceae) from the northwestern Pacific and Mediterranean with the description of Grateloupia asiatica sp. nov. . J. Phycol. , 37 : 433 – 442 .
- Kawaguchi , S , Wang , H-W , Horiguchi , T , Lewis , JA and Masuda , M . 2002 . Rejection of Sinkoraena and transfer of some species of Carpopeltis and Sinkoraena to Polyopes (Rhodophyta, Halymeniaceae) . Phycologia , 41 : 619 – 635 .
- Kraft , GT . 1977 . The morphology of Grateloupia intestinalis from New Zealand, with some thoughts on generic criteria within the family Cryptonemiaceae (Rhodophyta) . Phycologia , 16 : 43 – 51 .
- Lamouroux , JVF . 1813 . Essai sur les genres de la famille des thalassiophytes non articulées . Ann. Mus. Hist. Nat , 20 : 21–47, 115–139, 267–293
- McIvor , L , Maggs , CA and Stanhope , MJ . 2002 . RbcL sequences indicate a single evolutionary origin of multinucleate cells in the red algal tribe Callithamnieae . Mol. Phylogenet. Evol. , 23 : 433 – 446 .
- Newton L 1931 A Handbook of the British Seaweeds, British Museum (Natural History) London
- Nicholas KB Nicholas HB 1997 GeneDoc: a tool for editing and annotating multiple sequence alignments, Distributed by the author, http://www.psc.edu/biomed/genedoc
- Posada , D and Crandall , KA . 1998 . MODELTEST: testing the model of DNA substitution . Bioinformatics , 14 : 817 – 818 .
- Saunders , GW and Kraft , GT . 1996 . Small-subunit rRNA gene sequences from representatives of selected families of the Gigartinales and Rhodymeniales (Rhodophyta). 2. Recognition of the Halymeniales ord. nov. . Can. J. Bot. , 74 : 694 – 707 .
- Serrão , EA , Alice , LA and Brawley , SH . 1999 . Evolution of the Fucaceae (Phaeophyta) inferred from nrDNA-ITS . J. Phycol. , 35 : 382 – 394 .
- Sherwood , AR and Sheath , RG . 2000 . Biogeography and systematics of Hildenbrandia (Rhodophyta, Hildenbrandiales) in Europe: inferences from morphometrics and rbcL and 18S rRNA gene sequence analyses . Eur. J. Phycol. , 35 : 143 – 152 .
- Silva , PC , Basson , PW and Moe , RL . 1996 . Catalogue of the benthic marine algae of the Indian Ocean . Univ. Calif. Publ. Bot. , 79 : 1 – 1259 .
- Simon , C , Ar Gall , E and Deslandes , E . 2001 . Expansion of the red alga Grateloupia doryphora along the coasts of Brittany (France) . Hydrobiologia , 443 : 23 – 29 .
- Swofford DL 2002 PAUP* Phylogenetic Analysis Using Parsimony (*and Other Methods), Sinauer Associates Sunderland Massachusetts
- Verlaque , M . 2001 . Checklist of the macroalgae of Thau Lagoon (Hérault, France), a hot spot of marine species introduction in Europe . Oceanologica Acta , 24 : 29 – 49 .
- Villalard-Bohnsack , M and Harlin , MM . 1997 . The appearance of Grateloupia doryphora (Halmeniaceae, Rhodophyta) on the northeast coast of North America . Phycologia , 36 : 324 – 328 .
- Wang , HW , Kawaguchi , S , Horiguchi , T and Masuda , M . 2000 . Reinstatement of Grateloupia catenata (Rhodophyta, Halymeniaceae) on the basis of morphology and rbcL sequences . Phycologia , 39 : 228 – 237 .
- Wang , HW , Kawaguchi , S , Horiguchi , T and Masuda , M . 2001 . A morphological and molecular assessment of the genus Prionitis J Agardh (Halymeniaceae, Rhodophyta) . Phycol. Res. , 49 : 251 – 261 .
- Womersley HBS Lewis JA 1994 Family Halymeniaceae Bory 1828: 158 In The Marine Benthic Flora of Southern Australia. Part IIIA. Bangiophyceae and Florideophyceae (Acrochaetiales, Nemaliales, Gelidiales, Hildenbrandiales and Gigartinales sensu lato) (Womersley, H..B.S., editor) 167 218 Australian Biological Resources Study Canberra