Abstract
Detailed analysis of the morphology of Florisphaera profunda from plankton samples collected at three sites in the Atlantic and Pacific Oceans reveals wide variation in this deep ocean-dwelling coccolithophore. In addition to the two varieties described previously, we found a third distinctive form, Florisphaera profunda var. rhinocera var. nov. All three varieties occur at each of the sampling sites. The analysis of monthly samples from different levels in the lower photic zone (LPZ) (100–200 m) at the Hawaii Ocean Time series station suggests that the varieties have similar distributions, which are correlated to primary productivity and the availability of light. The analysis of coccolith and coccosphere size in F. profunda reveals the existence of several size modes in Florisphaera profunda var. profunda and F. profunda var. elongata. The biological significance of these modes, or morphotypes is not known. However, their co-occurrence in single samples from different oceanic areas suggests that they are not ecophenotypes. In the light of recent molecular genetic analyses of intraspecific groups within commonly occurring coccolithophores, the varieties and size morphotypes of F. profunda are of significant interest for the study of marine phytoplankton biodiversity. Coccolithophores inhabiting the LPZ may be adapted to the low light, high nutrient conditions of this layer and hold great potential as a means to reconstruct past oceanographic conditions such as the position of the nutricline. However, coccolithophore biodiversity in the LPZ is poorly documented and the number of species may be much higher than previously thought.
Introduction
Recent analyses of selected coccolithophore species have indicated that the biodiversity in this single-celled marine phytoplankton group may be severely underestimated, as fine scale ‘intraspecific’ morphological groups appear to display ecological and genetic differences (Bollmann, Citation1997; Knappertsbusch et al., Citation1997; Young & Westbroek, Citation1991; Sáez et al., Citation2003). Such morphological groups may in fact represent different species which diverged several million years ago, as already demonstrated for other microscopic planktonic organisms e.g., planktonic foraminifera (Darling et al., Citation1996; Norris & de Vargas, Citation2000; de Vargas et al., Citation2004). These findings are crucial to understanding the biodiversity, ecology and evolution of coccolithophores as well as for their application as proxies in palaeoenvironmental reconstructions (Bollmann et al., Citation2002). On the basis of these facts, detailed morphological as well as genetic analyses of other coccolithophore taxa, of which some 180 species occur in the ocean at the present day (Jordan & Kleijne, Citation1994), are necessary.
One important coccolithophore that displays intraspecific morphological variability is Florisphaera profunda Okada & Honjo. This unique, deep dwelling species is not clearly related to other coccolithophores and is therefore classified as incertae sedis (Young et al., Citation2003). Two varieties of F. profunda have so far been described: Florisphaera profunda var. profunda and Florisphaera profunda var. elongata (Okada & Honjo, Citation1973; Okada & McIntyre, Citation1977, Citation1979, Citation1980) () and several additional morphologies have also been figured (Okada, Citation1983; Cros & Fortuño, Citation2002). However, the two varieties of F. profunda appear to be difficult to distinguish (Young, Citation1998) and are therefore not always separately recorded in plankton or sediment studies. Furthermore, Kleijne (Citation1991) has suggested that Florisphaera profunda var. elongata and an unidentified holococcolithophorid ‘type B’ may be different stages of a single life cycle. Therefore, the goal of the present study is to re-assess the ecological and biological significance of morphological variation within F. profunda from plankton samples.
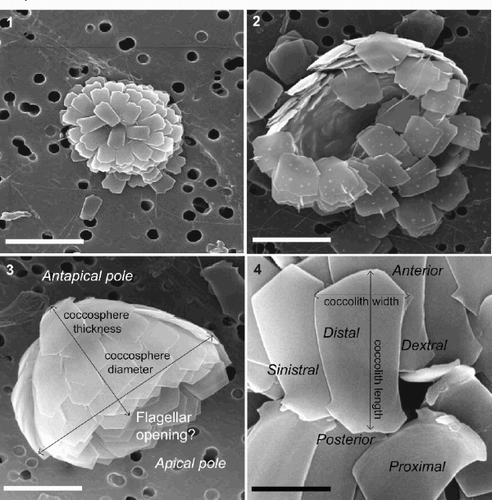
Figs 1–4. SEMs showing coccosphere and coccolith morphology of Florisphaera profunda var. profunda (1, 2) and Florisphaera profunda var. elongata (3, 4). . Complete coccosphere of F. profunda var. profunda viewed from antapical pole, composed of small, simple, straight-sided small coccoliths. . Partially incomplete coccosphere viewed from antapical pole, composed of unusual, short-fat coccoliths with distal spine. . Complete coccosphere of F. profunda var. elongata viewed from side with coccosphere terminology used in this study, composed of elongate coccoliths with anterior peak. . Close-up of F. profunda var. elongata coccoliths with coccolith terminology used in this study. Scale bars represent 5 μm (–) and 2.5 μm ().
The ecology of F. profunda is poorly understood. However, it appears to be adapted to low light, high nutrient conditions (Jordan et al., Citation1992; Brand, Citation1994; Cortés et al., Citation2001) and may even be heterotrophic (Brand, Citation1994). The species dominates coccolithophore assemblages in the LPZ (>100 m) throughout the year in the subtropical oligotrophic central gyres (Cortés et al., Citation2001; Haidar & Thierstein, Citation2001) and is a common component of fossil coccolithophore assemblages in marine sediments dating back to four million years (Okada, Citation1983; Young, Citation1998). Florisphaera profunda therefore holds great potential as a tool for the reconstruction of past oceanographic conditions, such as changes in the position of the nutricline (Molfino & McIntyre, Citation1990; McIntyre & Molfino, Citation1996; Henriksson, Citation2000; Kinkel et al., Citation2000), surface water turbidity (Ahagon et al., Citation1993) and primary productivity (Beaufort et al., Citation1997; Citation2001).
Material and methods
The morphology of F. profunda coccospheres and coccoliths was studied in filtered plankton samples from the LPZ at three locations in the Atlantic and Pacific Oceans; the Hawaii Ocean Time-series (HOT), 22°45′N, 158°0′W, the Bermuda Atlantic Time-series (BATS), 32°10′N, 64°30′W and European Station for Time-series in the Ocean, Canary Island Eastern Boundary Current (ESTOC EBC), Canary Islands, 28°65′N, 13°65′W. A small segment of each filter sample, housed at the Geological Institute of the Swiss Federal Institute of Technology (ETH), Zürich, was mounted on an aluminium stub and coated with 15 nm of gold. Using a Philips XL-30 scanning electron microscope SEM, a total of 1000 consecutive fields of view were scanned along a radial transect of each filter sample at a magnification of 2000 × (for details see Bollmann et al., Citation2002). This is equivalent to an area of ca. 2.66 mm2 or 17.5 ml of filtered seawater in the HOT samples and 8.75 ml in the Bermuda and Canary Islands samples. The overview images were then viewed on a PC and all complete, partially disintegrated, and totally collapsed F. profunda specimens were classified and measured. Coccosphere diameter and coccolith length and width (, ) were recorded using analySIS 3.0 for Windows® to an accuracy of 0.2 μm, as determined by micrometric calibration. From the HOT station, Hawaii, monthly plankton samples at the depth of maximum F. profunda abundance (100, 150 or 200 m), were analysed for the years 1994 and 1995. Additional LPZ samples with >ca. 8000 F. profunda cells l−1 were analysed, as well as samples from a depth transect from 0–200 m, passing through a shallow peak of F. profunda at 100 m during March 1995. Selected samples from BATS, Bermuda and Eastern Boundary Current (EBC), Canary Islands were studied for comparison to the HOT samples.
Terminology
In their original description of F. profunda, Okada & Honjo (Citation1973) use several informal morphological terms. However, these terms have not been adopted by all subsequent authors, and confusion has arisen with regard to the orientation of coccospheres and isolated coccoliths, and the differentiation between the two varieties.
The description of this artichoke-shaped coccosphere in terms of an apical and an antapical end was previously not possible because no flagellar opening had been observed. Okada & Honjo (Citation1973) therefore used the term shallow dome top in their original description of F. profunda. However, in many specimens we have observed an opening at the opposite end of the cell to the shallow dome top, which may represent the flagella opening (). We therefore suggest that the terms apical pole (the end with the flagellar opening) and antapical pole (shallow dome top of Okada & Honjo, Citation1973) should be used to orient coccospheres of F. profunda (). This is in keeping with the description of coccosphere morphology in other coccolithophores (Young et al., Citation1997).
The polygonal plate-shaped coccoliths that form F. profunda cannot be conveniently described by any of the lith-terms given by Young et al. (Citation1997). We therefore assign the term polygolith to this type of coccolith morphology, consisting of a single crystal of flat, polygonal-shaped calcite. Polygoliths of F. profunda have a weakly birefringent appearance in the polarizing light microscope (Perch-Nielsen, Citation1985) and are therefore easily overlooked. In the absence of any terminology for the two flat coccolith surfaces, we name these proximal and distal (). The proximal surface of F. profunda coccoliths faces inwards towards the centre of the coccosphere and the distal surface faces outwards. In keeping with the coccosphere terminology adopted above, the end of the coccolith oriented toward the antapical pole is termed the ‘anterior’ (). The anterior end of F. profunda coccoliths is always wider than the opposite, posterior end. This difference can be used to orientate the coccoliths and, in the absence of a visible flagellar opening, the coccosphere upon which it sits. By applying the system proposed for other flat, elongate coccoliths such as Ceratolithus (Young et al., Citation1997), the terms dextral and sinistral can be used to describe the right and left edges of F. profunda coccoliths when viewed from the distal surface, with the anterior end up ().
Results
Morphology and taxonomy
The main difference between the two varieties of F. profunda described by Okada & Honjo (Citation1973) is the shape of the apical end of their coccoliths. In this respect, the apical end of F. profunda var. profunda is straight or zig-zagged and F. profunda var. elongata is rounded or possesses an obtuse angled peak (–). However, Okada & Honjo (Citation1973) also stated that the coccospheres of F. profunda var. profunda and F. profunda var. elongata differ in terms of their size. This may have prompted subsequent authors to adopt coccolith size as a distinguishing feature instead of the shape of the apical end, even though the size of coccoliths and coccospheres in both varieties overlap significantly (Okada & Honjo, Citation1973). Using the shape of the apical end of the coccoliths as a distinguishing feature, we were able to identify the two varieties of F. profunda described by Okada & Honjo (Citation1973) in 26 plankton samples from the Atlantic and Pacific Oceans. Our size measurements demonstrate that whilst F. profunda var. elongata reaches a greater maximum size, both varieties overlap considerably in terms of coccosphere diameter and coccolith length and width (, ). Furthermore, additional morphological variation was present within both varieties, including striations on the distal surface of F. profunda var. elongata and the presence of a small dorsal spine on rare specimens of F. profunda var. profunda (). Some of these features have been already reported in plankton samples from other locations (e.g. Cros & Fortuño, Citation2002, fig. 85D).
In addition to the two existing varieties of F. profunda, we discovered a third distinctive form which differs from the other two by having strongly asymmetrical, sail-shaped coccoliths with a prominent horn-like spine on the dextral side of their anterior end (–). Specimens of this form are immediately recognisable and occurred in the majority of samples analysed. A single specimen with this morphology was figured by Okada (Citation1983, plate 1, ) from 125 m in the central Pacific, but was not reported since. Given its distinctive morphology and occurrence in samples containing the two existing varieties from the Atlantic and Pacific Oceans, we propose this form as a new variety Florisphaera profunda var. rhinocera.
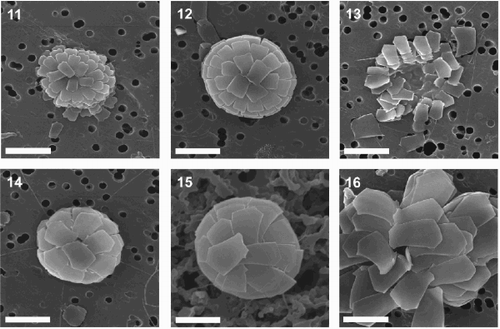
Figs 5–8. SEMs showing morphology of Florisphaera profunda var. rhinocera var. nov. . Complete coccosphere viewed from antapical pole, composed of straight-sided, rhombic coccoliths with prominent anterior spine (Holotype). . Partially flattened coccosphere viewed from antapical pole, composed of curved coccoliths with indistinct anterior spine. . Flattened coccosphere viewed from the side, composed of curved, sail-shaped coccoliths with small anterior and dextral spines. . Collapsed coccosphere viewed from side, composed of straight-sided to slightly curved coccoliths with prominent anterior spine. Scale bars represent 5 μm.

Florisphaera profunda var.
rhinocera var. nov. ( )
Unnamed variety of F. profunda Okada, Citation1983: 185, plate 1, .
DERIVATION OF NAME: On account of distinctive anterior spine, which resembles the horn of the extant mammal rhinoceros (family Rhinoceroteridae). Feminine adjective.
LATIN DESCRIPTION: Coccosphaera constans ex 30–100 coccolithis simplicus. Multum delicata. Diameter est 7.1–12 μm longus (medius 9.1 μm). Coccolithi quadranguli ad falcate aut forma veli sunt visione proximale aut distale. Plani aut varie convexi latere distale praeter axem longum. Recti aut leviter curvati, fronte levigata ad inaequabile levante leviter prope dexterum latus. Spina evidens in dextero margine frontis. Spina plerumque acuta atque varie longa est. Latera recta ad dextra curvata, plerumque levigata, se contrahenta prope tergum. Spina frontis processus ingentis incrementi crassitudinis lateris in dextero margine saepe est. Formae infrequentes parvam spinam in dextero margine, proiectam in partem anteriorem, aut distalem superficiem leviter pectinatam habere possunt. Margo laevus minime saepe spissatum est ac minimam spinam obtusam efficere potest. Tergum plerumque rectum, saepe spissatum, leviter curvatum et minime saepe tortuosum est. Coccolithi marginibus curvatis tenuem, rotundatam dexteram extensionem habere possunt. Spinam frontis atque ingentem dextram inconcinnitatem aut curvaturam usas esse ad disponendum recte coccolithos singulos oportet. Londitudo 1.7–3.8 μm (media 2.7 μm), latitudo 0.9–3.1 μm (media 1.0 μm).
DESCRIPTION: Coccosphere composed of ca. 30–100 simple coccoliths. Very delicate. Diameter ca. 7.1–12 μm (mean ca. 9.1 μm). Coccoliths rhombic to almost crescentic or sail-shaped when viewed from proximal or distal surface. Flat or with variable distal convexity along long axis. Straight or slightly curved, smooth to irregular anterior rising gently towards dextral side. Distinctive spine on dextral edge of anterior. Spine is usually sharp and of variable length. Sides straight to dextrally curved, usually smooth, tapering towards posterior. Anterior spine is often a continuation of strong lateral thickening on dextral edge. Rare specimens may possess a small spine on dextral edge, projecting in an anterior direction, or a slightly scalloped distal surface. Sinistral edge is only very rarely thickened and may give rise to a very small blunt anterior spine. Posterior usually straight, often thickened, slightly curved and more rarely zig-zagged. Coccoliths with curved edges may possess a weak, rounded dextral projection. Anterior spine and strong dextral asymmetry or curvature should be used to orientate isolated coccoliths. Length ca. 1.0–3.8 μm (mean ca. 2.7 μm), width ca. 0.9–3.1 μm (mean ca. 1.9 μm).
DIFFERENTIAL DIAGNOSIS: Coccospheres of this variety are distinguished from those of Florisphaera profunda var. profunda and Florisphaera profunda var. elongata by their strong asymmetry and the presence of a spine on the dextral corner of the anterior end of their coccoliths.
HOLOTYPE: , Plankton sample H61–100, collected from 100 m water depth at 14:04 on 05.03.1995 at HOT time series station, Hawaii, North Pacific (Latitude N° – 22.75, Longitude W° – 158). Sample stored at Geological Institute, ETH, Zürich, Switzerland.
DISTRIBUTION: North Atlantic Ocean, North Pacific, Equatorial Pacific, Caribbean Sea.
Morphometry
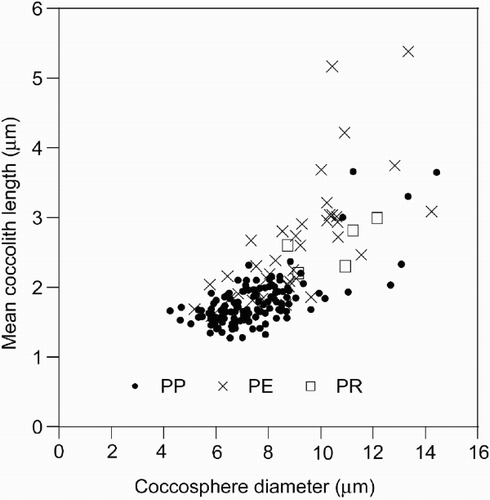
Our measurements of coccosphere and coccolith size indicate that wide size variation occurs within F. profunda (). Because of the delicate nature of F. profunda, coccosphere diameter could only be measured on ca. 200 intact and un-deformed specimens. Nevertheless, this dimension appears to be broadly correlated with the mean coccolith diameter on a coccosphere (). Each of the three varieties of F. profunda have different coccosphere size ranges, with F. profunda var. rhinocera forming medium sized coccospheres (ca. 8–12 μm coccosphere diameter) in relation to the other two (F. profunda var. profunda: 4–14 μm; F. profunda var. elongata: 5–15 μm).
Fig. 9. Coccosphere and coccolith size of F. profunda from HOT, BATS and EBC plankton samples. Coccosphere diameter and mean coccolith length of all complete, undeformed cells upon which at least three coccoliths could be measured. The individual distributions of the three varieties of F. profunda are shown: PP – F. profunda var. profunda, PE – F. profunda var. elongata, PR – F. profunda var. rhinocera. Coccosphere diameter and mean coccolith length is correlated with a coefficient (r) of 0.46.
By measuring the length and width of isolated coccoliths from collapsed F. profunda specimens we were able to determine that coccolith size is relatively constant on a single coccosphere (standard deviation ca.<0.2 μm). Plotting of the average coccolith size of all specimens in which three or more coccoliths could be measured, reveals that coccolith length and width are linearly correlated in the three varieties of F. profunda with a coefficient of 0.59–0.93 ( A–C).
Fig. 10. Graphs showing mean coccolith size distribution and morphotypes of F. profunda from HOT, BATS and EBC plankton samples. A–C. Mean coccolith length and width of all complete, deformed or collapsed specimens upon which three or more coccoliths could be measured is plotted as a scatter alongside the smoothed frequency distribution of mean coccolith length, calculated using a bin of 0.2 μm. Mean coccolith length and width is correlated with a coefficient (r) of: (A) – 0.84, (B) – 0.93 and (C) – 0.59. In F. profunda var. profunda and F. profunda var. elongata several modes or size morphotypes are visible. These are labelled S, M, L and XL, and their possible subdivision is indicated by dashed lines on the distribution of mean coccolith length. D–F. Mean coccolith size distribution and morphotypes of F. profunda in a single plankton sample from October 1995 at 150 m depth at HOT (sample H67–150m). Morphotypes F. profunda var. profunda S and M and F. profunda var. elongata S, M and L are visible in this sample. No coccospheres of F. profunda var. elongata XL were found, however, isolated coccoliths corresponding in size to this morphotype were encountered.
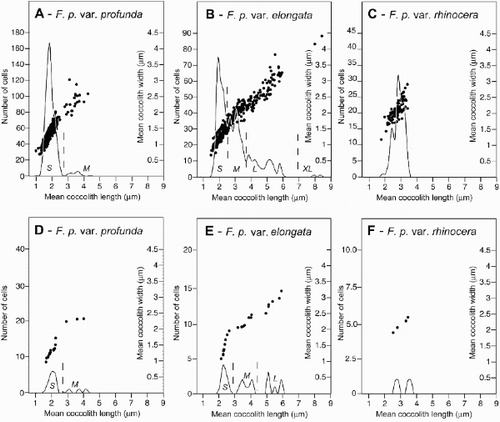
The distribution of mean coccolith length in F. profunda var. rhinocera is unimodal (). However, F. profunda var. profunda and F. profunda var. elongata display distinctly bi- to polymodal mean coccolith length distributions. The coccolith size distribution of F. profunda var. profunda is composed of a small (mean length 1.9 μm) and a medium sized mode (mean length 3.4 μm), which can be easily separated at 2.5 μm mean coccolith length ( A). The mean coccolith length distribution of F. profunda var. elongata on the other hand is less clear, but may be composed of four modes ( B): small (mean length 2.1 μm; <2.5 μm), medium (mean length 3.1 μm; 2.5–4 μm), large (mean length 4.9 μm; 4–6 μm) and extra large (>7 μm). Many of these size modes can be distinguished in the mean coccolith length distribution of single samples from the three localities ( D, E). We suspect that they represent different size groups within the two varieties (–). However, the low number of intact, un-deformed specimens in which coccosphere diameter could be reliably measured prevented the reliable documentation of these size groups of coccospheres ().
Distribution in plankton samples
All three varieties of F. profunda were present in the majority of samples analysed. In most samples, F. profunda var. profunda and F. profunda var. elongata occurred in comparable numbers (average ca. 1500–2000 cells l−1) and accounted for the majority of the F. profunda specimens. The new variety F. profunda var. rhinocera was generally less abundant than the other two (average ca. 500 cells l−1) but was recorded at all three sampling sites and reached densities of >2000 cells l−1 in some samples.
The analysis of monthly samples from HOT at the depth of maximum F. profunda abundance in the LPZ during 1994–1995 show a clear seasonal distribution of each of the varieties with high cell densities in summer and autumn 1994 and spring 1995, and low cell densities in winter 1994 and 1995 and summer 1995 (). All three exhibit similar fluctuations in their cell densities throughout this period, which mirror those of total F. profunda, as reported by Cortes et al. (2001).
Fig. 17. Graphs showing Florisphaera profunda cell density at the HOT time series station. A Total F. profunda cell density at the depth of maximum abundance during 1994–1995. B–D. Cell density of the three varieties and six size morphotypes at the depth of maximum total F. profunda abundance: F. profunda var. profunda (B), F. profunda var. elongata (C) and F. profunda var. rhinocera (D). Total F. profunda abundance data from Cortés et al. (Citation2001).
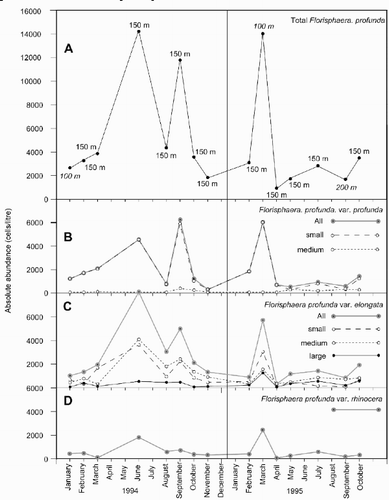
By calculating the average coccolith length on cells of F. profunda, we were able to classify specimens of F. profunda var. profunda and F. profunda var. elongata into the six size morphotypes described above. The vast majority of F. profunda var. profunda cells encountered at HOT belong to the small size morphotype. Consequently, cells of this size morphotype exhibit a similar distribution pattern to that of total F. profunda var. profunda during 1994–1995 (). The medium size morphotype of F. profunda var. profunda occurred in very low absolute numbers (<500 cells l−1) at the depth of maximum F. profunda, though its abundance relative to the small size morphotype was much higher during summer and autumn 1995 when total F. profunda was low. The small and medium size morphotypes of F. profunda var. elongata accounted for the majority of cells of this variety at HOT. They occur in similar absolute numbers throughout 1994–1995 and exhibit distinct fluctuations, which follow that of total F. profunda var. elongata during this period. The large size morphotype of F. profunda var. elongata occurred in low numbers (<500 cells l−1) in all HOT samples. During 1994–1995 it showed minor fluctuations, including an increase in absolute abundance during the shallow peak of F. profunda at 100 m in March 1995 (). The extra large size morphotype of F. profunda var. elongata occurred during this month at HOT, but below the depth of maximum F. profunda abundance at 150 m. Cells belonging to this biggest morphotype (mean coccolith length >7 µm) were not recorded in any other samples at HOT.
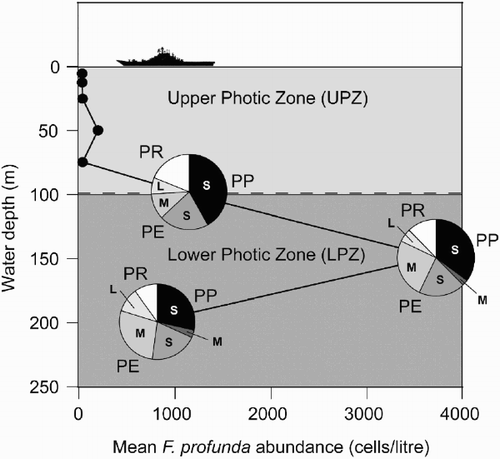
All varieties of F. profunda were present in samples collected from 100, 150 and 200 m depth at HOT. For each variety, its highest abundance during any given month always occurred at the depth of maximum F. profunda cell density. However, the proportion of each of the varieties at the three sampling depths suggests that F. profunda var. elongata is more abundant at deeper levels in the LPZ relative to F. profunda var. profunda and F. profunda var. rhinocera (). This is the result of an increase in the relative abundance of the medium and large morphotypes of F. profunda var. elongata and a reduction in F. profunda var. rhinocera and the small morphotype of F. profunda var. profunda with depth ().
Fig. 18. Diagram showing Florisphaera profunda cell density at different depths in the photic zone during 1994–1995. Mean total F. profunda cell density for 1994–1995 is shown by black line, with the relative abundance of each of the three varieties of F. profunda and their size-morphotypes at 100, 150 and 200 m in the LPZ indicated. PP – F. profunda var. profunda, PE – F. profunda var. elongata , PR – F. profunda var. rhinocera. Total F. profunda abundance data from Cortés et al. (Citation2001).
In order to investigate whether there were any dominant ecological controls on the distribution of the varieties of F. profunda, their cell densities and relative abundances in all LPZ samples analysed from 1994–1995 at HOT were compared to several in situ environmental parameters including light, temperature, nutrients and primary productivity (Cortés et al., Citation2001). Pearson product-moment correlation analysis revealed no high correlations, suggesting that the distribution of the three varieties cannot be related to a single environmental parameter. Likewise, the cell densities and relative abundances of the size morphotypes of F. profunda var. profunda and F. profunda var. elongata were not well correlated to any abiotic parameter in our samples.
Stepwise multiple regression models (MR) were therefore calculated to explain the variability in the cell densities of the F. profunda varieties and size morphotypes described above. The best models for the distribution of the three varieties of F. profunda were:
These models suggest that the abundances of all three varieties of F. profunda are influenced by light and primary productivity. In their analysis of total F. profunda in the LPZ at HOT, Cortés et al. (Citation2001) concluded that the species was most abundant when light was low. In their MR model, the availability of nutrients explained a smaller but significant percentage of the variability in total F. profunda cell density. This is also recorded in our model for F. profunda var. profunda above. Whilst the MR model for F. profunda var. elongata only explains 48% of the cell density variability in this variety, the importance of depth in this model confirms our suggestion, that it may prefer deeper levels in the LPZ.
Multiple regression analysis of the size morphotypes of F. profunda var. profunda and F. profunda var. elongata was less conclusive. The large and extra large size morphotypes of F. profunda var. elongata were too rare in most samples to perform stepwise MR. However, we were able to explain 68% of the variance in the cell density of the small morphotype of F. profunda var. profunda in the LPZ at HOT using the model below. The similarity of this model to that calculated for F. profunda var. profunda is explained by the fact that cells belonging to this size morphotype account for the vast majority of F. profunda var. profunda encountered in our samples.
Discussion and conclusions
Our analysis of the shape and size of the calcareous skeletons of F. profunda reveals that several morphological groups exist in this coccolithophore species. The two varieties of F. profunda described by Okada & Honjo (Citation1973) could be easily distinguished on the shape of the anterior end of their calcite coccoliths. At all three sampling sites, a third separate morphological variety of F. profunda, characterised by having strongly asymmetrical coccoliths with a horn-like spine was also present. Morphometric analysis of F. profunda from our plankton samples reveals the presence of several size groups within F. profunda var. profunda and F. profunda var. elongata. These size groups occur in many samples from the three areas and seem to represent separate size morphotypes of these taxa.
Similar intraspecific morphological variation has been reported within several coccolithophores inhabiting the upper photic layers of the ocean, e.g., Gephyrocapsa, (Bollmann, Citation1997), Calcidiscus leptoporus (Kleijne, Citation1993; Knappertsbusch et al., Citation1997; Quinn et al., Citation2004). Analyses on the distribution (Knappertsbusch et al., Citation1997), ecology (Renaud et al., Citation2002) and life cycle (Geisen et al., Citation2002) of the intraspecific groups of C. leptoporus have indicated that these may represent separate species. This has subsequently been confirmed by laboratory culture experiments (Quinn et al., Citation2003) and molecular genetic analyses (Sáez et al., Citation2003). We now know that this coccolithophore is composed of at least two ‘sibling’ species: Calcidiscus leptoporus (Murray & Blackmann) Loeblich & Tappan as emended by Sáez et al., Citation2003 and Calcidiscus quadriperforatus (Kamptner) Quinn & Geisen in Sáez et al., Citation2003, which may have diverged as long as 11 million years ago.
The discovery of ‘sibling’ species in Calcidiscus and other coccolithophores, e.g., Umbilicosphaera sibogae and Coccolithus pelagicus (Sáez et al., Citation2003) is changing our view of biodiversity in this phytoplankton group. In the light of these discoveries, the occurrence of intraspecific morphological groups in F. profunda is of great interest and requires further investigation. Much less is known about the coccolithophores inhabiting the LPZ of the ocean. They are morphologically quite different from the coccolithophores in the upper photic zone (Young, Citation1994) and appear to be adapted to low light, high nutrient conditions (Jordan et al., Citation1992), or may even be heterotrophic (Brand, Citation1994).
Our analysis suggests that the three varieties and six size groups of F. profunda may have a widespread geographic distribution in the Atlantic and Pacific Oceans. They co-occur in single samples and exhibit similar seasonal distributions and ecologies at the HOT time series station, suggesting that they are not ecophenotypes of a single species. Such a hypothesis may be tested in laboratory culture under varying environmental conditions (Bollmann, Citation1997; Quinn et al., Citation2003) or with molecular genetics (Sáez et al., Citation2003). However, despite our recent attempts F. profunda has not yet been successfully isolated and maintained in culture. In fact, only a small proportion (ca. 20%) of all living coccolithophore species have so far been cultured (Probert & Houdan, Citation2004) and the taxonomy of this phytoplankton group therefore remains mainly based on the morphology of their calcareous skeletons. Nevertheless, the application of new techniques for the combined morphometric and molecular genetic analysis of microscopic plankton (de Vargas et al., Citation2004) is likely to improve this situation.
Acknowledgements
The results presented here form part of a project funded by the Swiss Federal Institute for Technology (ETH), (Grant No. 0–20974–02). The authors would like to thank the reviewers for their useful comments and Andrea Fiorentino for the Latin translation.
References
References
- Ahagon , N , Tanaka , Y and Ujiie , H . 1993 . Florisphaera profunda, a possible nannoplankton indicator of late Quaternary changes in sea-water turbidity at the northwestern margin of the Pacific . Mar. Micropalaeon. , 22 : 255 – 273 .
- Beaufort , L , de Garidel-Thoron , T , Mix , AC and Pisias , NG . 2001 . ENSO-like forcing on oceanic primary production during the late Pleistocene . Science , 293 : 2440 – 2444 .
- Beaufort , L , Lancelot , Y , Camberlin , P , Cayre , O , Vincent , E , Bassinot , F and Labeyrie , L . 1997 . Insolation cycles as a major control of Equatorial Indian Ocean primary production . Science , 278 : 1451 – 1454 .
- Bollmann , J . 1997 . Morphology and biogeography of Gephyrocapsa coccoliths in Holocene sediments . Marine Micropaleontology , 29 : 319 – 350 .
- Bollmann , J , Henderiks , J and Brabec , B . 2002 . Global calibration of Gephyrocapsa coccolith abundance in Holocene sediments for paleotemperature assessment . Paleoceanography , 17 : 1 – 9 .
- Bollmann , J , Cortes , MY , Haidar , AT , Brabec , B , Close , A , Hofmann , R , Palma , S , Tupas , L and Thierstein , HR . 2002 . Techniques for quantitative analyses of calcareous marine phytoplankton . Mar. Micropaleon. , 44 : 163 – 185 .
- Brand LE 1994 Physiological ecology of marine coccolithophores In: Coccolithophores (Winter, A.& Siesser, W.G. editors) 39 47 Cambridge University Press Cambridge
- Cortés , MY , Bollmann , J and Thierstein , HR . 2001 . Coccolithophore ecology at the HOT station HOT, Hawaii . Deep. Sea Res. II , 48 : 1957 – 1981 .
- Cros , L and Fortuño , J-M . 2002 . Atlas of northwestern Mediterranean coccolithophores . Sci. Mar. , 66 ( Supplement 1 )
- Darling , KF , Kroon , D , Wade , CM and Brown , AJL . 1996 . Molecular phylogeny of the planktic foraminifera . J. Foram. Res. , 26 : 324 – 330 .
- de Vargas C Sáez A Thierstein HR 2004 Super-species in the calcareous plankton In: Coccolithophores – From Molecular Processes to Global Impact (Thierstein, H. & Young, J.R. editors) 271 298 Springer Berlin
- Geisen , M , Billard , C , Broerse , ATC , Cros , L , Probert , I and Young , JR . 2002 . Life-cycle associations involving pairs of holococcolithophorid species: Intraspecific variation or cryptic speciation? . Eur. J. Phycol. , 37 : 531 – 550 .
- Haidar , AT and Thierstein , HR . 2001 . Coccolithophore dynamics off Bermuda (N. Atlantic). . Deep Sea Res. II , 48 : 1925 – 1956 .
- Henriksson , AS . 2000 . Coccolithophore response to oceanographic changes in the equatorial Atlantic during the last 200,000 years . Palaeogeography, Palaeoclimatology, Palaeoceanography , 156 : 161 – 173 .
- Jordan RW Kleijne A 1994 A classification system for living coccolithophores In: Coccolithophores (Winter, A. & Siesser, W.G. editors) 83 105 Cambridge University Press Cambridge
- Jordan RW Weaver PPE Shackelton JJ 1992 The paleoecology and paleoceanography of Florisphaera profunda in Late Quaternary sediments In: Fourth International Conference on Paleoceanography (ICP IV), 158. Berichte–Reports Geologisches–Paläontologisches Institüt Universität Kiel 57
- Kinkel , H , Baumann , K-H and Cepek , M . 2000 . Coccolithophores in the equatorial Atlantic Ocean: response to seasonal and Late Quaternary surface water variability . Mar. Micropaleon. , 39 : 87 – 112 .
- Kleijne , A . 1991 . Holococcolithophorids from the Indian Ocean, Red Sea, Mediterannean Sea and North Atlantic Ocean . Mar. Micropaleon. , 17 : 1 – 76 .
- Kleijne A 1993 Morphology, taxonomy and distribution of extant coccolithophorids (Calcareous nanoplankton) Proefschrift Vrije Universiteit Amsterdam
- Knappertsbusch , M , Cortes , MY and Thierstein , HR . 1997 . Morphologic variability of the coccolithophorid C. leptoporus in the plankton, surface sediments and from the Early Pleistocene . Mar. Micropalaeon. , 30 : 293 – 317 .
- McIntyre , A and Molfino , B . 1996 . Forcing of Atlantic equatorial and subpolar millennial cycles by precession . Science , 274 : 1876 – 1870 .
- Molfino , B and McIntyre , A . 1990 . Precessional forcing of nutricline dynamics in the equatorial Atlantic . Science , 249 : 766 – 769 .
- Norris , RD and de Vargas , C . 2000 . Evolution all at sea . Nature , 405 : 23 – 24 .
- Okada H 1983 Modern nanofossil assemblages in sediments of coastal and marginal seas along the western Pacific Ocean In: Reconstruction of Marine Environments (Meulenkamp, J.E. editor) 171 187 Utrecht Micropaleontology Bulletin Utrecht
- Okada , H and Honjo , S . 1973 . The distribution of oceanic coccolithophorids in the Pacific . Deep Sea Res. , 20 : 355 – 374 .
- Okada , H and McIntyre , A . 1977 . Modern coccolithophores of the Pacific and North Atlantic Oceans . Micropaleontology , 23 : 1 – 55 .
- Okada , H and McIntyre , A . 1979 . Validation of Florisphaera profunda var elongata . Int. Nanoplank. News , 1 : 2
- Okada , H and McIntyre , A . 1980 . Validation of Florisphaera profundax var elongata . Int. Nanoplank. News. , 2 : 81
- Perch-Nielsen K 1985 Cenozoic calcareous nanofossils In: Plankton Stratigraphy (Bolli, H.M., Saunders, J.B. & Perch-Nielsen, K. editors) 427 554 Cambridge University Press Cambridge
- Probert I Houdan A 2004 The laboratory culture of coccolithophores In: Coccolithophores – From Molecular Processes to Global Impact (Thierstein, H. & Young, J.R. editors) 217 249 Springer Berlin
- Quinn , PS , Thierstein , HR , Brand , LE and Winter , A . 2003 . Experimental evidence for the species character of Calcidiscus leptoporus morphotypes . J. Paleon. , 77 : 825 – 830 .
- Quinn PS Sáez A Baumann K-H Steel BA Sprengel C Medlin LK 2004 Coccolithophorid biodiversity: Evidence from the cosmopolitan species Calcidiscus leptoporus In: Coccolithophores – From Molecular Processes to Global Impact (Thierstein, H. & Young, J.R. editors) 299 236 Springer Berlin
- Renaud , S , Ziveri , P and Broerse , ATC . 2002 . Geographic and seasonal differences in the morphology and dynamics of the coccolithophore Calcidiscus leptoporus . Mar. Micropalaeon. , 46 : 363 – 385 .
- Sáez , AG , Probert , I , Geisen , M , Quinn , PS , Young , JR and Medlin , LK . 2003 . Pseudo-cryptic speciation in coccolithophores . Proc. Nat. Acad. Sci. USA , 100 : 7163 – 7168 .
- Young JR 1998 Neogene In: Calcareous Nanofossil Biostratigraphy (Bown, P.R., editor) 225 265 Kluwer Academic Publishers Amsterdam
- Young JR 1994 Functions of coccoliths In: Coccolithophores (Winter, A. & Siesser, W.G. editors) 63 82 Cambridge University Press
- Young JR Geisen M Cros L Kleijne A Sprengel C Probert I Østergaard J 2003 A guide to extant coccolithophore taxonomy J. Nanoplank. Res. Special Issue 1
- Young , JR and Westbroek , P . 1991 . Genotypic variation in the coccolithophorid species Emiliania huxleyi . Mar. Micropaleon. , 18 : 5 – 23 .
- Young , JR , Bergen , JA , Bown , PR , Burnett , JA , Fiorentino , A , Jordan , RW , Kleijne , A , van Niel , BE , Romein , AJ and Von-Salis , K . 1997 . Guidelines for coccolith and calcareous nanofossil terminology . Palaeontology , 40 : 875 – 912 .