Abstract
Iron, one of the structural elements of organic components that play an essential role in photosynthesis and nitrogen assimilation of plants, is available at extremely low concentrations in large parts of the Southern Ocean's surface waters. We tested the hypothesis that photosynthesis is the primary target of iron stress in phytoplankton living in this specific environment, resulting in a reduced carbohydrate production. Cultures of a small Antarctic diatom, Chaetoceros brevis, were exposed to two different photon irradiances under iron-rich and iron-poor conditions. Under both light regimes growth rate was reduced only slightly by iron starvation, as expected because the iron requirement of a small-celled species such as C. brevis is low. Even so, iron-starved cells differed markedly from iron-replete cells: for low and high irradiance, respectively, they had a 20 and 27% lower content of light-harvesting pigments (chlorophyll a and c 2 and fucoxanthin), a 8 and 15% decrease in light absorption and a 15 and 17% decrease in quantum yield of photosystem II. The diurnal production of water-extractable carbohydrates was reduced by 28 and 31%, which resulted in a low supply of energy and carbon skeletons from these storage products. This may well have influenced protein synthesis. The nocturnal consumption of carbohydrates was also reduced, which, together with the almost proportional decrease in cellular C and N content, suggests that the C and N metabolism were tightly tuned in iron-stressed cultures. The decrease in C and N content correlated with a decrease in cell volume. Our results indicate that iron limitation is likely to affect the ability of phytoplankton to maintain high rates of protein synthesis within the deep wind-mixed layer of the Southern Ocean. In addition, growth at the surface could be inhibited by too much light: iron-poor cultures of C. brevis grown at low irradiance showed enhanced sensitivity to photoinhibition.
Introduction
Recent in situ iron fertilization experiments (Boyd et al., Citation2000; Dalton, Citation2002; Gervais et al., Citation2002) have confirmed the evidence obtained earlier that phytoplankton growth in the Southern Ocean is limited by iron (De Baar & Boyd, Citation2000). Iron plays a crucial role in processes in plants that require electron transfer reactions, including photosynthesis and nitrogen assimilation. One of the most noticeable effects of iron deficiency on photosynthesis is a decrease in cellular content of chlorophyll (chl) a and other pigments involved in light harvesting (Glover, Citation1977; Doucette & Harrison, Citation1990; Greene et al., Citation1991; Van Leeuwe & Stefels, Citation1998). Iron limitation also reduces the synthesis of proteins of the photosynthetic apparatus, like the D1-protein in photosystem II (PSII) (Greene et al., Citation1992; Vassiliev et al., Citation1995). In addition, the functioning of the Photosynthetic Electron Transport (PET) chain is affected by a decrease in iron-sulphur complexes, e.g. ferredoxin (Erdner et al., Citation1999; McKay et al., Citation1999). As a consequence, photosynthetic efficiency is reduced in iron-limited cells (Geider et al., Citation1993; Davey & Geider, Citation2001).
Iron is also a structural component of the metallo-enzymes that are involved in nitrate uptake, nitrite and nitrate reductase (Gao et al., Citation1993). The activity of nitrate reductase is low in iron-deficient phytoplankton (Timmermans et al., Citation1994, Citation1998). If this impairs the ability to metabolize nitrate, it could lead to high cellular carbohydrate : protein and carbon : nitrogen (C : N) ratios due to a decrease in cellular nitrogen content and the accumulation of glucan. These are well-known features of nitrogen-limited phytoplankton cells (Myklestad, Citation1988). So far, however, no increase in C : N ratios in iron-deplete phytoplankton has been observed (Greene et al., Citation1991; Maldonado & Price, Citation1996; Davey & Geider, Citation2001). In fact, Rueter and Ades (Citation1987) had already suggested that nitrogen assimilation of iron-deplete cells of the freshwater alga Scenedesmus quadricauda (Chlorophyceae) was not as strongly affected as photosynthesis, because the cells incorporated a large fraction of total carbon into proteins, as measured by 14C fractionation.
The effect of iron limitation on C metabolism or N metabolism could be light dependent. In light-limited cells, more iron is needed to enlarge the photosynthetic apparatus in order to increase light absorption (Raven, Citation1990; Strzepek & Price, Citation2000). Stefels and Van Leeuwe (Citation1998) also found indications based on dimethylsulphoniopropionate (DMSP) measurements that cells of Phaeocystis sp. were energy-limited under low light, low iron conditions, whereas cells were bordering on nitrogen deficiency induced by iron limitation under high light, low iron conditions.
Here we elaborate further on the ‘photosynthesis = primary target’ – hypothesis by studying carbohydrate metabolism. Carbohydrates are the first products of the Calvin-Benson cycle, the activity of which is dependent on ATP and NADPH generated by photosynthesis. Under saturating light conditions, a significant part of the carbon fixed by phytoplankton is stored as polysaccharides (Vårum et al., Citation1986). For diatoms the main storage carbohydrate is β-1,3-glucan, which is easily extracted in water (Myklestad, Citation1988). This glucan can facilitate continued protein synthesis in darkness by the provision of energy and carbon skeletons (Cuhel et al., Citation1984; Granum & Myklestad, Citation2001). We investigated the hypothesis that photosynthesis is the primary target of iron limitation, which would lead to a reduction in the diurnal water-extractable carbohydrate production. We studied this at two different irradiances in laboratory experiments with the Antarctic diatom Chaetoceros brevis.
Materials and methods
Cell culture
The single celled Antarctic diatom Chaetoceros brevis (no. CCMP 163, diameter approx. 4 μm) was cultured in a 8 h : 16 h light : dark regime in filtered (0.2 μm) natural seawater medium. The iron concentration was 15 nM (measured by organic ligand solvent extraction followed by atomic absorption spectrophotometry: Nolting and De Jong, Citation1994); 140 μM NaNO3, 7.5 μM K2HPO4 and 60 μM Na2SiO3 were added. The medium was supplemented with vitamins and trace metals (excluding iron) as described by Admiraal and Werner (Citation1983). Except for silicate, all major nutrients were cleaned from iron contamination with a chelex column (Aquil). Low iron availability was further achieved by adding 1 mM Na2-EDTA.
Experimental set-up
Three cultures were maintained separately for several months at 4°C, with regular transfer of the cells into fresh medium. Several weeks before the start of the experiment, subsets of the cultures were placed at two different photon irradiances (light source TL-Biolux, Philips), 25 and 100 μmol m − 2s − 1. Each of these six cultures was used to inoculate two 3-l polycarbonate Fernbach bottles with 1.5 l medium, which were placed at the pre-adapted irradiance. The cultures were covered with parafilm and were gently shaken several times a day to facilitate gas exchange between the medium and the air. After 1 week, 1 μM iron (FeCl3) was added to one of these bottles (picked at random). At the moment of iron addition (t = 0), cell density in the cultures was 6 – 7 × 107 l − 1.
At t = 0, 3 and 5 days, samples were taken for cell volume, cell number and polysaccharide concentration, at the beginning and at the end of each light period. In the morning, in vivo chl a fluorescence parameters (see below) were measured. At the end of the light period, samples were taken for pigment analysis and POC/PON analysis (also described below). At the end of the experiment, samples were taken for in vivo light absorption measurements.
Cell counts
Cell numbers and cell volume were determined with an automated particle counter (Coulter, model ZM). The count tube (diameter 30 μm) was calibrated with polystyrene particles (Coulter no. 9966067, diameter 8.7 μm). Samples were diluted with artificial seawater to a cell concentration of 20 – 60 cells per μl; 200 μl was counted per sample. Growth rates were calculated by fitting the model for exponential growth:
where N is cell number at different times, t is time in days and μ is growth rate (in d − 1). The regression coefficient (r2) of the fitted curves varied between 0.9910 and 0.9999 (n = 9).
Pigment analysis
Samples of 20 – 100 ml were filtered through 25-mm GF/F Whatman filters under gentle vacuum pressure ( < 200 mm Hg). The filters were frozen in liquid nitrogen immediately after filtration and stored at − 80°C. Later, filters were freeze-dried and extracted in 90% acetone for 48 h. Pigments were separated and quantified by HPLC according to Kraay et al. (Citation1992). The HPLC system consisted of a Waters 2690 Separations Module and a Waters 996 Photodiode Array Detector (Milford, MA, USA). A Waters DeltaPak (Milford, MA, USA) reversed-phase column (C18, 5 μm, 100 Å, 150 × 3.9 mm, fully end capped) was used. Pigment standards were obtained from DHI Water & Environment (Hørholm, Denmark). For β-carotene no standard was available; the conversion factor applied was taken from zeaxanthin because both pigments have similar absorption characteristics (Jeffrey et al., Citation1997).
In vivo absorption spectra measurements
The in vivo spectral absorption cross section (a*λ, in m2 mg − 1 chl a or in m2 cell − 1, λ = 350 – 700 nm) was determined on cell suspensions in a Varian-Cary 3E double beam spectrophotometer equipped with an integrating sphere. Spectra were corrected for background noise. The spectrally averaged absorption coefficient for photosynthetically available radiation (PAR) normalized to chl a, a*PAR (in m2 mg − 1 chl a), was calculated from the convolution of a*λ (400 – 700 nm) with the emission spectrum for the lamps used in our experiments (Dubinsky et al., Citation1986).
Chlorophyll a fluorescence measurements
In vivo chl a fluorescence was measured with a pulse-amplitude modulated fluorometer (PAM 2000, Walz, Effeltrich, Germany). Between 10 and 100 ml of culture was filtered through a 10-mm GF/F filter at a vacuum pressure less than 300 mm Hg. The filtration was stopped before the filter dried up to prevent cells from being damaged. The filter was quickly transferred to a custom-made cuvette filled with seawater (cooled to 4°C) and the fibre optic probe was inserted into the cuvette. After application of a 5-s far-red pulse, the algae were darkened for 5 min, before a second far-red pulse was applied. Then the minimal fluorescence (F0) and the maximal fluorescence (Fm) were measured to determine the maximum quantum yield of photosystem II (PSII) (Schreiber et al., Citation1994): Fv : Fm = (Fm − F0)/Fm, where Fv is the variable fluorescence.
Photosynthesis-irradiance response curves
After another far-red pulse, the algae on the filter were exposed to 10 irradiances up to 370 μmol m − 2 s − 1. The actinic light was provided by the red LED (650 nm) of the fluorometer. The effective quantum yield of PSII (ΦPSII) was determined each time after 30 s light exposure. The relative PSII electron transport rate (rETR) at each irradiance was calculated as: rETR = ΦPSII·irradiance (Genty et al., Citation1989).
The photosynthesis-irradiance response curve (as rETR vs irradiance) was fitted following recommendations of Frenette et al. (Citation1993). If no inhibition was observed, the model of Webb et al. (Citation1974) was used:
where rETRmax = maximum relative electron transport rate under optimal light, α = initial slope, β = photoinhibition constant, E = irradiance. If a curve showed inhibition, the exponential function of Platt et al. (Citation1980) was applied:
where rETRs = maximum, potential relative electron transport rate in the absence of photoinhibition under optimal light. In this case, rETRmax was calculated as follows:
The light saturation parameter Ek was calculated as rETRmax/α.
POC and PON analysis
Samples of 20 – 100 ml were filtered through pre-combusted (350°C) 10-mm GF/F Whatman filters under gentle vacuum pressure ( < 200 mm Hg). The filters were stored at − 80°C until analysis. The filters were analysed for particulate carbon (POC) and nitrogen (PON) on a CHNS-analyser type EA 1110 (Interscience, Breda, The Netherlands).
Carbohydrate analysis
Samples of 20 – 100 ml were filtered through pre-combusted (350°C) 25-mm GF/F Whatman filters under gentle vacuum pressure ( < 200 mm Hg). The filters were stored at − 20°C until analysis. They were extracted in water (Milli-Q) in sealed glass test tubes placed in a water bath for 1 h at 80°C. Afterwards, the tubes were cooled and centrifuged for 5 min at 715 g. The polysaccharide concentration was determined with the colorimetric TPTZ-method described by Myklestad et al. (Citation1997), with minor modifications. D( + )-Glucose was used as the standard.
Statistics
Differences between treatments were tested with multi-factor analyses of variance (ANOVA), following the procedure described by Underwood (Citation1997). Factors included were iron, light and, where applicable, the number of days after iron addition and the time of day (beginning/end light period). To examine the strength of a relationship between two variables, Pearson correlation coefficients were calculated.
Results
Growth rate and cell volume
Iron and light availability both had a significant effect on growth rate (). Maximum growth rates were measured for cells cultured at high irradiance and high iron conditions. The combination of low iron and low irradiance resulted in the lowest growth rates. Growth rates of iron-enriched cultures were approximately 6% higher than those of iron-limited cultures, both at low and high irradiance. At the end of the experiment, iron-limited cells were 12 – 14% smaller than iron-replete cells ().
Table 1. Physiological characteristics of the Antarctic diatom Chaetoceros brevi s grown in batch cultures under high and low light with and without iron addition
Pigment content
As expected for a diatom, cells contained chl a and c 2, fucoxanthin, diadinoxanthin, diatoxanthin and β-carotene. Diatoxanthin was detected only in C. brevis cells exposed to high irradiance. Cells exposed to a low irradiance had higher total pigment content than those exposed to a high irradiance, under both iron-rich and iron-poor conditions (). Highest diadinoxanthin contents were recorded for iron-replete cells exposed to a high irradiance (0.08 pg cell − 1). A reduction in cellular content of all pigments was observed as a result of iron limitation. There was a significant interaction between the effect of iron and light for the cellular content of all pigments except β-carotene (). At the end of the experiment, the chl a content of the iron-stressed cells was reduced by approximately 16% at high irradiance and 27% at low irradiance. There was a significant interaction between the factors iron and light for the ratios of chl a : C and chl a : N; these ratios were reduced only by iron limitation at low irradiance (). There was a small but significant effect of iron and light on the chl c 2 to chl a ratio and the fucoxanthin to chl a ratio (). The diadinoxanthin to chl a ratio and the β-carotene to chl a ratio were both higher at high than at low irradiance. For both ratios, there was a significant interaction between iron and light: they were reduced more by iron limitation at high irradiance. The ratio of diatoxanthin to diadinoxanthin was higher at high (0.023) than at low irradiance (0) but was not influenced by iron deficiency.
Table 2. The (relative) pigment content of Chaetoceros brevi s cells grown for 5 days in batch cultures under high and low light with and without iron
In vivo absorption spectra
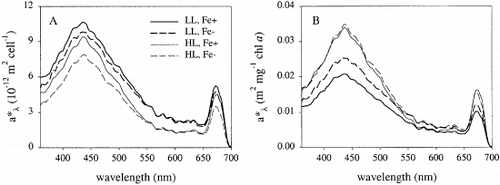
At the end of the experiment, cell-specific absorption was highest for cells exposed to low irradiances (). Cellular absorption capacity was higher for iron-replete than iron-deficient cells, at both low and high irradiance (8 and 15%, respectively). The difference in absorption was most pronounced at 430 and 675 nm. The light absorption per unit chl a was highest for cells cultured at high irradiance (). The absorption coefficient (a*PAR) was significantly affected by iron and light limitation and varied between 0.02 and 0.04 m2 mg chl a − 1 (). There was a significant interaction between iron and light: a*PAR increased more in response to iron limitation at low irradiance than at high irradiance.
Chlorophyll a fluorescence
There was a significant effect of iron concentration and irradiance on the maximal quantum yield of photosystem II (Fv : Fm) (). At the end of the experiment, the ratio Fv : Fm was higher for cells exposed to a low irradiance than for those receiving a high irradiance. Fv : Fm was lower for iron-deficient cultures than for iron-replete cultures, at both low and high irradiance (15 and 17%, respectively).
Photosynthesis-irradiance response curves
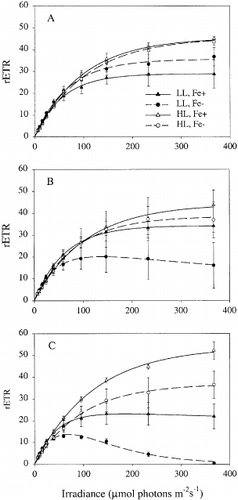
PE-curves measured at t = 0, 3 and 5 days after iron addition are presented in . At t = 0, the maximum relative rate of photosynthesis (rETRmax) was highest for cells pre-cultured at high irradiance. Differences between the PE-curves of the iron-replete and iron-deplete treatments gradually increased over the course of the experiment ( – C). At t = 5, rETRmax and the light saturation parameter Ek (rETRmax/α) were significantly affected by iron limitation (). There was no significant effect of iron availability on α, but there was a significant interaction between iron and light for this parameter. The algae growing at low irradiance and low iron showed inhibition if exposed to high irradiance. There also was a significant interaction between iron and light for the photoinhibition parameter (β): an increase in β due to iron limitation was observed only at low irradiance.
Table 3. Photosynthesis-irradiance curve parameters for Chaetoceros brevi s cells grown for 5 days in batch cultures under high and low light with and without iron
POC and PON
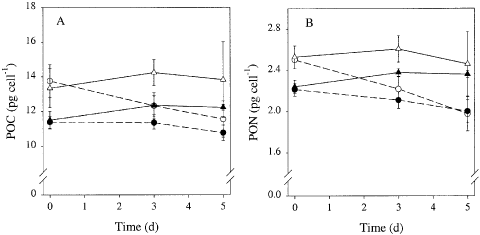
There was a significant effect of iron concentration and irradiance on the cellular carbon content (ANOVA, iron: p = 0.002, light: p < 0.001, iron× light: p = 0.54). Carbon content was higher at high irradiance than at low irradiance (). During the course of the experiment the carbon content in iron-deficient cells decreased relative to that of iron-replete cells at both irradiances. The cellular nitrogen content was also significantly affected by iron and light limitation (ANOVA, iron: p < 0.001, light: p < 0.001, iron × light: p = 0.32), in a qualitatively similar manner (). At the end of the experiment, the C : N ratio was highest in cultures grown at high irradiance () and was slightly (4%) higher in iron-deplete cultures than in iron-replete cultures.
Polysaccharides
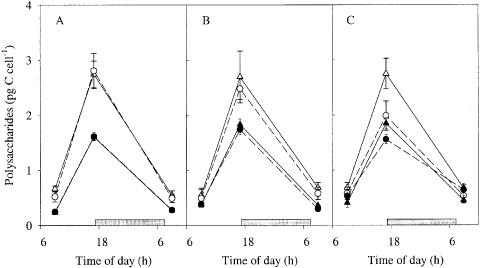
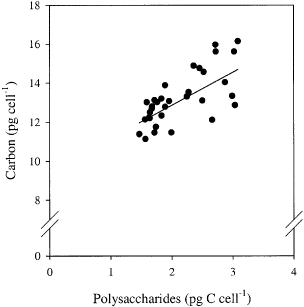
The net diurnal cellular production of polysaccharides was highest at high irradiance (). Over the course of the experiment, production was lower in iron-deplete cultures at both irradiances, but remained unchanged in iron-replete cells. After 5 days, the production was higher in iron-enriched cultures for both irradiances (). The percentage increase in polysaccharide quota during the light period was highest in iron-replete cultures: 370% at low irradiance and 330% at high irradiance. In iron-deplete cultures the increase was 220 and 290%, respectively. There was a significant effect of iron and light on the net nocturnal cellular polysaccharide consumption (ANOVA, iron: p = 0.012, light: p = 0.003, iron× light: p = 0.13). The decrease in the total polysaccharide concentration in the cultures during the dark period was around 70% for iron replete cells, at both irradiances. In iron-deplete cultures the decrease was around 50% (low irradiance) and 60% (high irradiance). About 20% of total carbon was polysaccharide (assuming the polysaccharides mainly consist of glucose, which contains 40% C by weight). The cellular carbon quota at the end of the day correlated with the cellular polysaccharide C quota, irrespective of the treatments (, linear correlation if all data are pooled: r = 0.71, n = 34). The rate of diurnal polysaccharide production correlated positively with the PE-curve parameters rETRmax (r = 0.75, n = 34) and the rETR at the cultured irradiance (r = 0.83, n = 34).
Discussion
Effects of iron limitation on photosynthesis and carbohydrate metabolism
Low iron concentrations led to a decrease in the amount of light harvesting complexes in cells of Chaetoceros brevis, accompanied by a reduced capacity to absorb light and a lower efficiency of the PET chain. These changes in photochemical characteristics are evidence that photosynthesis was the prime target of iron starvation, but the decline in the diurnal production of water-extractable carbohydrates is even stronger evidence for this notion. Indeed, iron-limited phytoplankton cells have physiological characteristics of cells that are energy limited, with a reduced metabolism. In contrast, cells that are nitrogen-limited tend to accumulate storage polysaccharides (Sakshaug & Holm-Hansen, Citation1977; Geider & La Roche, Citation1994). In fact, the diel change in the polysaccharide content and not the overall polysaccharide content was found to be an indicator of iron stress.
Cellular carbon and nitrogen quota both decreased in response to iron limitation. A decrease in C and N has also been observed in iron-limited Phaeodactylum tricornutum (Greene et al., Citation1991), Thallasiosira weissflogii (Milligan and Harrison, Citation2000) and Actinocyclus sp. (Muggli et al., Citation1996). The decrease in carbon content can be ascribed to a reduction in the rate of carbon assimilation due to the affected photosynthesis. The reduced build-up of water-extractable polysaccharides in the iron-stressed cells at t = 5 supports this notion. Cellular nitrogen quota might be affected directly by iron limitation through changes in the activity of enzymes involved in nitrogen assimilation, or more indirectly by changes in the supply of photosynthetically derived reductant to these enzymes or by the supply of carbon skeletons. Milligan and Harrison (Citation2000) found a reduction in the activity of nitrate and nitrite reductase in iron-stressed cells of the marine diatom Thalassiosira weissflogii that, however, did not limit N assimilation: the decrease in cellular N quota was allegedly caused by a diminished supply of NADPH for nitrite reduction, causing excretion of nitrite. Interestingly, no significant change in the total carbohydrate pool in response to iron limitation was observed by Milligan and Harrison (Citation2000). A difference between our study and that of Milligan and Harrison (Citation2000) is that we focussed on the water-extractable fraction, not the total carbohydrate content. A bulk measurement includes both storage glucan and structural carbohydrates of the cell membrane. The latter are of course not available for dark protein synthesis and hardly ever show diel variability. Our data indicate that limitation of nitrogen assimilation by restricted carbohydrate supply cannot be ruled out because the diurnal production of water-extractable polysaccharides was low in iron-limited cells. The ratio of C : N increased only slightly, which, combined with the observed decrease in carbohydrate consumption during the dark period in iron-stressed cells, suggests that C and N-metabolism were tightly coupled. This may have been the result of a diminished supply of NADPH for nitrite reduction, as suggested by Milligan and Harrison (Citation2000). Further investigations should answer this question. The reductions in cellular pigment, C and N content in iron-stressed cells are probably related to the decrease in cell size. A decrease in cell size is commonly observed in iron-stressed phytoplankton cells and is suggested to be an adaptation to low iron availability (Geider & La Roche, Citation1994). Interestingly, cellular pigment content decreased more than cell volume and C and N content, especially at low irradiance, leading to a decrease in chl a : C and chl a : N. This has been observed previously by Greene et al. (Citation1991).
A decrease in carbohydrate synthesis due to iron limitation has also been observed in higher plants. In citronella (Cymbopogon winterianus), the amount of 14C incorporated into an operationally defined sugar fraction was found to be reduced (Srivastava et al., Citation1998), and in sugar beet (Beta vulgaris) net diurnal starch accumulation rate per unit of leaf area was severely reduced upon iron stress (Arulanantham et al., Citation1990). The starch accumulation rate correlated well with the rate of photosynthesis. All this corroborates our finding of a high correlation between the carbohydrate accumulation rate and the electron transport rate.
Growth rate was strongly reduced by light limitation but only little by iron limitation. A possible explanation for the small impact of iron limitation on growth rate is that C. brevis has lower iron requirements for growth than larger diatoms such as C. dichaeta (Timmermans et al., Citation2001). Hence, iron availability was probably not limiting at the start of the experiment. However, iron-deplete cultures presumably ran into iron limitation by their own growth and consumption of the available iron, as indicated by an increased impairment of photosynthesis with time. Given the observed impact of iron limitation on PE-characteristics and carbohydrate production, more pronounced effects on growth can be anticipated under prolonged iron stress.
Combined effects of light and iron
An expected enhancement of the effects of iron limitation on growth rate due to a decrease in iron-use efficiency at low light was not observed even though the cellular chl a content was more strongly affected by iron limitation at low irradiance than at high irradiance. This discrepancy may be explained by the stronger increase in the absorption coefficient a*PAR in response to iron deficiency at low irradiance, which would counteract the effects of the decrease in pigment content at this irradiance. An increase in a*PAR due to iron limitation has also been observed by Greene et al. (Citation1991) in the diatom Phaeodactylum tricornutum and by Van Leeuwe and De Baar (Citation2000) in the prasinophyte Pyramimonas sp.. It has been attributed to a decrease in pigment content, which reduces the ‘package-effect’, i.e. the shading of thylakoid membranes (Berner et al., Citation1989).
The ratio of the accessory light harvesting pigments (chl c 2 and fucoxanthin) to chl a varied little among the different experimental treatments. The ratios of diatoxanthin + diadinoxanthin to chl a and of diatoxanthin to diadinoxanthin were highest at high irradiance. Synthesis and de-epoxidation of diadinoxanthin are processes that play a photoprotective role by increasing dissipation of excitation energy in photosystem II (Olaizola et al., Citation1994). An increase in the ratio of diatoxanthin to diadinoxanthin in response to iron limitation has been observed in a few studies, where it was presented as an adaptive response to enhanced radical formation caused by iron limitation (Geider et al., Citation1993; Van Leeuwe & Stefels, Citation1998). In C. brevis, however, radical formation did not seem to be a major problem at the selected irradiances, as indicated by the very low cellular content of diatoxanthin. Apparently, iron-deplete cells preferentially invested in light harvesting capacity, not in photoprotection, as indicated by the decrease in the ratio of diatoxanthin + diadinoxanthin to chl a. Notably, β-carotene, another pigment that can function as a photoprotector (Middleton & Teramura, Citation1993), also decreased relative to chl a in response to iron limitation.
Iron limitation affected the maximum quantum yield of photosystem II (Fv : Fm) and the maximum relative electron transport rate (rETRmax) to a similar extent at both irradiances. The light utilization efficiency (α) was not affected by iron limitation, in agreement with the findings of Greene et al. (Citation1991). Van Leeuwe and De Baar (Citation2000) and Davey and Geider (Citation2001) found a decrease in α (based on 14C measurements). Apparently, in these studies the decrease in the package effect in iron-limited phytoplankton cells did not fully compensate for the decrease in the quantum yield of photosynthesis (see Greene et al., Citation1991). In our study, there was a significant interaction between iron and light for α, which was probably related to the stronger decrease in the package effect at low irradiance.
In agreement with the observed reduction in photoprotective pigments, our PE curves showed a strong inhibition at high irradiance for iron-limited cells, especially if cultured at low irradiance. This suggests that these cells are sensitive to photoinhibition if exposed to high irradiance. Inhibition has not been observed in PE-curves based on 14C incorporation measurements in earlier studies using similar irradiances (Greene et al., Citation1991; McKay et al., Citation1997), but longer incubation periods were used (usually 30 min to 1 h). Moreover, PE curves measured by PAM cannot be compared to 14C incorporation because different processes are measured by these methods (electron transport and carbon assimilation, respectively).
Some caution is needed in the quantitative comparison of the impact of iron between the two irradiances. Analysis of the interaction between iron and light availability is complicated by the possible increase in photo-redox cycling of Fe-EDTA chelates at high irradiance which may increase the availability of iron to phytoplankton (Hudson & Morel, Citation1990). Furthermore, since growth rate differed between the two irradiances and iron availability was probably related to cell number (see above), the changes in the availability of iron through the course of time may have differed between the light treatments.
Conclusion and ecological considerations
Iron stress appears primarily to affect photosynthesis in C. brevis, causing a reduction in the diurnal production of water-extractable carbohydrates. This may have implications for phytoplankton of the Southern Ocean, where cells experience frequent changes in irradiance due to deep mixing caused by high surface wind stress (Mitchell et al., Citation1991). Under such conditions, the carbohydrate pool can serve as an energy buffer or reserve, allowing cells to store radiation energy during the short periods spent near the surface where light is available at photosynthetically saturating levels; in this way an energy and carbon source, and thus time to adapt, is provided when irradiance decreases (Post et al., Citation1985). Our results suggest that this process is affected in iron-limited diatom cells. Near the surface, cell growth may also be limited but in a quite different way, namely by too much light: iron-stressed cultures of C. brevis which had been exposed to low irradiance showed enhanced sensitivity to photoinhibition.
Acknowledgements
We thank B.Venema for carbon and nitrogen analysis, R. Visser for pigment measurements and W. van de Poll for assistance with PAM-fluorometry. We acknowledge J. Stefels and two anonymous reviewers for valuable comments on the manuscript. This project was partly funded by the European Commission's Environmental and Climate Research Programme, CARUSO (CARbon dioxide Uptake by the Southern Ocean, EC contract # ENV4-CT 97-0472).
References
References
- Admiraal , W and Werner , D . 1983 . Utilization of limiting concentrations of ortho-phosphate and production of extracellular organic phosphates in cultures of marine diatoms . J. Plankton Res , 5 : 495 – 513 .
- Arulanantham , AR , Rao , IM and Terry , N . 1990 . Limiting factors in photosynthesis: VI. Regeneration of ribulose 1,5-bisphosphate limits photosynthesis at low photochemical capacity . Plant Physiol , 93 : 1466 – 1475 .
- Berner , T , Dubinsky , Z , Wyman , K and Falkowski , PG . 1989 . Photoadaptation and the “package” effect in Dunaliella tertiolecta (Chlorophyceae) . J. Phycol , 25 : 70 – 78 .
- Boyd , PW , Watson , AJ , Law , CS , Abraham , ER , Trull , T , Murdoch , R , Bakker , DCE , Bowie , AR , Buesseler , KO , Chang , H , Charette , M , Croot , PL , Downing , K , Frew , R , Gall , M , Hadfield , M , Hall , J , Harvey , M , Jameson , G , Laroche , J , Liddicoat , M , Ling , R , Maldonado , MT , McKay , RM , Nodder , S , Pickmere , S , Pridmore , R , Rintoul , S , Safi , K , Sutton , P , Strzepek , R , Tanneberger , K , Turner , S , Waite , A and Zeldis , J . 2000 . A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by iron fertilization . Nature , 407 : 695 – 702 .
- Cuhel , RL , Ortner , PB and Lean , DRS . 1984 . Night synthesis of protein by algae . Limnol. Oceanogr , 29 : 731 – 744 .
- Dalton , R . 2002 . Ocean tests raise doubts over use of algae as carbon sink . Nature , 420 : 722 – 722 .
- Davey , MS and Geider , RJ . 2001 . Impact of iron limitation on the photosynthetic apparatus of the diatom Chaetoceros muelleri (Baccillariophyceae) . J. Phycol , 37 : 987 – 1000 .
- de Baar HJW Boyd PW 2000 The role of iron in plankton ecology and carbon dioxide transfer of the global oceans In The Changing Ocean Carbon Cycle (Hanson, R.B. Ducklow, H.W. & Field, J.G., editors) 61 140 Cambridge University Press, Cambridge
- Doucette , GJ and Harrison , PJ . 1990 . Some effects of iron and nitrogen stress on the red tide dinoflagellate Gymnodinium sanguineum . Mar. Ecol. Prog. Ser , 62 : 293 – 306 .
- Dubinsky , Z , Falkowski , PG and Wyman , K . 1986 . Light harvesting and utilization by phytoplankton . Plant Cell Physiol , 27 : 1335 – 1349 .
- Erdner , DL , Price , NM , Doucette , GJ , Peleato , ML and Anderson , DM . 1999 . Characterization of ferredoxin and flavodoxin as markers of iron limitation in marine phytoplankton . Mar. Ecol. Prog. Ser , 184 : 43 – 53 .
- Frenette , JJ , Demers , S , Legendre , L and Dodson , J . 1993 . Lack of agreement among models for estimating the photosynthetic parameters . Limnol. Oceanogr , 38 : 679 – 687 .
- Gao , Y , Smith , GJ and Alberte , RS . 1993 . Nitrate reductase from the marine diatom Skeletonema costatum . Plant Physiol , 103 : 1437 – 1445 .
- Geider , RJ and La Roche , J . 1994 . The role of iron in phytoplankton photosynthesis and the potential for iron-limitation of primary productivity in the sea . Photosynth. Res , 39 : 275 – 301 .
- Geider , RJ , La Roche , J , Greene , RM and Olaizola , M . 1993 . Response of the photosynthetic apparatus of Phaeodactylum tricornutum (Bacillariophyceae) to nitrate, phosphate, or iron starvation . J. Phycol , 29 : 755 – 766 .
- Genty , B , Briantais , JM and Baker , NR . 1989 . The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence . Biochim. Biophys. Acta , 990 : 87 – 92 .
- Gervais , F , Riebesell , U and Gorbunov , MY . 2002 . Changes in size-fractionated primary productivity and chlorophyll a in response to iron fertilization in the Southern Polar Frontal Zone . Limnol. Oceanogr , 47 : 1324 – 1335 .
- Glover , H . 1977 . Effects of iron deficiency on Isochrysis galbana (Chrysophyceae) and Phaeodactylum tricornutum (Bacillariophyceae) . J. Phycol , 13 : 208 – 212 .
- Granum , E and Myklestad , SM . 2001 . Mobilization of beta-1,3-glucan and biosynthesis of amino acids induced by NH4 + addition to N-limited cells of the marine diatom Skeletonema costatum (Bacillariophyceae) . J. Phycol , 37 : 772 – 782 .
- Greene , RM , Geider , RJ and Falkowski , PG . 1991 . Effect of iron limitation on photosynthesis in a marine diatom . Limnol. Oceanogr , 36 : 1772 – 1782 .
- Greene , RM , Geider , RJ , Kolber , Z and Falkowski , PG . 1992 . Iron-induced changes in light harvesting and photochemical energy conversion processes in eukaryotic marine algae . Plant Physiol , 100 : 565 – 575 .
- Hudson , RJM and Morel , FMM . 1990 . Iron transport in marine phytoplankton: Kinetics of cellular and medium coordination reactions . Limnol. Oceanogr , 35 : 1002 – 1020 .
- Jeffrey SW Mantoura RFC Wright SW 1997 Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods UNESCO publishing
- Kraay , GW , Zapata , M and Veldhuis , MJW . 1992 . Separation of chlorophylls c1, c2, and c3 of marine phytoplankton by reversed-phase-C18-high-performance liquid chromatography . J. Phycol , 28 : 708 – 712 .
- Maldonado , MT and Price , NM . 1996 . Influence of N substrate on Fe requirements of marine centric diatoms . Mar. Ecol. Prog. Ser , 141 : 161 – 172 .
- McKay , RM , Geider , RJ and La Roche , J . 1997 . Physiological and biochemical response of the photosynthetic apparatus of two marine diatoms to Fe stress . Plant Physiol , 114 : 615 – 622 .
- McKay , RM , La Roche , J , Yakunin , AF , Durnford , DG and Geider , RJ . 1999 . Accumulation of ferredoxin and flavodoxin in a marine diatom in response to Fe . J. Phycol , 35 : 510 – 519 .
- Middleton , EM and Teramura , AH . 1993 . The role of flavonol glycosides and carotenoids in protecting soybean from UV-B damage . Plant Physiol , 103 : 741 – 752 .
- Milligan , AJ and Harrison , PJ . 2000 . Effects of non-steady-state iron limitation on nitrogen assimilatory enzymes in the marine diatom Thalassiosira weissflogii (Bacillariophyceae) . J. Phycol , 36 : 78 – 86 .
- Mitchell , BG , Brody , EA , Holm-Hansen , O , McClain , C and Bishop , J . 1991 . Light limitation of phytoplankton biomass and macronutrient utilization in the Southern Ocean . Limnol. Oceanogr , 36 : 1662 – 1677 .
- Muggli , DL , Lecourt , M and Harrison , PJ . 1996 . Effects of iron and nitrogen source on the sinking rate, physiology and metal composition of an oceanic diatom from the subarctic Pacific . Mar. Ecol. Prog. Ser , 132 : 215 – 227 .
- Myklestad , SM . 1988 . Production, chemical structure, metabolism, and biological function of the (1-3)-linked, beta-d-glucans in diatoms . Biol. Oceanogr , 6 : 313 – 326 .
- Myklestad , SM , Skånøy , E and Hestmann , S . 1997 . A sensitive and rapid method for analysis of dissolved mono- and polysaccharides in seawater . Mar. Chem , 56 : 279 – 286 .
- Nolting , RF and de Jong , JTM . 1994 . Sampling and analytical methods for the determination of trace metals in surface seawater . Int. J. Environ. Anal. Chem , 57 : 189 – 196 .
- Olaizola , M , La Roche , J , Kolber , Z and Falkowski , PG . 1994 . Non-photochemical fluorescence quenching and the diadinoxanthin cycle in a marine diatom . Photosynth. Res , 41 : 357 – 370 .
- Platt , T , Callegos , CL and Harrison , WG . 1980 . Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton . J. Mar. Res , 38 : 687 – 701 .
- Post , AF , Dubinsky , Z , Wyman , K and Falkowski , PG . 1985 . Physiological responses of a marine planktonic diatom to transitions in growth irradiance . Mar. Ecol. Prog. Ser , 25 : 141 – 149 .
- Raven , JA . 1990 . Predictions of Mn and Fe use efficiencies of phototrophic growth as a function of light availability for growth and of C assimilation pathway . New Phytol , 116 : 1 – 18 .
- Rueter , JG and Ades , DR . 1987 . The role of iron nutrition in photosynthesis and nitrogen assimilation in Scenedesmus quadricauda (Chlorophyceae) . J. Phycol , 23 : 452 – 457 .
- Sakshaug , E and Holm-Hansen , O . 1977 . Chemical composition of Skeletonoma costatum (Grev.) Cleve and Pavlova (monochrysis) lutheri (Droop) Green as a function of nitrate-, phosphate- and iron-limited growth . J. Exp. Mar. Biol. Ecol , 29 : 1 – 34 .
- Schreiber , U , Bilger , W and Neubauer , C . 1994 . Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis . Ecol. Stud , 100 : 49 – 70 .
- Srivastava , NK , Misra , A and Sharma , S . 1998 . The substrate utilization and concentration of 14C photosynthates in citronella under Fe deficiency . Photosynthetica , 35 : 391 – 398 .
- Stefels , J and van Leeuwe , MA . 1998 . Effects of iron and light stress on the biochemical composition of Antarctic Phaeocystis sp. (Prymnesiophyceae). I. Intracellular DMSP concentrations . J. Phycol , 34 : 486 – 495 .
- Strzepek , RF and Price , NM . 2000 . Influence of irradiance and temperature on the iron content of the marine diatom Thalassiosira weissflogii (Bacillariophyceae) . Mar. Ecol. Prog. Ser , 206 : 107 – 117 .
- Timmermans , KR , Stolte , W and de Baar , HJW . 1994 . Iron-mediated effects on nitrate reductase in marine phytoplankton . Mar. Biol , 121 : 389 – 396 .
- Timmermans , KR , van Leeuwe , MA , de Jong , JTM , McKay , RML , Nolting , RF , Witte , HJ , van Ooyen , J , Swagerman , MJW , Kloosterhuis , H and de Baar , HJW . 1998 . Iron stress in the Pacific region of the Southern Ocean: evidence from enrichment bioassays . Mar. Ecol. Prog. Ser , 166 : 27 – 41 .
- Timmermans , KR , Gerringa , LJA , de Baar , HJW , van der Wagt , B , Veldhuis , MJW , de Jong , JTM , Croot , PL and Boye , M . 2001 . Growth rates of large and small Southern Ocean diatoms in relation to availability of iron in natural seawater . Limnol. Oceanogr , 46 : 260 – 266 .
- Underwood AJ 1997 Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance Cambridge University Press, Cambridge
- van Leeuwe , MA and de Baar , HJW . 2000 . Photoacclimation by the Antarctic flagellate Pyramimonas sp. (Prasinophyceae) in response to iron limitation . Eur. J. Phycol , 35 : 295 – 303 .
- van Leeuwe , MA and Stefels , J . 1998 . Effects of iron and light stress on the biochemical composition of Antarctic Phaeocystis sp. (Prymnesiophyceae). II. Pigment composition . J. Phycol , 34 : 496 – 503 .
- Vassiliev , IR , Kolber , Z , Wyman , KD , Mauzerall , D , Shukla , VK and Falkowski , PG . 1995 . Effects of iron limitation on photosystem II composition and light utilization in Dunaliella tertiolecta . Plant Physiol , 109 : 963 – 972 .
- Vårum , KM , Østgaard , K and Grimsrud , K . 1986 . Diurnal rhythms in carbohydrate metabolism of the marine diatom Skeletonema costatum (Grev.) Cleve . J. Exp. Mar. Biol. Ecol , 102 : 249 – 256 .
- Webb , WL , Newton , M and Starr , D . 1974 . Carbon dioxide exchange of Alnus rubra: a mathematical model . Oecologia , 17 : 281 – 291 .