Abstract
The diatom genus Haslea Simonsen was erected to accommodate spindle-shaped valves with acute apices, convex margins, straight raphe and approximate central pores, inconspicuous areas, and transapical striae crossed at a right angle by a longitudinal pattern with a slit apically oriented in each areola. Investigations on mudflats of the French Atlantic coast revealed the presence of a sigmoid diatom with characteristics of both Gyrosigma Hassall and Haslea. Following a thorough morphological study by light and scanning electron microscopy in addition to biochemical and molecular analyses, we propose the transfer of Gyrosigma nipkowii Meister to Haslea nipkowii (Meister) Poulin & Massé, comb. nov. Despite a deviant sigmoidicity, this taxon shows diagnostic features of Haslea, with longitudinal slits running externally from one pole to the other, all merging with a peripheral slit at the apex, and the presence of small areolae beyond the helictoglossa. Unique to H. nipkowii are the unequal T-shaped distal raphe fissures, the overlapping and deflected proximal raphe ends, and the overhanging axial costa on the primary side of the valve. This taxon synthesizes C25 highly branched isoprenoids (HBIs) typical of the genus Haslea, with a double bond always present between C6 and C5. Phylogenetic analyses of the plastid-encoded 16S ribosomal gene for seven species of Haslea and two sigmoid taxa established the monophyly of the genus Haslea including H. nipkowii.
Introduction
In his synopsis of naviculoid diatoms, Cleve (Citation1894) created two sections under Naviculae to accommodate linear to lanceolate forms. The Fusiformes on one hand exhibited parallel striae, not crossed by longitudinal lines, but having puncta arranged in longitudinal, straight rows, while the Orthostichae showed puncta arranged in parallel, transverse and longitudinal rows crossing each other at a right angle, and the presence of a stauros. Cleve also pointed out that some forms of the section Orthostichae seem to be connected with Gyrosigma Hassall.
Later Patrick (Citation1959) established a series of new subgenera in the heterogeneous Navicula Bory. She placed the section Orthostichae under the subgenus Cuspidata and kept separate the subgenus Fusiformes, for thinly silicified valves with indistinct areas and transapical striae crossed at a right angle by a longitudinal pattern. A few years later, Hustedt (Citation1961 – 1966) placed Patrick's subgenus, as well as Cleve's Naviculae orthostichae pro parte, under Naviculae fusiformes. He circumscribed the Fusiformes by the delicate appearance of the cells and the typical spindle-shaped valve outline with convex margins, sometimes almost parallel in the middle region. He recognized the approximate location of the central raphe endings and the obscure polar fissures. He observed the typical striation of the valve, consisting of transapical striae crossed at a right angle by longitudinal striae. Finally he noted the occasional transapical development of the central nodule.
Based on his study of the raphe structures in diatoms, Schrader (Citation1973) pointed out the need to transfer the section Naviculae fusiformes to an independent genus, and discussed its close affinity to the Gyrosigma/Pleurosigma complex. At the same time, Simonsen (Citation1974) erected the new genus Haslea to accommodate mainly the Naviculae fusiformes of Cleve (Citation1894) and the subgenus Fusiformes of Patrick (Citation1959). The diagnosis provided by Simonsen (Citation1974) follows very closely the one produced by Hustedt (Citation1961 – 1966), and he clearly described the areolate striae, which consist of an apically oriented external slit in each areola. As previously depicted by Schrader (Citation1973), Simonsen (Citation1974) also discussed the affinity of Haslea to the genera Gyrosigma and Pleurosigma W. Smith, based on the similarity of the central nodule. Later Cox (Citation1977, Citation1979) reported on the symmetry and valve structure in naviculoid diatoms as revealed by electron microscopy. She stated that both external proximal and distal raphe endings curve to the same side in Haslea crucigera (W. Smith) Simonsen, a characteristic recently highlighted by Massé et al. (Citation2001 a). She also confirmed the occurrence of ribs ( = central bars as defined by Schrader, Citation1973, and Cardinal et al., Citation1986) on both sides of the internal raphe ridge in Haslea, a feature also present in Gyrosigma and Pleurosigma. Finally, she pointed out the similarity of the external ornamentation in G. littorale (W. Smith) Griffith & Henfrey to Haslea in which the striation is composed of external longitudinal and continuous slits parallel to the raphe system which merge to a peripheral slit or stria at or near the poles. Unique but not widespread in Haslea is the occurrence of a false stauros which corresponds to the thickening of some central transapical interstriae or virgae on both sides of the central nodule (Cox, Citation1979). Robert & Prat (Citation1973) reported on the ultrastructure of the generitype, Haslea ostrearia (Gaillon) Simonsen, associated with oyster beds and known to produce a blue pigment. Ricard (Citation1987) followed previous descriptions of the genus Haslea, emphasizing the ornamentation, which consists of rectangular areolae with a membrane perforated by a slit extended along the apical axis.
Very recently, Massé et al. (Citation2001 a) have studied four species of Haslea, including two new taxa, making a thorough examination of the valvar ultrastructure by both light and electron microscopy. In all specimens examined they reported the occurrence of a transapical arrangement of the areolae crossed at a right angle by a longitudinal pattern. The areolae are quadrangular to square internally, but externally they appear as continuous longitudinal slits through the basal siliceous layer, which all merge with a peripheral slit at or near the poles. They also recognized the constant presence of a raphe rib or costa associated with the raphe fissures covering them partially over most of the valve length, except at the centre or near the apices. Finally the central nodule is always bordered by uneven central bars while the central raphe endings are very close to each other and the polar endings are curved to the same side of the valve. They noted that some species exhibited a pseudo-stauros formed by the thickening of some two to three transapical costae crossing the centre of the valve.
Mudflat samples collected from two different locations along the Atlantic coast in France included sigmoid diatom specimens sharing characteristics known to occur in the genera Gyrosigma and Haslea. These samples also synthesized unusual terpenoids observed in both genera. The aims of this study were to describe the ultrastructure of these sigmoid diatom specimens, which we identified as Gyrosigma nipkowii Meister, and to determine their phylogenetic placement relative to six species of Haslea and two other sigmoid taxa in analyses of the plastid-encoded 16S ribosomal gene.
Materials and methods
Sampling
Oyster pond sediments in the Bay of Marennes-Oléron (Charente-Maritime) and intertidal mudflats in the Bay of Bourgneuf (Vendée) on the French Atlantic coast were sampled in November 1999 and January 2000, respectively, to isolate diatom cells producing highly branched isoprenoids (HBIs). The top 1 cm of the sediment was collected using a sterile syringe and preserved in the dark in a cooler. Some diatom samples were fixed with 1% formaldehyde and later extracted from the sediment using the Ludox methodology as described by Blanchard et al. (Citation1988). In the laboratory, diatom specimens were isolated under the light microscope by manually transferring cells to 250 ml Erlenmeyer flasks. Diatom cells were grown in F/2-enriched seawater medium (Guillard, Citation1975) under controlled conditions at a temperature of 14°C, at an irradiance of 100 μmol m − 2 s − 1 provided by cool-white fluorescent tubes under a light-dark cycle of 14 : 10 h. Cultures of several species of Gyrosigma, Haslea and Pleurosigma were also maintained and details of sampling are presented in .
Table 1. Collection sites and dates at which species of the diatom genera Haslea, Gyrosigma and Pleurosigma were isolated for molecular analyses. GenBank Accession numbers are indicated
Herbarium material
Original diatom slides were loaned to us from the Institut für Systematische Botanik und Botanischer Garten in Zurich (Z) and from the Diatom Herbarium at the Academy of Natural Sciences in Philadelphia (ANSP) for further comparisons with our material from France. We first examined the holotype slide of Gyrosigma nipkowii from the Meister collection in Zurich [sediment sample from the Bay of Amoy, now Xiamen, in China collected in 1931 by M. Voigt] (Meister, Citation1932). Additionally we also examined the holotype slide of Gyrosigma pallidum from the Philadelphia General Collection [G.C. 62630; sediment sample from tidal flats in the Yaquina Estuary, Oregon, USA collected in 1967 by R.Z. Riznyk] (Riznyk, Citation1973).
Microscopy
Diatom material was prepared according to Hendey (Citation1974). Permanent slides of cleaned diatom material were mounted using a high refractive index medium (Hyrax®) and examined by light microscopy (LM) (Olympus Provis and Leica Diaplan). A few drops of cleaned material were air-dried on coverslips, mounted with carbon tape onto aluminium stubs and coated with Au-Pd. Preparations were examined by scanning electron microscopy (SEM) (JEOL 6400F). Some diatom material was gently centrifuged and cross-sections prepared following Nassiri et al. (Citation1998). Transapical thin sections (250 – 500 nm) were obtained using a diamond knife, then mounted on a glass plate, rinsed and coated with Au-Pd for SEM observations (Massé et al., Citation2001 b).
Hydrocarbon analysis
Diatom cells were harvested from the culture flasks by filtration at the end of the exponential growth phase. Filters were freeze-dried and hydrocarbons extracted with hexane. The total hexane extract (THE) was saponified (6% KOH in methanol/water (80/20), 1 h at 80°C) and the non-saponifiable lipids (NSL) re-extracted by hexane. NSL fractions were examined by gas chromatography (GC) coupled to a 5970 Series Mass Selective Detector (12 m fused silica HP-1 column). Data were collected using Hewlett Packard MS-Chemstation software. Individual HBI alkenes were identified on the basis of their published gas chromatographic characteristics.
DNA extraction, PCR and sequencing
Total DNA was isolated from cells using the DNeasy plant mini kit from Qiagen. 16S rRNA genes were amplified using primers DIA16S3 (5′-CATGCAAGTCGTACGRGAGT) and DIA16S4 (5′-CAGTCACTAATTCTACCTTAG). For all amplifications a 50 μl reaction mixture was composed of 1X LA PCR Buffer II, 0.4 mM each dNTP, 0.2 μM each primer, 2.5 units LA Taq (TaKaRa, BioWhittaker) and 100 ng DNA template. The PCR conditions were an initial denaturation step of 1 min at 94°C, 30 cycles each consisting of 30 s at 95°C, 45 s at 46°C and 2 min at 72°C, and a final extension step of 10 min at 72°C. PCR-amplified products were cloned in pCR2.1-TOPO plasmid from TOPO TA cloning kit (Invitrogen) and clones were sequenced by the ESGS-Cybergene company (Evry, France). Sequence analysis was carried out using the Mac DNASIS software system (Hitachi Software Engineering, Co., Ltd.). Gene sequences have been archived on GenBank for the following taxa with their respective accession numbers: Gyrosigma fasciola (Ehrenberg) Griffith & Henfrey (AF 514847), Pleurosigma intermedium W. Smith (AF 514848), Haslea crucigera (AF 514849), Haslea nipkowii (Meister) Poulin & Massé, comb. nov. (AF 514850), Haslea sp. (AF 514851), Haslea ostrearia (AF 514852), Haslea pseudostrearia Massé, Rincé & Cox (AF 514853), Haslea salstonica Massé, Rincé & Cox (AF 514854), and Haslea wawrikae von Stosch (AF 514855).
Sequence analysis
Non coding 16S plastid rDNA sequences including sequences from GenBank for Odontella sinensis (Greville) Grunow were manually aligned using the Bioedit sequence Alignment Editor (Hall, Citation1999). Maximum parsimony (MP) and maximum likelihood (ML) analyses were performed with PAUP* (Swofford, Citation1998). In all analyses, gaps were considered as missing characters. For MP and ML analyses, a branch and bound search was done with branches collapsed if maximum branch length is zero and MulTrees options. The nucleotide substitution model used for ML was selected using ModelTest version 3.06 (Posada & Crandall, Citation1998) and PAUP* (Swofford, Citation1998) according to the approach outlined by Huelsenbeck & Crandall (Citation1997). The robustness of trees was assessed for both methods using bootstrap proportions (Felsenstein, Citation1985) with 1000 bootstrap replications. For MP, branch and bound search was used with the same options cited above. For ML, heuristic search was selected due to computing time with the following setting: addition sequence: as-is, TBR, multrees, branches collapsed. Decay indices for MP were obtained using the software AutoDecay 5.02 (Eriksson, Citation1998). All trees were rooted with Odontella sinensis.
Results
Taxonomy
Haslea nipkowii (Meister) Poulin & Massé, comb. nov. ( – ).
Figs 1 – 5. Light and scanning electron micrographs of Haslea nipkowii from the French Atlantic coast. Figs 1, 2. Cells with two plate-like chloroplasts along margins. Fig. 3. Living cell in girdle view. Fig. 4. Cleaned valve in phase contrast optics. Fig. 5. External valve view in SEM. All scale bars represent 10 μm.
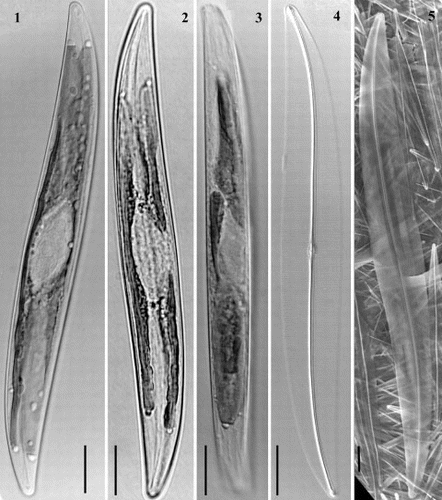
Figs 6 – 8. Light micrographs of Haslea nipkowii from the French Atlantic coast. Fig. 6. Half valve showing a distinct white spot at centre (arrowhead) with opposing thin central bar (arrow) and wide axial costa. Fig. 7. Central valve showing transapical striae crossed at right angle by a longitudinal pattern with distinct white spot (arrowhead) and opposing narrow central bar (arrow). Fig. 8. Apex showing bifurcated distal raphe fissure. All scale bars represent 10 μm.
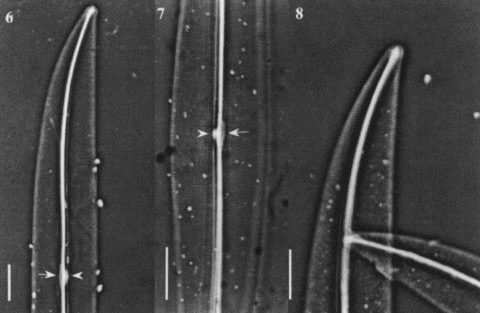
Figs 9 – 12. Light micrographs of Gyrosigma spp. Figs 9, 10. Gyrosigma nipkowii (holotype slide # 3203024 from Meister collection (Z), Bay of Amoy, China). Fig. 9. Whole valve showing a distinct white spot at centre (arrowhead) with opposing central bar (arrow) and wide axial costa. Fig. 10. Broken valve. Figs 11, 12. Gyrosigma pallidum (holotype slide G.C. # 62630 from Riznyk collection (ANSP), Yaquina Estuary, USA). Whole valve exhibiting a distinct axial costa, a narrow central bar (arrow) with an opposing white spot (arrowhead). All scale bars represent 10 μm.
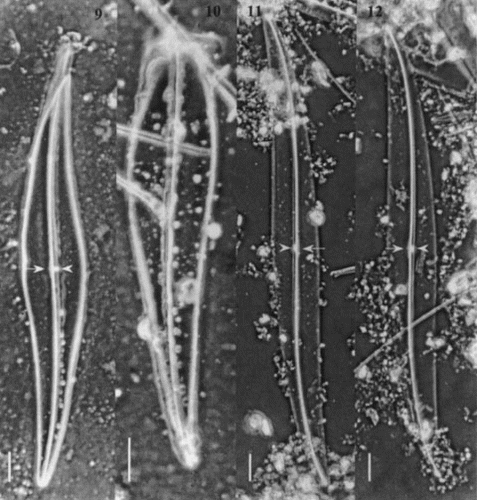
Figs 13 – 19. Light micrographs of Gyrosigma spp. Figs 13 – 15. Gyrosigma nipkowii (holotype slide # 3203024). Fig. 13. Half valve. Fig. 14. Valve centre showing axial costa, narrow central bar with opposing white spot. Note the slightly deflected proximal raphe ends toward the primary side. Fig. 15. Valve apex. Figs 16 – 19. Gyrosigma pallidum (holotype slide G.C. # 62630). Fig. 16. Half valve. Figs 17, 18. Centre of valve exhibiting a distinct axial costa, a narrow central bar (arrow) with an opposing white spot (arrowhead). Note striae pattern crossed at 90°. Fig. 19. Valve apex showing T-shaped distal raphe fissure (arrow). Scale bars represent: Figs 13 – 18, 10 μm; Fig. 19, 5 μm.
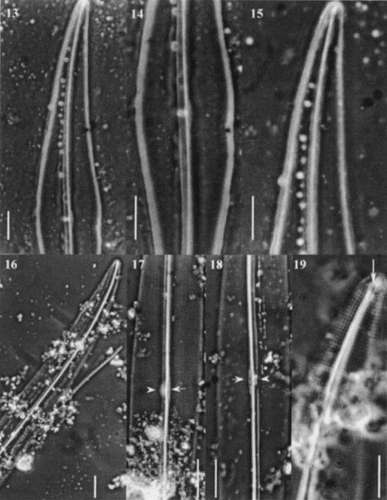
Figs 20 – 28. Scanning electron micrographs of Haslea nipkowii from the French Atlantic coast. Fig. 20. Internal valve view midway between apex and centre showing the stria pattern and the axial costa completely overhanging the raphe system. Fig. 21. Internal valve at centre showing the proximal raphe ends, the narrow central bar (black arrowhead) with opposing bulge (white arrowhead) which correspond to the white spot visible in LM. Fig. 22. Valve centre in internal view exhibiting small transapical extensions of the central bar. Figs 23, 24. Internal views of apex showing the quadrangular areolae, the straight helictoglossa and the two peripheral longitudinal slits reuniting at the far tip of the valve. Note the recessed axial costa in Fig. 24. Fig. 25. Internal valve centre showing roundish areolae, a recessed axial costa, straight proximal raphe ends and a narrow central bar. Fig. 26. External view of valve at centre showing the longitudinal slits, the wider primary side and the proximal raphe ends slightly deflected toward the same side and gently overlapping. Fig. 27. Internal view of valve showing the axial costa and the first series of areolae with the foramen opening at an angle (arrow). Fig. 28. Same specimen tilted (46°) showing the foramen of the first series of areolae (arrow).
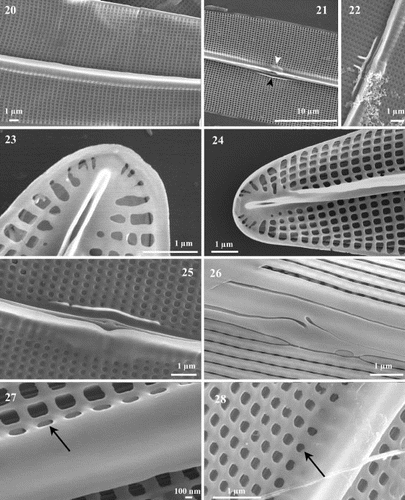
Figs 29 – 32. Scanning electron micrographs of Haslea nipkowii from the French Atlantic coast. Fig. 29. External valve view midway between apex and centre showing the wider axial area on the primary side bordered by the first longitudinal slit which appears unopened because of the angled position of the foramen underneath. Figs 30, 31. Apex in external view showing the uneven T-shaped terminal fissure of the raphe, the terminal area in the shape of a Bishop's mitre, the abutting longitudinal slits with the two peripheral slits that merge together at the far tip of the pole. Fig. 32. Internal view of apex showing the straight helictoglossa and the merging of the two peripheral slits.
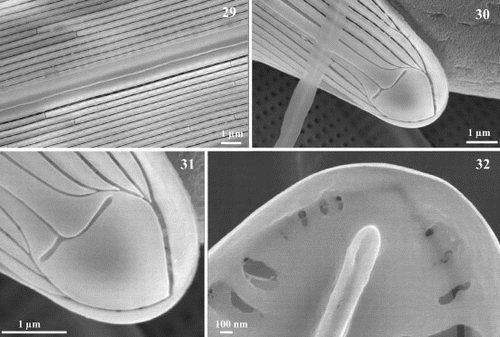
Figs 33 – 40. Scanning electron micrographs of transapical thin sections of Haslea nipkowii. Fig. 33. Thin section midway between apex and centre showing the general structure of the frustule. Fig. 34. Thin section near the apex. Figs 35 – 37. Thin sections midway between apex and centre. Details showing the larger areola with internal and external openings (single arrow), the C-cedilla (Ç) shape of the raphe fissure (single line) and the prominent and overhanging axial costa (double arrow). Fig. 38. Thin section at valve centre showing the large areola, the simpler raphe fissure (single line), the recessed axial costa (double arrow) and the slightly raised central bar (double line). Figs 39, 40. Thin sections showing the cingular valvocopula and copula. Scale bars represent: Figs 33 – 35, 37, 38, 1 μm; Figs 36, 39, 40, 100 nm.
Basionym: Gyrosigma nipkowii Meister (Citation1932), Kieselalgen aus Asien, p. 43, pl. 17, figs 134 – 136.
Holotype: Collection F. Meister (Z). Sample # 2377, Materialien von Herrn Voigt [material received from Mr. Voigt], Amoy Bucht, Grundprobe [Bay of Amoy, now the Bay of Xiamen in China, sediment sample]. Slide # 3203024, Gyrosigma nipkowii with specimens encircled (, ).
Synonym: Gyrosigma pallidum Riznyk (Citation1973), p. 124, pl. 9, , pl. 19, .
Holotype: Collection R.Z. Riznyk (ANSP). Sally's Bend sediment, Yaquina Estuary, Oregon, USA. Slide GC # 62630, many specimens present on the slide but none circled (, , ).
Description: Frustules rectangular and gently curved. Valves sigmoid, almost linear to very slightly linear-lanceolate, with two opposite, almost straight margins and two opposite, gently convex ones: 131 – 191 μm in length, 15.5 – 20 μm in width. Apices sub-acute to sub-rostrate. Raphe sternum straight and central over one-third of the apical axis becoming gently curved and displaced to the convexity toward the poles. Approximate central raphe endings co-axial internally and displaced externally to one side in a sigmoid fissure. Distal raphe branches terminate internally in a short apically oriented helictoglossa and in an external obtuse-angled or T-shaped fissure. Raphe fissures open unilaterally on an accessory rib, except at centre and near the apices. Raphe sternum bordered by an overhanging unilateral axial costa, except at the poles, while a short and narrow central bar is present at centre opposite the axial costa. Narrow axial area wider on the primary side of the valve, conspicuously internally thickened at centre over five to 10 striae, and distinct external terminal areas beyond the raphe fissures. Striae consisting of transapical and parallel rows of quadrate to roundish internal areolae crossed at a right angle by longitudinal rows: 23 – 25 transapical striae in 10 μm, 31 – 34 longitudinal striae in 10 μm. Valve surface covered externally by continuous longitudinal slits running parallel to the raphe, which merge with a peripheral stria near the apices.
Morphology
Live cells are weakly silicified with two apically elongate plate-like chloroplasts lying along each margin and extending slightly under the valve face (). Each plate shows a large central concavity. In girdle view, the frustules are rectangular and gently curved (). The sigmoid nature of the valves can be described as almost linear to slightly linear-lanceolate, resembling more or less a parallelogram, with two opposite, almost straight margins and two opposite, gently convex margins (). However, the holotype from Meister's collection shows two valves of a lanceolate outline () while the numerous specimens on Riznyk's slide exhibited a similar morphology to our specimens from the French Atlantic coast (). The apices are distinctly sub-acute to sub-rostrate (). The specimens examined from marine intertidal sediments of the bays of Marennes-Oléron and Bourgneuf are 131 – 191 μm long and 15.5 – 20 μm wide, which compares nicely with morphometric data from Meister's specimens (145 – 147 μm in length by 20.5 – 22 μm in width) and Riznyk's Gyrosigma pallidum (150 – 198 μm long and 17 – 20 μm wide).
The raphe sternum is centrally located and straight over most of its length, gently curving toward the convexity of the valve near the poles (), a feature that is easily visible also in Meister's and Riznyk's specimens (). The raphe consists of two branches, which open unilaterally in internal view on accessory ribs, except at the centre of the valve and the poles (). The proximal raphe endings are co-axial and very close to each other, less than 1 μm apart (), while at the apices they terminate in a slightly raised, straight and apically oriented helictoglossa (). A conspicuous axial costa, very slightly indented at the valve's centre overhangs the raphe sternum over most of its length, except at the centre and near the poles (). Facing and opposite the axial costa at the centre, there is a thin and short central bar bordering the distal part of the striae (). Sometimes, in some specimens, two very short transapically expanded silica structures extend out of the central bar (). This feature is reminiscent of the pseudo-stauros observed in some species of the genus Haslea. Externally, the proximal raphe endings are slightly deflected to the axial area and overlap in a more or less sigmoid manner (, ), while the distal raphe ends terminate in a widely obtuse angled and uneven bifurcation or T-shape ().
In LM, hyaline areas tend to appear rather indistinct (), but a small terminal area is visible beyond the raphe fissures (). A unilaterally wider axial area is always present with a conspicuous thickening at centre shown as a small whitish dot (; arrowheads). All specimens exhibited this peculiar characteristic whether they were from Meister's, Riznyk's or our material (, ,; arrowheads). In SEM, the valves exhibit a narrow and linear axial area, which is conspicuously more developed on the primary side of the valve, both internally and externally, with a clearly marked internal thickening or bulging at the centre of the valve over 5 to 10 striae (, ). On the other hand, the central area is simply undifferentiated (). Each pole is characterized by a marked terminal area in the shape of a Bishop's mitre just beyond the unequal bifurcate raphe fissures ().
The valve's ornamentation is barely visible in LM, and only the transapical striae are discernible (, ). In SEM, the internal valve view shows a distinct striation consisting of uniseriate rows of parallel, transapical areolae crossed at a right angle by a longitudinal pattern (). The areolae are quadrate to rectangular over most of the valve surface, except near the central region where they are circular (). Externally, the valve surface is composed of continuous, longitudinal strips of silica running parallel to the raphe, which are intersected by thin, continuous, evenly spaced and straight slits (, ). These slits (or striae) merge near the apices with a peripheral stria from each margin, these latter reuniting themselves beyond the terminal area at the far tip of the apex (, 31). A row of smaller areolae surrounds the helictoglossa at the far tip of the valve, connecting externally to the reunited marginal slit (, ).
In addition, SEM examination of transapical sections of diatom valves revealed a highly consistent arrangement of the raphe-associated structures (). On the external primary side of the valve in Haslea nipkowii, which corresponds to the wider unilaterally developed axial area (), the first continuous longitudinal slit-like stria connects internally to the first series of very large areolae opening by a foramen which is positioned at an obtuse angle in relation to other areolae (, ; single arrow). Overhanging the raphe sternum lies, internally, a unilaterally, well-developed axial costa (; double arrow). Internal raphe fissures open laterally on an accessory rib over most of the valve's length (; single line), except at the centre and the poles (; single line). Thin cross-sections revealed a far less marked axial costa near the centre or the poles (; double arrow), while it is fairly well-developed elsewhere along the apical axis of the valve when the raphe fissures appear clearly laterally located (; double arrow). Facing the axial costa and bordering the raphe sternum, a narrow central bar can be seen as a gently raised structure, which is totally absent away from the central region of the valve (; double line). Finally, thin sections revealed the cingulum which reunites the two valves (), each composed with the valvocopula and the copula. The copula is tapered and underlies the valvocopula ().
Meister's holotype material was only available for LM observations. It consisted of one complete valve and one half-valve (). Unfortunately, we were unable to find additional specimens from the dry sample received (sample # 2377). As stated in Meister's (Citation1932) protologue, the valves are lanceolate with the margins in the middle region appearing slightly angular. The axial area is reported to be moderately large and it may correspond in fact to the axial costa, which is more prominent on only one side of the valve as depicted in our material (, ). Opposite the axial costa at the centre it is possible to see a thin central bar, as in our specimens (, ; arrow). The prominent white spot visible in LM (, 13, 14; arrowhead) on one side at the valve's centre in Meister's specimens corresponds to the internal thickening of the axial costa we observed in our specimens both in LM and SEM (, ; white arrowhead). The pattern of ornamentation is identical to that of our specimens, as is the number of striae: 21 – 22 transapical striae in 10 μm and 28 – 29 longitudinal striae in 10 μm.
The LM examination of Riznyk's material has confirmed the morphological similarity that exists between specimens from the western coast of the USA and the French Atlantic coast (, ). Every single valvar feature examined in LM occurs in both collections with similar variability. Biometric data are also within the same range of variation: 150 – 198 μm in length by 17 – 20 μm in width with 21 – 24 transapical striae in 10 μm and 27.5 – 30.5 longitudinal striae in 10 μm.
Hydrocarbons
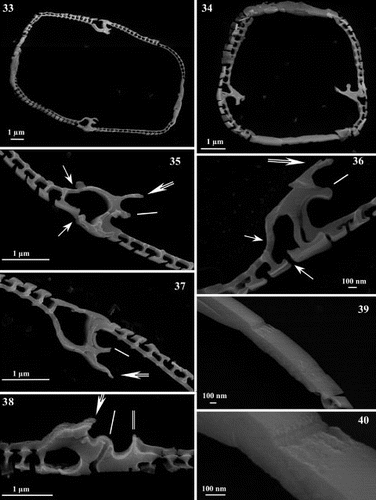
The GC-MS total ion chromatogram of the non-saponifiable lipid fraction obtained from a monospecific culture of Haslea nipkowii revealed the presence of phytol, n-C21:6 (heneicosahexaene) and tri-unsaturated highly branched isoprenoids (C25:3 E, Z; I – III; ). By comparison of mass spectra and retention indices with authenticated compounds, I and II were identified as (9E)-2,6,10,14-tetramethyl-7-(3-methylpent-4-enyl) pentadec-5,9-diene (I) and (9E)-2,10,14-trimethyl-6-methylene-7-(3-methylpent-1-enyl) pentadec-9-ene (II) previously characterized in cultures of other Haslea species (Belt et al., Citation1996; Allard et al., Citation2001). All Haslea species investigated produced C25 HBIs exhibiting a C6 which is always unsaturated. In addition, the stereochemistry of the tri-substituted double bond (C9 – C10) is always E. In contrast, in all the sigmoid taxa investigated, mixtures of both E and Z isomers (C9 – C10) are always observed. Furthermore, the C7 and not the C6 is always unsaturated (Belt et al., Citation2000 a,(Citation b ).
Fig. 41. I – III: Numbering scheme (I) and structures of C25 HBI alkenes characterized for Haslea nipkowii. I – II: Structures of C25 HBI alkenes previously characterized for Haslea species. IV – V: Structures of C25 HBI alkenes previously characterized for sigmoid taxa.
Fig. 42. Partial total ion current chromatogram of the non-saponifiable lipid fraction of the diatom Haslea nipkowii showing the presence of three C25 HBI isomers, n-C21:6 (heneicosahexaene) and phytol, the side chain of chlorophyll.
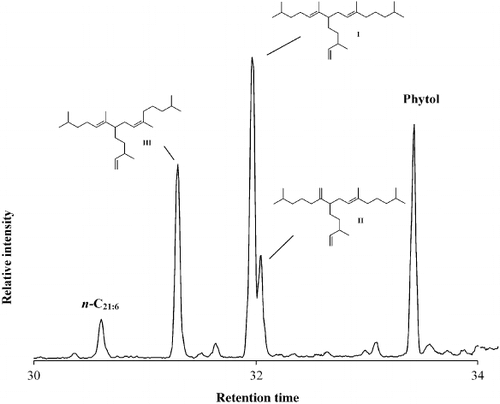
Surprisingly, in the diatom Haslea nipkowii, a third triene (III) was identified as the Z isomer of 2,6,10,14-tetramethyl-7-(3-methylpent-4-enyl) pentadec-5,9-diene (I). Thus, the double bond positions are characteristic of Haslea spp. while the E/Z isomerism at C9 – C10 is a structural feature associated with HBIs from sigmoid species (Belt et al., Citation2001).
Molecular analysis
The alignment was unambiguous (gaps were introduced at only five sites). It was 1338 nucleotides in length, of which 80 were variable and 33 were parsimony informative. In the parsimony analysis, only one most parsimonious tree of 104 steps was obtained, with a consistency index (CI) of 0.7885 and a retention index (RI) of 0.6000 ().
Fig. 43. Single most parsimonious tree for 16S sequences of Haslea, Gyrosigma and Pleurosigma species, rooted with Odontella sinensis. The tree is 104 steps long with a consistency index of 0.7885 and a retention index of 0.6000. Branch lengths are proportional to the nucleotide changes indicated by the scale bar below the tree. Numbers above or below branches are bootstrap values based on 1000 replications of the MP or ML analyses, respectively. Decay index values (d) are also provided.
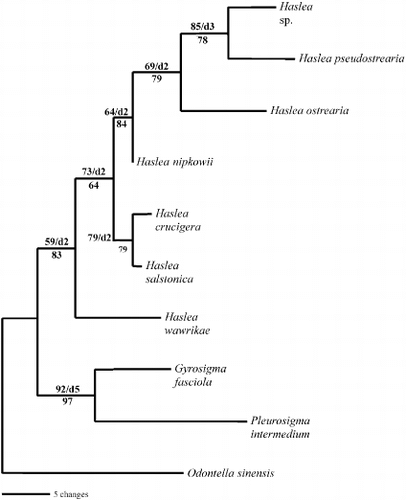
For ML analysis, the model suggested by Modeltest (Posada & Crandall, Citation1998) using the Modeltest Hierarchical Likelihood Ratio Tests (hLRTs) was the general time reversible model of DNA substitution (Rodriguez et al., Citation1990), following a gamma distribution with a shape parameter of 0.8140 and considering a proportion of invariable sites of 0.7936 (nucleotide frequencies A = 0.28180, C = 0.20580, G = 0.27950, T = 0.23290 and rate matrix: [A-C] = 1.0000, [A-G] = 4.9263, [A-T] = 1.0000, [C-G] = 1.0000, [C-T] = 12.2331, [G-T] = 1.0000). The ML and MP trees had the same topologies and, consequently, only the most parsimonious tree is shown ().
The molecular analysis showed that the first divergence in our ingroup separates the representatives of the genus Haslea from the Gyrosigma and Pleurosigma taxa. The Gyrosigma/Pleurosigma group is strongly supported whereas the Haslea clade is weak. Further investigations into the monophyly of Haslea should include more representatives of Haslea, Gyrosigma and Pleurosigma, as well as other sigmoid genera such as Donkinia Ralfs and Rhoicosigma Grunow. Within the Haslea clade, H. nipkowii was the sister taxon of the Haslea sp. – H. pseudostrearia – H. ostrearia group. All of these species share the absence of a pseudo-stauros, whereas H. salstonica and H. crucigera possess this structure. Haslea wawrikae does not have a pseudo-stauros but is the only planktonic species of all Haslea with special features such as a long siliceous thread terminating the apex. However, our data set is too limited and further analyses with more species should be performed in order to confirm the phylogenetic value of the presence/absence of the pseudo-stauros character in the genus Haslea. Even if our dataset is relatively small, both in terms of species and in informative sites, the molecular analysis clearly placed H. nipkowii within the Haslea clade and far from Gyrosigma. These results were in accordance with the morphological characters and the chemical signature previously presented, and were strong support for our proposed new combination of H. nipkowii.
Discussion
It should not be too difficult to assign specimens to the genus Haslea because of its characteristic features, the linear-lanceolate valve's outline, acute apices and right-angle areolar striae. However whereas Haslea spp. have spindle-shaped naviculoid valves, our specimens exhibit a distinct sigmoid morphology of the valves. In this, our specimens share a morphological feature with Gyrosigma and Pleurosigma, two well-established sigmoid genera. In addition, the striation in our specimens is composed of transapical rows of areolae that are crossed at a right angle by a longitudinal pattern, yet another diagnostic criterion characterizing the genus Gyrosigma. An additional confusion is added by the external proximal raphe endings that are deflected and slightly overlapping, resembling some species of Pleurosigma (Cardinal et al., Citation1984; Sterrenburg, Citation1991 a; Stidolph, Citation1992).
Despite these characters linking our material of Gyrosigma nipkowii with Gyrosigma and Pleurosigma, it exhibits several diagnostic features of the genus Haslea, which correlates with the molecular analysis (). We therefore feel justified in transferring this species to Haslea. Although the sigmoidicity of the valve in H. nipkowii is a character of the genus Gyrosigma, this feature has to be evaluated with some flexibility. Sterrenburg (Citation1991 a) admitted that the overall morphology of the valve is not a reliable character for recognizing all species of Gyrosigma and Pleurosigma, as evidenced by the occurrence in the genus Pleurosigma of non-sigmoid taxa such as P. planctonica Simonsen and P. directum Grunow. The valves of H. nipkowii show other features that are diagnostic of the genus, particularly the 90°-angle between transapical and longitudinal striae which form externally continuous, parallel and apically oriented narrow slits abutting at each pole with a peripheral slit (Massé et al., Citation2001 a). Some sigmoid taxa (e.g. Gyrosigma littorale, G. sterrenburgii Stidolph) show a trace of this character, with the merging of 2 – 3 slit-like areolae on the external valve face (Cox, Citation1979; Sterrenburg, Citation1992), but for most Gyrosigma species, each areola is externally perforated by an apically oriented narrow slit which is almost never united with adjacent ones. At the apex beyond the terminal area, the areolae are shorter than on the rest of the valve, but they should not be confused with the small pores or the crescent of apical micropores observed at the far ends of the valves in some species of Gyrosigma (Schoeman & Archibald, Citation1986; Stidolph, Citation1992; Sterrenburg, Citation1995). There is no such equivalent crescent in Haslea.
The raphe system observed in our specimens confirms the variability of this character in Haslea, in which the proximal raphe ends of many species can be straight and gently displaced to one side, markedly deflected to one side or slightly overlapped and deflected to one side (Massé et al., Citation2001 a). There is also great variability in the aspect of the distal raphe endings, which can be straight and dilated or expanded, strongly deflected to the primary side of the valve or simply unevenly T-shaped. There is no consistency at the generic level for the polar termination of the raphe fissures, as in Gyrosigma and Pleurosigma (Cardinal et al., Citation1989; Sterrenburg, Citation1990, Citation1991 a,Citation b , Citation1992; Stidolph, Citation1992).
Central bars, originally defined by Schrader (Citation1973) and later adopted by Cardinal et al. (Citation1989), correspond to a siliceous narrow thickening of the internal basal layer bordering the central nodule and the proximal raphe branches. However, it appears that central bars are too highly variable to be considered a stable character delineating sigmoid diatom species (Sterrenburg, Citation1991 a). In Haslea, central bars vary but to a lesser extent. In H. nipkowii, there is usually a straight or very slightly bent, very thin and short bar positioned on only one side in the middle region bordering the nodule and the proximal raphe branches. Occasionally the bar has very short transapical extensions or none (Massé et al., Citation2001 a). Associated with central bars is the thickening of transapical virgae which contribute to forming a pseudo-stauros at the centre, a highly remarkable characteristic best observed in LM in H. crucigera, H. crucigeroides (Hustedt) Simonsen and H. salstonica (Cardinal et al., Citation1986; Massé et al., Citation2001 a).
Monophyly of the genus Haslea including H. nipkowii () supports the systematic value of the ornamentation pattern of the valves. Haslea crucigera and H. salstonica are shown to be sister taxa, and both possess a pseudo-stauros, which thus appears to be a synapomorphy (). Although a pseudo-stauros is missing in H. nipkowii, the very short transapical extensions on one side of the central bar resemble one (). In addition, there is some variability in the pseudo-stauros in H. crucigera with the thickening of one to three virgae (Cardinal et al., Citation1986; Massé et al., Citation2001 a).
Therefore, in spite of a deviant sigmoid morphology, we consider our diatom specimens from the French Atlantic coast to belong to Haslea, sharing several diagnostic features with the genus. Although this species could be placed in Gyrosigma based on the shape of the valve and the stria pattern, the most striking characters in H. nipkowii are the unique external endings of the unequal T-shaped distal raphe fissures, the overlapping and deflected proximal raphe ends, and the overhanging axial costa on the primary side of the valve. Like other taxa in the genus Haslea, H. nipkowii shows external narrow longitudinal slits that run apically from one pole to the other, all merging with a peripheral slit at the far end. Yet another character of H. nipkowii and other species within the genus is the presence of smaller areolae in a single bent row beyond the helictoglossa internally and the terminal area externally.
It is now well established that several species belonging to the genus Haslea are able to synthesize highly branched isoprenoids (Volkman et al., Citation1994; Belt et al., Citation1996; Allard et al., Citation2001). These C25 hydrocarbons identified from cultures of the diatoms H. ostrearia, H. crucigera, H. pseudostrearia and H. salstonica have two to six double bonds and several common structural features (see I, II). Indeed, the C6 is always unsaturated with a double bond between C6 and C5 or C6 and C17 and the C7 is never saturated. All the compounds possess a vinyl moiety (C24 – C25). Recently Belt et al. (Citation2000 a,Citation b ) reported C25 trienes, tetraenes and pentaenes in cultures of the sigmoid diatoms, Pleurosigma intermedium and Pleurosigma sp. (see IV, V). However in these alkenes, C6 was always saturated and a tri-substituted double bond at the main HBI branch point (C7 – C20) was present. In addition total ion current chromatograms and NMR spectroscopic studies always revealed the presence of mixtures of E/Z geometric isomers (C9 – C10), whereas only E isomers were detected in the HBI content of all Haslea species investigated.
In summary, Haslea nipkowii produces HBI alkenes that are in the group of regioisomeric chemicals found in all investigated Haslea species, whereas the additional presence of stereoisomerism is more indicative of sigmoid genera. Therefore, it is interesting to note that H. nipkowii shares not only ultrastructural features known to occur in both sigmoid genera and Haslea but also some unusual biochemical properties.
Further morphological and molecular studies will help us better understand the phylogeny of Haslea in regard to sigmoid genera such as Gyrosigma or Pleurosigma as well as non-sigmoid but related genera like Navicula. Such research will assist the search for stable characters with which to circumscribe diatom genera.
Acknowledgements
We thank A. Barreau of the Service commun de microscopie, Université de Nantes, for his assistance and unlimited access to the SEM, and E.J. Cox, G. Reid, Y. Rincé and F.A.S. Sterrenburg for personal communications. We are grateful to H.R. Preisig from the Institut für Systematische Botanik und Botanischer Garten in Zurich and C.W. Reimer from the Diatom Herbarium, Academy of Natural Sciences in Philadelphia for the loan of holotype material. Special thanks to the Canadian Museum of Nature for a research grant to M. Poulin and the University of Plymouth for a research assistantship to G. Massé. Finally the ISOMer at the Université de Nantes is acknowledged for some travel support provided to M. Poulin to visit the laboratory.
References
References
- Allard , WG , Belt , ST , Massé , G , Naumann , R , Robert , J-M and Rowland , SJ . 2001 . Tetra-unsaturated sesterterpenoids (Haslenes) from Haslea ostrearia and related species . Phytochem , 56 : 795 – 800 .
- Belt , ST , Cooke , DA , Robert , J-M and Rowland , SJ . 1996 . Structural characterization of widespread polyunsaturated isoprenoid biomarkers: a C25 triene, tetraene and pentaene from the diatom Haslea ostrearia Simonsen . Tetrahedron Lett , 37 : 4755 – 4758 .
- Belt , ST , Allard , WG , Massé , G , Robert , J-M and Rowland , SJ . 2000a . Highly branched isoprenoids (HBIs): identification of the most common and abundant sedimentary isomers . Geochim. Cosmochim. Acta , 64 : 3839 – 3851 .
- Belt , ST , Allard , WG , Massé , G , Robert , J-M and Rowland , SJ . 2000b . Important sedimentary sesterterpenoids from the diatom Pleurosigma intermedium . Chem. Commun , 2000 ( 6 ) : 501 – 502 .
- Belt , ST , Allard , WG , Johns , L , König , WA , Massé , G , Robert , J-M and Rowland , SJ . 2001 . Variable stereochemistry in highly branched isoprenoids from diatoms . Chirality , 13 : 415 – 419 .
- Blanchard , G , Chrétiennot-Dinet , M-J , Dinet , A and Robert , JM . 1988 . Méthode simplifiée pour l'extraction du microphytobenthos des sédiments marins par le gel de silice Ludox . C. R. Acad. Sci. Paris. Sér III , 307 : 569 – 576 .
- Cardinal , A , Poulin , M and Bérard-Therriault , L . 1984 . Les diatomées benthiques de substrats durs des eaux marines et saumâtres du Québec. 4. Naviculales, Naviculaceae (à l'exclusion des genres Navicula, Donkinia, Gyrosigma et Pleurosigma) . Naturaliste can , 111 : 369 – 394 .
- Cardinal , A , Poulin , M and Bérard-Therriault , L . 1986 . Les diatomées benthiques de substrats durs des eaux marines et saumâtres du Québec. 5. Naviculales, Naviculaceae; les genres Donkinia, Gyrosigma et Pleurosigma . Naturaliste can , 113 : 167 – 190 .
- Cardinal , A , Poulin , M and Bérard-Therriault , L . 1989 . New criteria for species characterization in the genera Donkinia, Gyrosigma and Pleurosigma (Naviculaceae, Bacillariophyceae) . Phycologia , 28 : 15 – 27 .
- Cleve , PT . 1894 . Synopsis of the naviculoid diatoms. Part 1 . K. Svenska Vetensk.-Akad. Handl , 26 : 1 – 194 .
- Cox , EJ . 1977 . Raphe structure in naviculoid diatoms as revealed by the scanning electron microscope . Nova Hedwigia Beih , 54 : 261 – 274 .
- Cox , EJ . 1979 . Symmetry and valve structure in naviculoid diatoms . Nova Hedwigia Beih , 64 : 193 – 206 .
- Eriksson T 1998 AutoDecay ver. 4.0. T. Eriksson, Department of Botany, Stockholm University, Stockholm
- Felsenstein , J . 1985 . Confidence limits on phylogenies: an approach using bootstrap . Evolution , 39 : 783 – 791 .
- Guillard RRL 1975 Culture of phytoplancton for feeding marine invertebrates In Culture of Marine Invertebrate Animals (Smith, W.L. & Chanley, M.H., editors) 26 60 Plenum Press, New York
- Hall , TA . 1999 . BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/nt . Nucleic Acids Symposium Series , 41 : 95 – 98 .
- Hendey , NI . 1974 . The permanganate method for cleaning freshly gathered diatoms . Microscopy , 32 : 423 – 426 .
- Huelsenbeck , JP and Crandall , KA . 1997 . Phylogeny estimation and hypothesis testing using maximum likelihood . Ann. Rev. Ecol. Syst , 28 : 437 – 466 .
- Hustedt F 1961 – 1966 Die Kieselalgen Deutschlands, Österreichs und der Schweiz In Dr. L.Rabenhorst's Kryptogamen-Flora von Deutschland, Österreich und der Schweiz (Rabenhorst, L., editor) Volume 7, Part 3 1 816 Akademische Verlagsgesellschaft, Leipzig
- Massé , G , Rincé , Y , Cox , EJ , Allard , G , Belt , ST and Rowland , SJ . 2001a . Haslea salstonica sp. nov. and Haslea pseudostrearia sp. nov. (Bacillariophyta), two new epibenthic diatoms from the Kingsbridge estuary, United Kingdom . C. R. Acad. Sci. Paris, Life Sciences , 324 : 617 – 626 .
- Massé , G , Poulin , M , Belt , ST , Robert , J-M , Barreau , A , Rincé , Y and Rowland , SJ . 2001b . A simple method for SEM examination of sectioned diatom frustules . J. Microsc , 204 : 87 – 92 .
- Meister F 1932 Kieselalgen aus Asien Gebrüder Borntraeger, Berlin
- Nassiri , Y , Robert , J-M , Rincé , Y and Ginsburger-Vogel , T . 1998 . The cytoplasmic fine structure of the diatom Haslea ostrearia (Bacillariophyceae) in relation to marennine production . Phycologia , 37 : 84 – 91 .
- Patrick , R . 1959 . New subgenera and two new species of the genus Navicula (Bacillariophyceae) . Not. Nat , 324 : 1 – 11 .
- Posada , D and Crandall , KA . 1998 . Modeltest: testing the model of DNA substitution . Bioinformatics , 14 : 817 – 818 .
- Ricard M 1987 Diatomophycées In Atlas du phytoplancton marin (Sournia, A., editor) 2 1 297 Éditions du Centre National de la Recherche Scientifique, Paris
- Robert , J-M and Prat , D . 1973 . Ultrastructure de la diatomée Navicula ostrearia Bory révélée par le microscope électronique à balayage . C. R. Acad. Sci. Paris, Sér. D , 277 : 1981 – 1983 .
- Rodriguez , F , Oliver , JL , Marin , A and Medina , JR . 1990 . The general stochastic model of nucleotid substitution . J. Theor. Biol , 142 : 485 – 501 .
- Riznyk , RZ . 1973 . Interstitial diatoms from two tidal flats in Yaquina Estuary, Oregon, USA . Bot. Mar , 16 : 113 – 138 .
- Schoeman , FR and Archibald , REM . 1986 . Gyrosigma rautenbachiae Cholnoky (Bacillariophyceae): its morphology and taxonomy . Nova Hedwigia , 43 : 129 – 157 .
- Schrader , H-J . 1973 . Types of raphe structures in the diatoms . Nova Hedwigia, Beih , 45 : 195 – 230 .
- Simonsen , R . 1974 . The diatom plankton of the Indian Ocean Expedition of R/V ‘Meteor’ 1964 – 1965. ‘Meteor’ Forschergeb . Reihe D , 19 : 1 – 107 .
- Sterrenburg FAS 1990 Studies on the genera Gyrosigma and Pleurosigma (Bacillariophyceae). A new phenomenon: co-existence of dissimilar raphe structures in populations of several species In Ouvrage dédié à la mémoire du professeur Henry Germain (1903 – 1989) (Ricard, M., editor) 235 242 Koeltz Scientific Books, Koenigstein
- Sterrenburg , FAS . 1991a . Studies on the genera Gyrosigma and Pleurosigma (Bacillariophyceae). The typus generis of Pleurosigma, some presumed varieties and imitative species . Bot. Mar , 34 : 561 – 573 .
- Sterrenburg , FAS . 1991b . Studies on the genera Gyrosigma and Pleurosigma (Bacillariophyceae). Light microscopical criteria for taxonomy . Diatom Res , 6 : 367 – 389 .
- Sterrenburg , FAS . 1992 . Studies on the genera Gyrosigma and Pleurosigma (Bacillariophyceae). The type of the genus Gyrosigma and other attenuati sensu Peragallo . Diatom Res , 7 : 137 – 155 .
- Sterrenburg , FAS . 1995 . Studies on the genera Gyrosigma and Pleurosigma (Bacillariophyceae). Gyrosigma balticum (Ehrenberg) Rabenhorst, G. pensacolae sp. n. and simulacrum species . Bot. Mar , 38 : 401 – 408 .
- Stidolph , SR . 1992 . Observations and remarks on the morphology and taxonomy of the diatom genera Gyrosigma Hassall and Pleurosigma W. Smith. III. Gyrosigma sterrenburgii sp. nov., and Pleurosigma amara sp. nov . Diatom Res , 7 : 345 – 366 .
- Swofford DL 1998 PAUP* Phylogenetic analysis using parsimony (* and other methods) version 4.0b8 Sinauer Associates, Sunderland, MA
- Volkman , JK , Barrett , SM and Dunstan , GA . 1994 . C25 and C30 highly branched isoprenoid alkenes in laboratory cultures of two marine diatoms . Org. Geochem , 21 : 407 – 417 .