Abstract
A new heterotrophic armoured dinoflagellate is described from sand habitats in eastern Australia. Cabra matta gen. nov., sp. nov., lacks plastids and an eyespot. The thecal plate formula is Po 4′ 4” ‘x’ 3c ?s 5′′’ 1′′”. Its plate pattern differs from all currently described dinoflagellate genera, but is most similar to the genus Roscoffia. Cabra matta shows some similarity to species currently placed in the family Podolampaceae, however its evolutionary affinities and hence its position within the dinoflagellate systematic hierarchy remain unresolved.
Introduction
Benthic, sediment-dwelling dinoflagellates have been relatively little studied in comparison to their planktonic counterparts. In Australia, before 1990, only three species of benthic dinoflagellates had been recorded (Gillespie et al., Citation1986; Ruinen, Citation1938). Recently, studies on the diversity of sediment-dwelling dinoflagellates worldwide have been increasingly frequent (e.g. Faust, Citation1995; Faust & Steidinger, Citation1998; Hoppenrath, Citation2000 a,Citation b ; Horiguchi & Kubo, Citation1997; Horiguchi et al., Citation2000; Quod et al., Citation1999; Ten-Hage et al., Citation2000; Yoshimatsu et al., Citation2000). Several new genera have been named, for example Plagiodinium Faust & Balech Citation1993, Amphidiniella Horiguchi Citation1995, Halostylodinium Horiguchi & Yoshizawa-Ebata Citation2000 and Bysmatrum Faust & Steidinger Citation1998. Some species of thecate sand-dwelling dinoflagellates have been found to possess unusual thecal plate patterns that are difficult to categorize based on existing taxonomic criteria, and may not be easily assigned to any existing family (Faust & Balech, Citation1993; Hoppenrath, Citation2000 b; Horiguchi, Citation1995). These include the new genera Amphidiniella, Plagiodinium and Halostylodinium, as well as some species currently assigned to Adenoides Balech Citation1956, Amphidiniopsis Woloszynska 1928, Herdmania Dodge Citation1981, Planodinium Saunders & Dodge 1984, Roscoffia Balech Citation1956, Sabulodinium Saunders & Dodge 1984 and Thecadinium Kofoid & Skogsberg 1928. There may be considerable diversity yet to be described in sand-dwelling dinoflagellates.
This study forms part of a series of studies on the benthic flagellates of Australia (Al-Qassab et al., Citation2002; Ekebom et al., Citation1996; Larsen & Patterson, Citation1990; Lee & Patterson, Citation2000; Murray & Patterson, Citation2002 a,Citation b ). In the course of our survey of the benthic dinoflagellates present in the Sydney region, we recorded a species with an unusual thecal plate pattern which is sufficiently different from all currently described species to constitute a new genus. It is described here.
Materials and methods
Samples were collected at Port Botany, Botany Bay (151°14′E, 34°00′S), and at Heron Island (151°55′E, 23°25′S), in eastern Australia. Botany Bay is a warm temperate relatively shallow enclosed embayment with a waterway area of 80 km2 (NSW Department of Land and Water Conservation, 2000). At Port Botany, the sediment was made up of 81% medium sand and 14% fine sand (Lee & Patterson, Citation2002). The sediment water temperature ranged from 12 – 25°C, and the salinity ranged from 26 – 37 psu throughout the year at Port Botany. Heron Island is a coral cay in the southern Great Barrier Reef. The sediment was made up of coarse coral sand. During the course of periodic sampling in Port Botany between 1998 – 2002, over which time approximately 40 samples were collected, cells of Cabra matta were rarely found, being found on just three sampling occasions, in June 2000, April 2001 and January 2002. Cells showed no detectable seasonal distribution. In samples from Heron Island, cells were found on one occasion only, in June 2003.
Samples were collected from the first 0.5 cm of sediment at low tide using a flat spoon. Cells were collected by two methods: extracting them from the sediment through melting seawater ice (Uhlig, Citation1964), and by placing coverslips over a layer of lens tissue directly on the sediment and observing the coverslips after 12 – 36 h (Larsen & Patterson, Citation1990). They were observed and photographed using a Leica DMR light microscope with differential interference contrast optics. Photographs were taken of living cells, and cells were measured from photographs. The standard deviations (s.d.) of the measurements were calculated. For scanning electron microscopy, cells were pipetted individually from samples, rinsed three times in filtered seawater and placed on polylysine coated coverslips, or filtered onto a 2 μm nucleopore filter. They were fixed in 2% osmium tetroxide in seawater for 20 min, rinsed in distilled water, and dehydrated in a series of increasing ethanol concentrations (15, 30, 50, 70, 90, 100%). The cells were critical point dried, before being sputter coated with gold. They were observed using a Phillips 505 scanning electron microscope at 15 kV.
Holotypes and isotypes are deposited at the School of Biological Sciences, University of Sydney. The International Code of Botanical Nomenclature (Greuter et al., Citation2000) was applied.
Results
| 1. | Cabra matta Murray and Patterson, gen. et sp. nov. | ||||
| 2. | . | ||||
Diagnosis
Cellula 32 – 40 μm longa, 23 – 33 μm lata (inter dorsum et ventrem), circa 17 μm inter latera, ovalis paene rectangularis, aspectu laterali complanata. Formula scutelli Po 4′ 4” ‘x’ 3c ?s 5′′’ 1′′”. Theca multis cavis et foraminibus parvulis ornata. Cingulum ascendens, cellulam non omnino circumdans. Nucleus in regione postica situs. Cellulae neque chloroplasti neque ocellum.
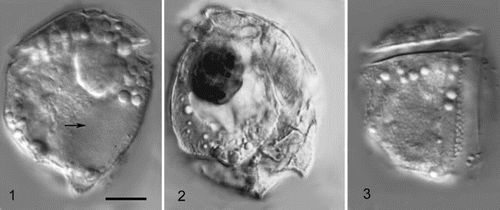
Figs 1 – 3. Cabra matta, gen. nov., sp. nov. Fig. 1. Low focus view of left lateral side. Arrow points to nucleus. Fig. 2. High focus view of right lateral side, showing dark (red) coloured inclusion, probably a food particle. Fig. 3. High focus view of left lateral side, showing suture between postcingular plates 2 and 3, and the areolate surface. All images taken using DIC optics. All images to same magnification, scale bar in Fig. 1 represents 10 μm.
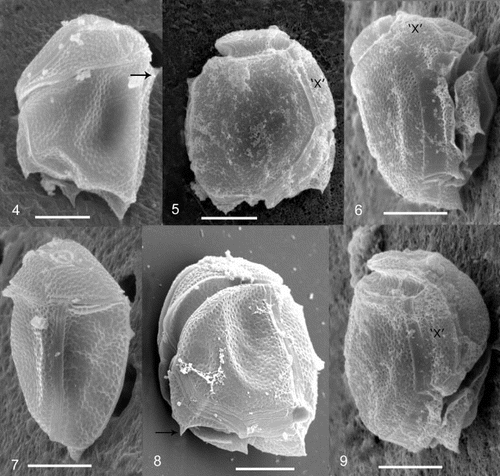
Figs 4 – 9. Cabra matta, gen. nov., sp. nov., SEM images. Fig. 4. Left lateral view, arrow points to dorsally pointed bump. Fig. 5. Right lateral view. Fig. 6. Ventral view. Fig. 7. Dorsal/left lateral view. Fig. 8. Posterior/left lateral view showing cingular plates. Arrow points to posteriorly pointed flange. Fig. 9. Ventral/right lateral view showing ‘x’ plate. The ‘x’ plate is indicated for orientation purposes. All scale bars represent 10 μm.
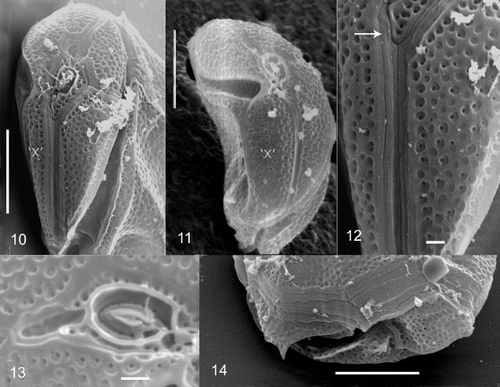
Figs 10 – 14. Cabra matta, gen. nov., sp. nov., SEM images. Fig. 10. Anterior view, showing epicone. Fig. 11. Anterior view, showing ‘x’ plate. Fig. 12. Anterior view of apical plates, arrow points to narrow first apical plate. Fig. 13. Apical pore and surrounding plates. Fig. 14. Lateral/posterior view, showing posterior plate. The ‘x’ plate is indicated for orientation purposes. Scale bars in 10, 11 and 14 represent 10 μm, and in 12 and 13, 1 μm.
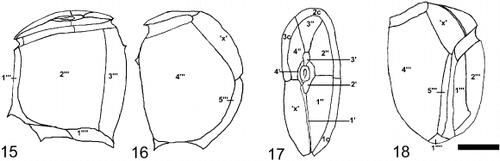
Figs 15 – 18. Cabra matta, gen. nov., sp. nov., line drawings of plate pattern. Fig. 15. Left lateral view. Fig. 16. Right lateral view. Fig 17. Apical view. Fig. 18. Ventral view. Scale bar represents 10 μm.
| 1. | Locality: Port Botany at Botany Bay, in intertidal sediment. | ||||
| 2. | Holotype: ; Isotype: | ||||
| 3. | Etymology: In the language of the Eora, the indigenous people of Botany Bay: ‘place where small organisms live’. | ||||
In lateral view, the cells are oval to rectangular and irregular, with the shape appearing different from the left as compared to the right side (, ). Cells are strongly laterally compressed. Cells are 32 – 40 μm long (mean 36 μm, s.d. 3.4 μm) and 23 – 33 μm wide (mean 28 μm, s.d. 3.3 μm), when measured between dorsal and ventral sides. The width between lateral sides is approximately 17 μm (n = 7). The hypotheca is much larger than the epitheca, which is relatively flat. The thecal plate pattern is an apical pore (Po), 4 apical plates (4’), 4 precingular plates (4”), an x plate (‘x’), 3 cingular plates (3c), and unknown number of sulcal plates (s), 5 postcingular plates (5′′’) and 1 antapical plate (1′′”) ( ). The cell surface is areolate with many indentations (approximately 0.5 μm), and small pores (approximately 0.15 μm) (, ). A small smooth margin is present along the edges of the plates (, 12). The first apical plate is very long and narrow, approximately 1 μm wide (). The apical pore is oval to rectangular in shape, approximately 3 μm long and 2 μm wide (). There is a ring of very fine pores (0.1 μm) surrounding an elliptical raised protrusion in the centre of the apical pore plate (). There are three small plates surrounding the apical pore region, one of which (3’) is deeply indented, posterior to it and without pores (). An ‘x’ plate is in the position of a fifth precingular plate (, ). The cingulum is strongly ascending and incompletely encircles the cell (). There are three cingular plates (). The cingular plates are areolate, but do not possess pores (). The number of sulcal plates could not be determined due to the deeply incised sulcus and the protruding first and fifth postcingular plates. There are five postcingular plates, the first of which is small, with a posteriorly pointed flange (). The third postcingular plate has a small dorsally pointed bump on the dorsal side of the cell (, 7). The nucleus is in the posterior dorsal side of the cell (). A large red body, probably a food body, is present in most cells (). Plastids and stigma are absent ().
Discussion
Alternative thecal plate pattern interpretations
Thecal plates have sometimes been interpreted slightly differently by different authors (for a discussion of the issue, see Fensome et al. (Citation1993) and Taylor (Citation1999)). In this study, the apical pore was designated as a single pore plate, as was also done for species of Roscoffia (Hoppenrath & Elbrächter, Citation1998; Horiguchi & Kubo, Citation1997). However, in the Podolampaceae this apical pore structure is considered to be two plates (Carbonell-Moore, Citation1994) comprising a pore plate, which is the ring of pores surrounding the raised protrusion, and a cover plate, which is the raised protrusion. In addition, an alternative plate pattern interpretation for the ‘x’ plate is as an additional precingular plate, as has been done for some species of Amphidiniopsis (Toriumi et al., Citation2002). In this case the number of plates in the precingular series would be five.
Comparison with other species
This species does not correspond with any currently known genera in its plate pattern and other cellular features. The species most similar to Cabra are members of the genus Roscoffia. There are two described species of Roscoffia, R. capitata Balech, Citation1956 and R. minor Horiguchi & Kubo, Citation1997, which have a general pattern of Po 3-4′ 0-1a 5” 3c 3-4s 5′′’ 1′′” (Balech, Citation1956; Hoppenrath & Elbrächter, Citation1998; Horiguchi & Kubo, Citation1997). The most similar features are the hypothecal plate arrangement, the presence of three cingular plates, the narrow first apical plate and the ascending or slightly ascending cingulum. The epithecal plate arrangement, apart from the incomplete cingulum and the possession of an ‘x’ plate, is also similar, except that R. minor possesses an anterior intercalary plate rather than four apical plates. Both species of Roscoffia possess a large obvious flagellar pore in the sulcus (Hoppenrath & Elbrächter, Citation1998; Horiguchi & Kubo, Citation1997). As the sulcal plates could not be determined in this study, it is not known whether Cabra also possesses a flagellar pore in the sulcus. Cabra differs from Roscoffia in that Roscoffia species do not possess incomplete cingula, their cingula are not as displaced, they are more symmetrically shaped, not as laterally compressed and they possess a sulcus which is wider and more distinct.
The position of the genus Roscoffia has not been resolved, however it has been suggested to be related to the family Podolampaceae (Hoppenrath & Elbrächter, Citation1998; Horiguchi & Kubo, Citation1997). In a recent molecular phylogenetic study, Roscoffia capitata was found to be a sister species to the newly described monotypic genus Lessardia (Saldarriaga et al., Citation2003), which was placed in the Podolampaceae.
Cabra also shows some similarity to species of the family Podolampaceae, in the order Peridiniales, which comprises the planktonic oceanic genera Podolampas Stein 1883, Blepharocysta Ehrenberg 1873, Lissodinium Matzenauer 1933, Lessardia Saldarriaga & Taylor Citation2003, Mysticella Carbonell-Moore Citation1994, Gaarderia Carbonell-Moore Citation1994, and Heterobractum Carbonell-Moore Citation1994 (Carbonell-Moore, Citation1994). The most similar features are the hypothecal plate arrangement, the apical pore structure and the presence of a long, narrow first apical plate. The general plate formula for this family was given as Po Pi CP 3′ 1a 5” 3c 4-5s 4-5′′’ 1′′”. Of these genera, Lissodinium, which contains some laterally compressed species, is most similar. The apical pore is similar in appearance, also possessesing a ring of pores, although, in Cabra matta, a canal plate appears to be lacking. The overall number of plates in the epithecal and hypothecal series is the same, although the plate that may be analogous to the anterior intercalary plate in Lissodinium touches the apical pore in Cabra, and so is tabulated as an extra apical plate. Despite the similarities, there are important differences between Cabra and species of Podolampaceae including the genus Lissodinium; the cingulum is imprinted in Cabra, as is typical in most dinoflagellates, rather than indistinct and broad, as in all genera currently placed in the Podolampaceae, and no genus or species in this family possesses an incomplete (and therefore an ‘x’ plate) or an ascending cingulum.
Because it is laterally compressed, this species also shows superficial similarities to other benthic dinoflagellates: Plagiodinium belizeanum Faust & Balech Citation1993, species of the genus Thecadinium, and the planktonic species Thecadiniopsis tasmanica Croome et al., Citation1987. Thecadinium has the general plate pattern: Po (present or absent) 3-5′ 0-2a 4-8” 5-6c 5-6s x (present or absent) 4-5′′’ 0-2p 1′′” (Hoppenrath, Citation2000 b). It contains both photosynthetic and heterotrophic species. The presence of an incomplete cingulum and an ‘x’ plate has only been recorded in one species, Thecadinium dragescoi (Hoppenrath, Citation2000 b). Cabra matta has a plate pattern which is quite different to Thecadinium dragescoi, which has eight (or seven, depending on interpretation) precingular plates and a more typical peridinioid hypothecal arrangement. Thecadinium dragescoi is probably more closely related to some species of the genus Amphidiniopsis than it is to other species of Thecadinium. Cabra matta differs from other species of Thecadinium in its epithecal plate pattern, and its ascending rather than descending or straight cingulum. Plagiodinium is a monotypic genus, and the species P. belizeanum is photosynthetic, does not possess an apical pore, and has a very different epithecal pattern to that of Cabra matta (Faust & Balech, Citation1993). The genus Thecadiniopsis is another monotypic genus that was established for a Thecadinium-like species which had a cingulum with ends that were displaced (Croome et al., Citation1987). Thecadiniopsis tasmanica differs from Cabra matta in its epithecal plate pattern, and the fact that its cingulum is descending rather than ascending.
We hesitate to assign this species to any currently known family, and until further comparative phylogenetic studies can be conducted using molecular genetic, ultrastructual or biochemical data, we place it in the order Peridiniales sensu Dodge (Citation1984), family uncertain.
Acknowledgements
We thank the Australian Federation of University Women and the Australian Biological Resources Study for funding. We thank A. Larkum for the provision of the Heron Island sample, and staff at the Electron Microscope Unit at the University of Sydney for technical advice and help. Ian Jackson and Christine Holzberg are thanked for help with the Latin translation.
References
References
- Al-Qassab , S , Lee , WJ , Murray , S , Simpson , AGB and Patterson , DJ . 2002 . Flagellates from stromatolites and surrounding sediments in Shark Bay, Western Australia . Acta Prot , 41 : 91 – 144 .
- Balech , E . 1956 . Étude des Dinoflagelles du sable de Roscoff . Rev. Alg , 2 : 29 – 52 .
- Carbonell-Moore , MC . 1994 . On the taxonomy of the family Podolampaceae Lindemann (Dinophyceae) with descriptions of three new genera . Rev. Paleo. Palyn , 84 : 73 – 99 .
- Croome , RL , Hallegraeff , GM and Tyler , PA . 1987 . Thecadiniopsis tasmanica gen. et sp. nov. (Dinophyta: Thecadiniaceae) from Tasmanian freshwaters . Br. Phycol. J , 22 : 325 – 333 .
- Dodge JD 1984 Dinoflagellate taxonomy In Dinoflagellates (Spector, D.L., editor) 17 42 Academic Press, Orlando
- Ekebom , J , Patterson , DJ and Vørs , N . 1996 . Heterotrophic flagellates from coral reef sediments (Great Barrier Reef, Australia) . Arch. Protist , 146 : 251 – 272 .
- Faust , MA . 1995 . Observation of sand-dwelling toxic dinoflagellates (Dinophyceae) from widely differing sites, including two new species . J. Phycol , 31 : 996 – 1003 .
- Faust , MA and Balech , E . 1993 . A further SEM study of marine benthic dinoflagellates from a mangrove island, Twin Cays, Belize, including Plagiodinium belizeanum gen. et sp. nov . J. Phycol , 29 : 826 – 832 .
- Faust , MA and Steidinger , KA . 1998 . Bysmatrum gen. nov. (Dinophyceae) and three new combinations for benthic scrippsielloid species . Phycologia , 37 : 47 – 52 .
- Fensome RA Taylor FJR Norris G Sarjeant WAS Wharton DI Williams GL 1993 A classification of living and fossil dinoflagellates American Museum of Natural History, Sheridan Press, Hanover
- Gillespie , NC , Lewis , RJ , Pearn , JH , Bourke , ATC , Holmes , MJ , Bourke , JB and Shields , WJ . 1986 . Ciguatera in Australia: occurrence, clinical features, pathophysiology and management . Med. J. Australia , 145 : 584 – 590 .
- Greuter , McNeill , WJ , Barrie , FR , Burdet , HM , De Moulin , V , Filgueras , TS , Nicolson , DH , Silva , PC , Skog , JE , Trehane , P , Turland , NJ and Hawksworth , DL . 2000 . International Code of Botanical Nomenclature (Saint Louis Code) . Regn. Vegetabile , 138 : 1 – 474 .
- Hoppenrath , M . 2000a . Morphology and taxonomy of Sinophysis (Dinophyceae, Dinophysiales) including two new marine sand-dwelling species from the North German Wadden Sea . Eur. J. Phycol , 35 : 153 – 162 .
- Hoppenrath , M . 2000b . Morphology and taxonomy of the marine sand-dwelling genus Thecadinium (Dinophyceae), with the description of two new species from the North German Wadden Sea . Phycologia , 39 : 96 – 108 .
- Hoppenrath , M and Elbrächter , M . 1998 . Roscoffia capitata (Dinophyceae) refound: notes on morphology and biology . Phycologia , 37 : 450 – 457 .
- Horiguchi , T . 1995 . Amphidiniella sedentaria gen. et sp. nov. (Dinophyceae), a new sand-dwelling dinoflagellate from Japan . Phycol. Res , 43 : 93 – 99 .
- Horiguchi , T and Kubo , F . 1997 . Roscoffia minor sp. nov. (Peridiniales, Dinophyceae): a new, sand-dwelling, armored dinoflagellate from Hokkaido, Japan . Phycol. Res , 45 : 65 – 69 .
- Horiguchi , T , Yoshizawa-Ebata , J and Nakayama , T . 2000 . Halostylodinium arenarium, gen. et sp. nov. (Dinophyceae), a coccoid sand-dwelling dinoflagellate from subtropical Japan . J. Phycol , 36 : 960 – 971 .
- Larsen , J and Patterson , DJ . 1990 . Some flagellates (Protista) from tropical marine sediments . J. Nat. Hist , 24 : 801 – 937 .
- Lee , WJ and Patterson , DJ . 2000 . Heterotrophic flagellates (Protista) from marine sediments of Botany Bay, Australia . J. Nat. Hist , 34 : 483 – 562 .
- Lee , WJ and Patterson , DJ . 2002 . Abundance and biomass of heterotrophic flagellates, and factors controlling their abundance and distribution in sediments of Botany Bay . Micr. Ecol , 43 : 467 – 481 .
- Murray , S and Patterson , DJ . 2002a . Amphidiniopsis korewalensis sp. nov., a new heterotrophic benthic dinoflagellate . Phycologia , 41 : 382 – 388 .
- Murray , S and Patterson , DJ . 2002b . The benthic dinoflagellate genus Amphidinium in south-eastern Australian waters, including three new species . Eur. J. Phycol , 37 : 279 – 298 .
- Quod , J , Ten-Hage , L , Turquet , J , Mascarell , G and Coute , A . 1999 . Sinophysis canaliculata sp. nov. (Dinophyceae), a new benthic dinoflagellate from western Indian Ocean Islands . Phycologia , 38 : 87 – 91 .
- Ruinen , J . 1938 . Notizen über Salzflagellaten II. Über die Verbreitung der Salzflagellaten . Arch. Protist , 90 : 210 – 258 .
- Saldarriaga , JF , Leander , BS , Taylor , FJR and Keeling , PJ . 2003 . Lessardia elongata gen. et sp. nov. (Dinoflagellata, Peridiniales, Podolampaceae) and the taxonomic position of the genus Roscoffia . J. Phycol , 39 : 368 – 378 .
- Taylor , FJR . 1999 . Charles Atwood Kofoid and his dinoflagellate tabulation system: an appraisal of the phylogenetic value of tabulation . Protist , 150 : 213 – 220 .
- Ten-Hage , L , Turquet , J , Quod , JP and Coute , A . 2000 . Coolia areolata sp. nov. (Dinophyceae), a new sand-dwelling dinoflagellate from the southwestern Indian Ocean . Phycologia , 39 : 377 – 383 .
- Toriumi , S , Yoshimatsu , S and Dodge , JD . 2002 . Amphidiniopsis uroensis sp. nov. and Amphidiniopsis pectinaria sp. nov. (Dinophyceae): two new benthic dinoflagellates from Japan . Phycol. Res , 50 : 115 – 124 .
- Uhlig , G . 1964 . Eine einfache Methode zur Extraktion der vagilen, mesopsammalen Mikrofauna . Helg. Wiss. Meer , 11 : 178 – 185 .
- Yoshimatsu , S , Toriumi , S and Dodge , JD . 2000 . Light and scanning electron microscopy of two benthic species of Amphidiniopsis (Dinophyceae), Amphidiniopsis hexagona sp. nov. and Amphidiniopsis swedmarkii from Japan . Phycol. Res , 48 : 107 – 113 .