Abstract
Halimeda Lamouroux constitutes a genus of calcified and segmented green seaweeds within the Bryopsidales. Molecular phylogenetic assessments have uncovered five principal monophyletic lineages within the genus. In the present study we define these lineages morphologically. We gathered morphological data from specimens used in the molecular analyses as well as from collections having a similar morphology and originating from the same geographical region. Starting from the lineages and their morphological synapomorphies, we define and illustrate five natural sections within Halimeda. All or most medullary siphons traversing the nodes between segments fuse into a single unit in specimens of lineage 1 (section Rhipsalis), and segments at the thallus base fuse with one another. Medullary siphons of specimens in lineage 2 (section Micronesicae) traverse the node without fusing. Medullary siphons of specimens in lineage 3 (section Halimeda) divide frequently below the nodes and become entangled among one another. The segments of specimens in this lineage possess a continuous uncorticated band along the distal perimeter instead of three or more pits as encountered in segments of specimens in all other lineages. Members of lineage 4 (section Pseudo-opuntia) possess club-shaped subperipheral utricles in their cortical region. Medullary siphons of specimens in lineage 5 (section Opuntia) fuse over only a short distance at the nodes and retain their identity. Apart from these synapomorphies, the lineages can be delimited further by a characteristic combination of symplesiomorphies and homoplasies. In addition we examined the morphology of H. bikinensis Taylor, a species not included in the molecular analyses, and discuss its ambiguous position in our sectional system.
Introduction
The green calcified seaweed genus Halimeda Lamouroux, (Citation1812) (Bryopsidales, Chlorophyta) occurs in reefs and lagoons across the tropics and subtropics (Barton, Citation1901; Taylor, Citation1950; Tsuda & Wray, Citation1977; Dong & Tseng, Citation1980; Hillis-Colinvaux, Citation1980, Citation1988; Drew & Abel, Citation1988; Tsuda & Kamura, Citation1991; Drew, Citation1995; Littler & Littler, Citation2000; Bandeira-Pedrosa et al., Citation2001). The characteristically segmented thalli are composed of ramifying siphons forming a medulla and a surrounding cortex (Barton, Citation1901; Hillis-Colinvaux, Citation1980). The siphons in the medulla string segments together and ramify into the cortex. There they rebranch frequently and terminate in a layer of inflated peripheral utricles. The latter adhere to one another and so enclose the segment's intersiphonal spaces (Barton, Citation1901; Hillis-Colinvaux, Citation1980). There, calcium carbonate precipitates as aragonite (Borowitzka & Larkum, Citation1977). Some medullary siphons surface in weakly calcified regions along the segment's distal perimeter where they adhere and may fuse. New segments (Hay et al., Citation1988), secondary holdfasts (Hillis-Colinvaux, Citation1980; Walters & Smith, Citation1994) or gametophores bearing bladder-like gametangia (Gepp, Citation1904; Kamura, Citation1966; Graham, Citation1975; Drew & Abel, Citation1988) develop from their tips. Thalli propagate clonally by means of ‘runner’ rhizoids (Hillis-Colinvaux, Citation1980) or fragmentation (Walters & Smith, Citation1994; Walters et al., Citation2002). Sexual reproduction occurs periodically; the gametes are released in concert in species-specific short intervals (Meinesz, Citation1980; Drew & Abel, Citation1988; Clifton, Citation1997; Clifton & Clifton, Citation1999).
The genus currently comprises 34 described extant species and several fossil taxa (Braga et al., Citation1996; Schlagintweit & Ebli, Citation1998; Hillis, Citation2000). All extant species and their taxonomic authorities are listed in . Hillis-Colinvaux (Citation1980) proposed five sections within the extant diversity based predominantly on patterns of medullary siphon anatomy at nodes between segments (Askenasy, Citation1888; Barton, Citation1901). These patterns often conflict with distributions of character states associated with utricle morphology and branching modes as well as with thallus habit across the taxa (Kooistra et al., Citation2002). Results of molecular phylogenetic studies in Kooistra et al. (Citation2002) indicate that most sections sensu Hillis-Colinvaux are not monophyletic.
Table 1. List of currently recognized Halimeda species and their taxonomic authorities. Species indicated with an asterisk were not examined in this study
The principal goals of this study are to demarcate monophyletic sections within Halimeda and to uncover their defining morphological traits. A morphological definition of these natural groups not only provides a helpful tool towards accurate identification of species but also allows, at least tentatively, placement of relatively recent fossil specimens in these sections. To achieve our goals, we inferred a maximum likelihood phylogeny from nuclear rDNA sequences of specimens across the taxonomic diversity and demarcated principal lineages therein. We then examined morphology and anatomy of the specimens included in the phylogeny in search of those traits whose states define one or more of these lineages. In addition, we included specimens in the morphological analyses for which no sequences were available but we used the latter specimens only to ascertain their fit into sections, not to redefine the sections.
Materials and methods
A list of specimens, together with their taxonomic identifications, herbarium codes and the GenBank accession numbers for their partial nuclear rDNA sequences is presented in . Details of preservation, taxonomic identification, DNA extraction, PCR and sequencing protocols can be found in Kooistra et al. (Citation2002). The 155 specimens of Halimeda used in this study were attributable to 32 of 34 currently recognized species (). All 49 specimens used for molecular analyses in this study as well as those used in previous publications on Halimeda by Kooistra and co-workers (Kooistra et al., Citation2002) are deposited in the GENT herbarium.
Table 2. List of Halimeda specimens used in this study
Phylogenetic analyses of the alignment were carried out using PAUP* version 4.0.b10 (Swofford, Citation2002). In all analyses, ambiguities were treated as uncertainties and gaps as missing data. Sequences of Udotea flabellum (Ellis & Solander) Howe and Penicillus capitatus Lamarck were used as the outgroup (Kooistra, Citation2002; Kooistra et al., Citation2002).
Hierarchical likelihood ratio tests (hLRT's) were performed using Modeltest v3.06 (Posada & Crandall, Citation1998). Resulting optimal parameters were then used to constrain maximum likelihood (ML) analysis. The ML analysis was carried out under the heuristic search option and tree bisection/reconnection branch swapping and was constrained using optimal hLRT parameter settings. Weighted (K = 2; Goloboff, Citation1993) maximum parsimony (MP) analysis was carried out under the heuristic search option and tree bisection/reconnection branch swapping. Bootstrap analyses (1000 replicates) were performed in weighted MP under the same settings.
Morphological analysis was also carried out on specimens used in the molecular analysis unless, in a few cases, not enough material was available. In that case, specimens unambiguously belonging to the same species and coming from the same geographical region were used. Additional specimens, for which no sequences were available, have also been examined (). Thallus and segment characteristics were noted. Anatomical details were gathered by dissection of segments as described in Hillis-Colinvaux (Citation1980) with the following modifications. The cortex was sectioned following decalcification. The medullary and nodal regions therein were examined after decalcification and removal of the surrounding cortical parts. In those cases where all nodal siphons fused into a single aggregate, nodal structures were also sectioned lengthwise. Scraped-off cortex fragments were used to examine segment surface. Observations on cortical structures were done using a slide with a cavity, allowing a better 3D impression. Camera lucida drawings were made using an Olympus BX51 microscope (Olympus, Tokyo, Japan).
Results
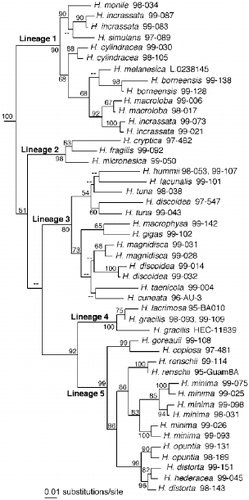
Hierarchical likelihood ratio tests performed on the sequence data set favoured a general-time-reversible base substitution model with estimated values for the following parameters: base frequencies: A = 0.206, C = 0.271, G = 0.305, T = 0.218; substitution rates: A⟷C = 1.211, A⟷G = 1.890, A⟷T = 1.524, C⟷G = 0.653, C⟷T = 3.525 relative to G⟷T = 1.000; proportion of invariable sites = 0.550; gamma shape parameter = 0.455. The tree resulting from our ML analysis constrained with these parameters is presented in . The topology is highly similar to those in Kooistra et al. (Citation2002). The tree-topology resulting from weighted MP analysis (not shown) differed only in a single aspect from that in : H. hummii and H. lacunalis did not form a clade. Five principal lineages marked in obtained high bootstrap support as did the clade containing lineages 4 and 5. Yet, the basal clades grouping these lineages obtained poor or insufficient support as in Kooistra et al. (Citation2002). All sequence pairs belonging to the same morphologically defined species obtained high bootstrap support.
Fig. 1. Maximum likelihood phylogram inferred from partial SSU nrDNA, ITS1, 5.8S rDNA and ITS2 of 47 specimens of Halimeda species and two outgroup species (see Table 2). – Ln likelihood = 9895.44751, tree-length = 1390 steps. The phylogram presented here has been redrawn with the outgroup taxa pruned away. MP bootstrap values ⩾ 50% are indicated below internodes. Lineages 1 – 5 are explained in text.
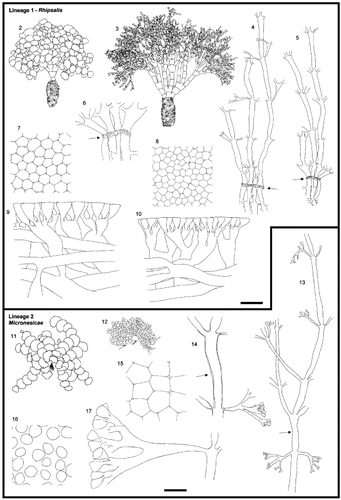
to illustrate the general morphology and anatomical characters of specimens in each of the five lineages.
Figs 2 – 17. (facing page) Morphology of Halimeda sections. Figs 2 – 10. Section Rhipsalis, Lineage 1. Figs 2, 3. General morphology. Fig. 2. H. simulans, H.0032. Fig. 3. H. cylindracea, HEC7612. Figs 4, 5. Medullary and nodal fusion. Fig. 4. H. cylindracea, H.0018. Fig. 5. H. simulans, H.0071. Fig. 6. Detail of nodal fusion. H. simulans, H.0071. Figs 7, 8. Surface view. Fig. 7. H. simulans, H.0071. Fig. 8. H. cylindracea, H.0018. Figs 9, 10. Cortical structures. Fig. 9. H. simulans, H.0071. Fig. 10. H. cylindracea, H.0018. Arrows indicate the location of the node. Figs 11 – 17. Section Micronesicae, Lineage 2. Figs 11, 12. General morphology. Fig. 11. H. fragilis, HEC14230. Fig. 12. H. micronesica, WLS184-02. Fig. 13. Medulla going through the nodal region. H. micronesica, H.0014. Fig. 14. Detail of a siphon at the node. H. fragilis, HV53. Figs 15, 16. Surface view. Fig. 15. H. cryptica, H.0237. Fig. 16. H. micronesica, WLS184-02. Fig. 17. Cortical structures. H. micronesica, WLS184-02. The cortical structures of H. micronesica are drawn from a slide prepared differently from those of all other species, because of the total lack of adhesion between utricles. Arrows indicate the location of the node. Scale bars represent: 25 mm for thalli, 500 μm for medulla, 250 μm for details of nodal structure, 60 μm for cortical structures and surface view.
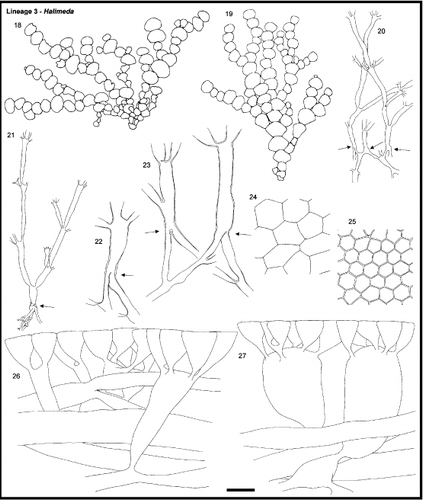
Figs. 18 – 27. Section Halimeda, Lineage 3. Figs. 18, 19. General morphology. Fig. 18. H. tuna, HV55. Fig. 19. H. lacunalis, HV306. Figs. 20, 21. Medullary and nodal fusion. Fig. 20. H. tuna, HV54. 21. H. lacunalis, HV306. Figs. 22, 23. Detail of nodal fusion. Fig. 22. H. lacunalis, H.0118. Fig. 23. H. tuna, H.0113. Figs. 24, 25. Surface view. Fig. 24. H. tuna, HV54. Fig. 25. H. lacunalis, HV306. Figs. 26, 27. Cortical structures. Fig. 26. H. tuna, H.0113. Fig. 27. H. taenicola, H.0037. The cortical structures of H. taenicola are highly variable between specimens and can be quite different from what is drawn in Fig. 27. Arrows indicate the location of the node. Scale bar represents: 25 mm for thalli, 500 μm for medullary, 250 μm for details of nodal structure, 60 μm for cortical structures and surface view.
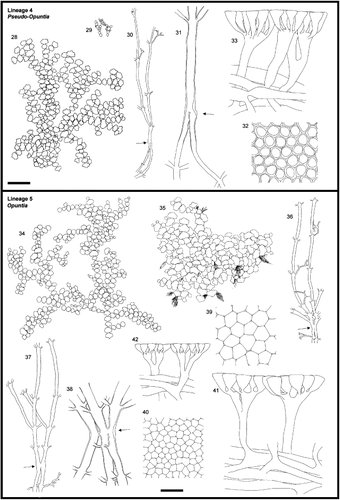
Figs. 28 – 42. Morphology of Halimeda lineages. Figs. 28 – 33. Section Pseudo-opuntia, Lineage 4. Fig. 28. General morphology. H. gracilis, C&PvR13865B. Fig. 29. General morphology. H. lacrimosa, redrawn from Hillis-Colinvaux (Citation1980). Fig. 30. Medullary and nodal fusion. H. gracilis, HV317. Fig. 31. Detail of nodal fusion. H. gracilis, H.0259. Fig. 32. Surface view. H. gracilis, HV317. Fig. 33. Cortical structures. H. gracilis, HV317. Figs. 34 – 42. Section Opuntia, Lineage 5. Fig. 34. General morphology. H. hederacea, HV1 Fig. 35. General morphology. H. distorta, HV199. Figs. 36, 37. Medullary and nodal fusion. Fig. 36. H. opuntia, HV19. Fig. 37. H. hederacea, HV9. Fig. 38. Detail of nodal fusion. H. copiosa, H.0265. Figs. 39, 40. Surface view. Fig. 39. H. distorta, HV199. Fig. 40. H. hederacea, HV9. Figs. 41, 42. Cortical structures. Fig. 41. H. distorta, HV199. Fig. 42. H. hederacea, HV9. Arrows indicate the location of the node. Scale bars represent: 25 mm for thalli, 500 μm for medullary, 250 μm for details of nodal structure, 60 μm for cortical structures and surface view.
Lineage 1, .
| 1. | Western Atlantic (Caribbean region): H. favulosa, H. incrassata, H. monile, H. simulans | ||||
| 2. | Indo-Pacific basin: H. borneensis, H. cylindracea, H. incrassata, H. macroloba, H. melanesica. | ||||
Most specimens were anchored in sandy substrata by means of a bulbous holdfast (, 3). Lower segments were large and barrel-shaped and the walls of their cortical siphons were strongly thickened thus giving rise to a stiff, stipe-like structure. In many species, segments on top of this so-called pseudo-stipe were moderately calcified, enlarged and partially fused in a fan- or squat-pillar-like structure (). Halimeda melanesica was also recovered in lineage 1, yet it lacked a bulbous holdfast and a pseudo-stipe. Nonetheless, the lowermost segments were also considerably larger than those in the upper region of the thallus. This species was encountered on wave-affected rock and rubble.
Nodes connecting segments in the middle thallus region possessed relatively thick-walled medullary siphons connecting with all their immediate neighbours by means of pores () thus giving rise to a single pack of interconnected medullary siphons. Notably, siphons did not fuse at the nodes in partially fused (basal) segments of H. borneensis and H. macroloba.
The cortex was dense and, depending on the species and the location of the examined segment in the thallus, consisted of three to many layers of moderately inflated utricles (, 10). In general, peripheral utricles were irregularly polygonal in surface view ().
Lineage 2, .
| 1. | Western Atlantic (Caribbean region): H. cryptica | ||||
| 2. | Indo-Pacific basin: H. fragilis, H. micronesica | ||||
The specimens of Indo-Pacific species were found in wave-affected biotopes (mostly H. micronesica), on shallow reef slopes and in channels with strong tidal currents (both H. fragilis and H. micronesica). Our specimens of H. cryptica originated from deep ( > 25m) cliffs facing the open sea. Segments of lineage 2 specimens appeared strongly calcified and brittle with flexible nodes. The specimens belonging to H. fragilis and H. micronesica were dull greyish green whereas those of H. cryptica were grass green on the segment side facing the light and white on the opposite side.
Specimens from this lineage possessed a single huge nodal siphon (H. cryptica) or several smaller ones passing through the nodes without fusion (H. fragilis and H. micronesica, ).
The cortex was relatively thin and consisted of a series of cylindrical utricles gradually becoming longer and broader from the periphery inwards (). Both Indo-Pacific species possessed primary utricles separating completely on decalcification of the segment and being round in surface view (). In contrast, the peripheral utricles of H. cryptica adhered to each other and were irregularly polygonal in surface view ().
Lineage 3, .
| 1. | Atlantic: H. discoidea, H. hummii, H. scabra Mediterranean H. tuna, Western Atlantic H. tuna | ||||
| 2. | Indo-Pacific basin: H. discoidea, H. gigas, H. lacunalis, H. macrophysa, H. magnidisca, H. taenicola | ||||
| 3. | Indo-Pacific and possibly Brazil: H. cuneata (Bandeira-Pedrosa et al., Citation2001) | ||||
Specimens of this lineage were found in semi-sheltered to exposed biotopes. In general, the thallus attached to hard substrata by means of a felt-like, discoid holdfast. Two major thallus morphologies were encountered: Halimeda lacunalis, H. hummii and H. cuneata possessed smooth, small and moderately calcified segments with flexible nodes whereas others such as H. discoidea, H. gigas and H. macrophysa had pliable, weakly calcified and large segments with broad but rather inflexible nodes. Yet, the division is not strict because H. magnidisca possessed large, pliable and weakly calcified segments with narrow and flexible nodes and thalli of H. taenicola were composed of small, moderately calcified segments with broad, inflexible nodes. These distinct thallus morphologies did not cluster in the phylogenetic tree. Our specimens of H. magnidisca deviated from the type material in that their holdfasts, though sand-encrusted, were not perfectly bulbous; the thalli grew on hard substrata covered with a thin layer of sand. On the other hand, we occasionally observed specimens of other lineage 3 species anchoring in unconsolidated substrata by a minute bulbous holdfast.
Medullary siphons branched frequently and entangled strongly below the distal perimeter of the segment to fuse in a single band in the segment's upper rim (). New segments emerged from anywhere along this band (). The nodal fusions were complete: the fused units continued into the subsequent segment as single, broad siphons () until they ramified.
In species with large pliable segments, the cortex consisted of a single or double layer of large and swollen sub-peripheral utricles leaving little space for calcification (H. discoidea, H. gigas, H. macrophysa, H, magnidisca) whereas in species with small segments and flexible nodes, the cortex contained one to several layers of variously formed sub-peripheral utricles (H. cuneata, H. lacunalis, see Hillis-Colinvaux, Citation1980, ; see also H. hummii in Ballantine, Citation1982). In all but one species (H. macrophysa), peripheral utricles adhered firmly, did not separate after we decalcified the segment, and showed an irregularly polygonal surface pattern ().
Lineage 4, .
| 1. | Atlantic (Caribbean region): H. gracilis, H. lacrimosa | ||||
| 2. | Indo-Pacific basin: H. gracilis | ||||
The specimens of this lineage were collected from relatively deep sites; they sprawled over rocky or partially unconsolidated substrata on reef slopes. Their fairly small segments were strongly calcified. In H. gracilis, three to several uncorticated pits were distributed along the segment's apical rim. In H. lacrimosa these pits appeared reduced and scattered over the upper part of the segment.
Medullary siphons fused completely at the nodes (, 31) though Hillis-Colinvaux (Citation1980) reported occasional occurrence of incomplete fusions in H. lacrimosa. The distance between subsequent ramifications in the subnodal region was larger than in other lineages and the siphons did not entangle among one another.
Secondary cortical utricles expanded only at their apex, the expanded areas forming a distinct layer. Numerous peripheral utricles sprouted from the broadened distal end of each secondary utricle (). Similarly, peripheral utricles broadened only slightly at their base and more strongly towards their distal end (). Peripheral utricles were round in cross-section. Around their tips, they formed lateral cell wall extensions that adhered to those of adjacent utricles in a hexagonal pattern. In the resulting surface view, the utricles appeared rounded as well as hexagonal, the prominence of each depending on the focal plane (). The peripheral utricles adhered to each other, although not strongly.
Lineage 5, .
| 1. | Atlantic (Caribbean region): H. copiosa, H. goreauii | ||||
| 2. | Indo-Pacific basin: H. distorta-hederacea species complex, H. minima, H. renschii, H. velasquesii | ||||
| 3. | Pan-tropical: H. opuntia | ||||
The specimens of this lineage were collected from various reef habitats. Most species showed a preference for a single habitat type: our specimens of H. renschii were found in moderately wave-exposed localities whereas those of H. copiosa, H. goreauii, H. minima and H. distorta always came from sheltered localities. Halimeda opuntia was ecologically plastic, abounding in a range of habitats from shaded sheltered lagoons and deep fore reefs to moderately exposed reef crests. Thallus shape was also strongly linked with habitat type: H. renschii thalli were erect, whereas those of specimens found in more sheltered habitats were pendant or sprawling (, 35). The segments of specimens belonging to this lineage were relatively small and heavily calcified.
Nodal medullary siphons fused briefly (in pairs and threes) without losing their identity ().
The cortex appeared thin: the few siphons emerging from the medulla usually did not ramify until close to the segment's periphery ().
Opuntioid lineages
The clade with lineages 4 and 5 possessed morphological synapomorphies as well. Specimens abounded in habitats under moderate to high grazing pressure and often revealed a sprawling mode of growth. The primary holdfast of full-grown thalli was often difficult to locate or was altogether absent. In the latter case, numerous secondary holdfasts attached the thallus to the substratum. Specimens of the sister species H. goreauii and (Atlantic) H. copiosa lacked such holdfasts.
The medullary siphons were generally narrower and smaller than those in the three other lineages.
The cortical siphons emerging from the medulla usually did not ramify until close to the segment's periphery. The large intersiphonal space was filled with aragonite, rendering the segments rigid and generally brittle. As in lineage 2, subperipheral utricles were cylindrical or widened only slightly towards their distal end.
Morphological observations on the type specimen of H. bikinensis
We have re-examined the type material of H. bikinensis (WRT46-156, MICH). The cortex was thin, the utricles were club-shaped, and the peripheral utricles possessed lateral cell wall extensions at their tips. In the subnodal medullary, siphon ramifications were widely spaced and trichotomous and did not become entangled with one another. Complete and incomplete fusions occurred together at the node. These character states were all typical for lineage 4 taxa. Furthermore, the segments were heavily calcified and brittle and possessed rhizoid tufts emerging from the uncorticated rim adjacent to the attachment region of daughter segments. These character states are also encountered in H. gracilis (lineage 4). What was peculiar, however, is that the type specimen of H. bikinensis also possessed an uncorticated rim along the distal segment perimeter, a character state typical for species in lineage 3. We attempted a molecular examination of the type specimen but unfortunately, its DNA was totally degraded.
Discussion
This study reveals that the five natural lineages from phylogenies based on partial nuclear rDNA sequences (Kooistra et al., Citation2002) possess readily recognizable morphological characters. Using the groups' morphological character states, we redefine sections established by Hillis-Colinvaux (Citation1980). Her sections based solely on medullary siphon patterns at the nodes between segments are already surprisingly close to the ones we establish here indicating that Barton (Citation1901) and Hillis-Colinvaux (Citation1980) were right in their notion that these patterns delimited natural groups. We define our sections only through synapomorphies, though we note the symplesiomorphies since each section is defined by a particular combination. Although we provide some ecological information with the sections, we refer to Kooistra et al. (Citation2002) for detailed historic ecological patterns in the evolution of the lineages and for habitat descriptions of species to Hillis-Colinvaux (Citation1980) and references therein.
History of subdivisions in Halimeda
De Toni (Citation1889) introduced sections in Halimeda taxonomy. He cited the descriptions of Agardh's (Citation1887) subgeneric groupings of implicit hierarchy as diagnoses for his sections Tunae, Pseudo-opuntiae, Opuntia and Rhipsales. His division is based mainly on thallus appearance, a notoriously unreliable feature (Hillis-Colinvaux, Citation1980; Kooistra et al., Citation2002). Moreover, several species in his sections are of uncertain status (Hillis-Colinvaux, Citation1980). Almost a century later, Hillis-Colinvaux (Citation1980) revised the generic subdivision. She redescribed three out of De Toni's four sections (Tunae, Opuntiae, Rhipsales) and diagnosed two novel sections (Micronesicae and Crypticae). She further altered the spelling of De Toni's section names to conform to the ICBN. Section Tunae was renamed Halimeda because it contains H. tuna, the type species of the genus. She based her sectional descriptions solely on nodal fusion patterns, although she noted that other characters accompanied these patterns.
A new sectional division
Section Rhipsalis J. Agardh ex De Toni, Lineage 1
Type species: Caribbean H. incrassata.
The defining characters of this lineage are the interconnecting pores of the nodal siphons and the segment agglutination in the basal thallus region in H. melanesica and in the thallus region above the pseudo-stipe in all other species. The bulbous holdfast and the pseudo-stipe are not diagnostic for this section because H. melanesica (lineage 1) lacks these traits whereas H. magnidisca, which is a member of lineage 3, does possess a bulbous holdfast and a stipe-like basal zone when growing on sand (Noble, Citation1986). Both the bulbous holdfast and the pseudo-stipe are adaptations to growth in unconsolidated substrata (Hillis-Colinvaux, Citation1980; Kooistra et al., Citation2002).
Hillis-Colinvaux (Citation1980) assigned H. melanesica to her section Micronesicae (our lineage 2) because the description (Valet, Citation1966) mentions only sparse siphon fusion if any at all. Yet placement in lineage 1 corroborates its morphology because minute pores connect the nodal medullary siphons (Kooistra et al., Citation2002).
Section Micronesicae Hillis-Colinvaux, Lineage 2
Type species: H. micronesica.
A single character defines this lineage: broadened siphons pass unfused through the nodes. Unfused nodal siphons appear to render nodes flexible minimizing drag in wave-affected environments (H. micronesica), and habitats with strong tidal currents (H. fragilis and H. micronesica). The single nodal medullary siphon observed in H. cryptica may result from secondary reduction related to the species' adaptation to deep sites. Yet such environments are not necessarily sheltered. There, thalli are exposed to current, swell from long surface waves and high-amplitude internal waves (Pinkel, Citation1983; personal observations).
The single siphon traversing the node between segments of H. cryptica enticed Hillis-Colinvaux (Citation1980) to propose a monotypic section Crypticae because it sets the species apart from all other Halimeda species. However, recovery of H. cryptica in lineage 2 indicates that section Crypticae Hillis-Colinvaux is obsolete.
Section Halimeda, Lineage 3
The defining traits of this lineage are the subnodal entanglement of medullary siphons and the presence of an uncorticated band along the distal part of the segment perimeter. New segments emerge from anywhere along this band.
De Toni (Citation1889) validly described this section as Tunae based on the description of a grouping of implicit hierarchy by Agardh (Citation1887). Hillis-Colinvaux (Citation1980) renamed the section Halimeda because a section containing the type species of the genus must have the same name as the genus (ICBN). However, she retained the original authorities, meaning that the full name of the section was Halimeda J. Agardh ex De Toni. The Saint Louis ICBN states that the name of a section containing the type species of the genus should not be followed by an author citation. This is here corrected.
Noble (Citation1986) did not allocate H. magnidisca to any of Hillis-Colinvaux's sections because the specimens examined by her possess segments and siphon fusion patterns typical for section Halimeda but bulbous holdfasts and stipitate lower segments typical of section Rhipsalis sensu Hillis-Colinvaux (Citation1980). Kooistra et al. (Citation2002) showed that this species is a member of lineage 3 (section Halimeda). Apparently, bulbous holdfasts have been acquired multiple times independently as an adaptation to growth on soft substrata.
Halimeda hummii was placed in section Opuntia sensu Hillis-Colinvaux (Citation1980) by Hillis et al. (Citation1998), but according to the molecular phylogeny in Kooistra et al. (Citation2002) this species belongs within lineage 3 (section Halimeda). Apparently, many characters have evolved in this species to states similar to those in lineage 5 (section Opuntia). Incidentally, some medullary siphons may fuse incompletely, but if present, they are always accompanied by completely fused siphons. The non-entangling behaviour of siphons in the subnodal region, too, is reminiscent of lineages 4 or 5 rather than of lineage 3. The difference from lineages 4 and 5 lies in the way new segments arise. In H. hummii, as in all other members of the Halimeda section, segments can emerge anywhere along the uncorticated band in the distal part of the segment perimeter whereas in lineages 4 and 5, new segments emerge only from uncorticated pits.
Section Pseudo-opuntia J. Agardh ex De Toni, Lineage 4
Type species: Indo-Pacific H. gracilis.
The defining character of this lineage is encountered in the cortical structure: secondary cortical utricles expand only at their apex and have a large and fairly constant number of peripheral utricles arranged around their distal end (Kooistra et al., Citation2002). The section was first validly described by De Toni (Citation1889) but later rendered obsolete by Hillis-Colinvaux (Citation1980). She moved H. gracilis, the only unambiguous species in it, to her section Halimeda. Complete nodal siphon fusion is the defining trait of Hillis-Colinvaux's (Citation1980) section Halimeda. Yet, her section is paraphyletic and the trait is a symplesiomorphy shared between specimens in lineages 3 and 4. Therefore we propose to re-establish De Toni's (Citation1889) section Pseudo-opuntia, with H. lacrimosa and H. gracilis as its members. This treatment renders both sections Halimeda and Pseudo-opuntia natural units.
The Pseudo-opuntia lineage shares its main nodal fusion pattern with the Halimeda lineage. The distinction lies in the behaviour of medullary siphons just below the nodes and in the way new segments arise. In members of section Halimeda, the medullary siphons ramify frequently below the nodes and consequently, become entangled with one another. In members of Pseudo-opuntia, the medullary siphons show no sign of entanglement in the subnodal zone because the distance between subsequent ramifications is relatively large. Moreover, in members of section Halimeda, segments arise from anywhere in the uncorticated band that spans the distal part of the segment perimeter. In section Pseudo-opuntia, segments arise from round to slightly elongated pits.
Section Opuntia J. Agardh ex De Toni, Lineage 5
Type species: H. opuntia.
This section has only a single defining character: the nodal medullary siphons fuse briefly in pairs or threes (and rarely in small groups) without losing their identity. Yet, H. lacrimosa (Hillis-Colinvaux, Citation1980), H. hummii (Ballantine, Citation1982) and H. borneensis can occasionally show similar patterns in the nodes, alongside the typical patterns in these species. Section Opuntia was erected by De Toni (Citation1889), and drastically expanded by Hillis-Colinvaux (Citation1980). The species composition of this section as in Hillis-Colinvaux (Citation1980) is maintained unaltered.
Opuntioid lineages
Lineages 4 and 5 could also be merged into a single section. The defining traits would then be a thin subperipheral cortex and heavily calcified segments. All but two species (H. lacrimosa, H. renschii) show a sprawling, or pendant, habit and live in sheltered to semi-exposed habitats. Nonetheless, we believe that the dominant patterns of nodal fusion and the shape of the peripheral and secondary utricles differ sufficiently between the two lineages to maintain them as different sections.
Morphological symplesiomorphies and homoplasies
Many characters are present in two or more lineages that are not sister clades. For example, the presence of uncorticated pits from which daughter segments can emerge is an ancestral trait. This character only changed state in the common ancestry of lineage 3. The central uncorticated pit appears to have stretched out laterally to occupy most of the upper segment rim (Kooistra et al., Citation2002). Unlike in all other lineages where new segments emerge exclusively from the uncorticated pits in the segment rim, new segments emerge from anywhere along this band.
Lineages 3 and 4 share patterns of nodal siphon fusion while all other lineages possess their own nodal pattern of siphon behaviour. In , complete fusion is a symplesiomorphy of these lineages.
Lineage 2 and the opuntioid clade (lineages 4 and 5) share generally well-calcified and often brittle segments. In addition, the utricles in the subperipheral cortex are generally not notably swollen. Yet, it should be noted that calcification and utricle shape are related because the more swollen the utricles, the less intersiphonal space remains to be filled with aragonite. Morphologically, it would be more parsimonious to let lineages 2 and 3 switch positions. Such a switch is not improbable because bootstrap support for the clade uniting lineages 3, 4 and 5 is below 50% and Kooistra et al. (Citation2002) showed that this alternative topology is not significantly worse using the Kishino-Hasegawa test option in PAUP*. It should be noted however, that even in this alternative topology strong calcification does not become a synapomorphy because several species in lineage 3 also possess strongly calcified segments.
Species not included in the phylogeny
Although we did not have access to specimens of the following taxa for molecular analyses, we expect that H. favulosa will be recovered in lineage 1 (section Rhipsalis), H. scabra and H. xishaensis will fall within lineage 3 (section Halimeda), and H. howensis will be recovered in lineage 5 (section Opuntia) because according to their descriptions in Hillis-Colinvaux (Citation1980), Dong & Tseng (Citation1980) and Kraft (Citation2001) these taxa share all synapomorphies and the proper combination of symplesiomorphies with these lineages. However, whether these species constitute genetically and biologically valid taxa or just plastic extremes within other species remains to be resolved.
Placement of H. bikinensis in our system remains puzzling. Its original description (Taylor, Citation1950) and those in Hillis (Citation1959) and Hillis-Colinvaux (Citation1980) as well as the anatomical characteristics we observed in the type permit placement in either lineage 3 or 4. The anatomy and general morphology of the type specimen indicate that it belongs to section Pseudo-opuntia rather than section Halimeda. The thin cortex, the club-shaped secondary utricles and the lateral cell wall extensions at the tips of peripheral utricles as well as the widely spaced, trichotomous ramifications of the subnodal medullary and the complete and incomplete nodal fusions occurring side by side are all typical for species in our section Pseudo-opuntia (lineage 4). However, the presence of the uncorticated rim along the distal segment perimeter of H. bikinensis is a synapomorphy of section Halimeda (lineage 3) in our molecular phylogeny. In section Pseudo-opuntia, the uncorticated region is limited to a series of round to slightly elongate pits along the perimeter (H. gracilis) or a number of reduced pits scattered over the upper part of the segment (H. lacrimosa).
If H. bikinensis groups within lineage 4 then the uncorticated rim is not a synapomorphy of lineage 3 whereas if it goes with lineage 3 then the apically inflated secondary utricles in the cortex do not define section Pseudo-opuntia. If the species forms a lineage on its own behalf, then only the entangling siphons below the nodes remain a synapomorphy of lineages 3, and taxa in lineage 4 do not possess a single synapomorphy.
Given the problems H. bikinensis creates in our sectional division, it is understandable that Hillis-Colinvaux (Citation1980) grouped species in lineages 3 and 4 in a single section Halimeda defined by complete fusion of medullary siphon in pairs and triplets at the nodes. According to the molecular phylogenies, however, her section is paraphyletic. It should be stressed also that Hillis-Colinvaux's (Citation1980) concept of H. bikinensis differs from that of Taylor (Citation1950). The rhizoidal tufts emerging from the uncorticated rim suggest a sprawling habit of the type specimen Taylor collected. This sprawling behaviour goes unnoticed in Hillis-Colinvaux (Citation1980). Instead, she states that thalli are erect. The general morphology of the specimen depicted by her corresponds very well to that of some Indo-Pacific Halimeda discoidea. Also, re-examination of specimens from the National History Museum (London) identified by her as H. bikinensis revealed only thoroughly swollen, albeit smallish, secondary utricles as encountered in some Indo-Pacific H. discoidea.
Key to the sections
In order to facilitate assignment of specimens to our sections, we present a key based on morphological characters. The two most problematic cases of morphological convergence (H. hummii and H. melanesica) key out even if a misstep occurs. Halimeda bikinensis also keys out separately.
| 1. | (1a) Siphons not fusing at the nodes between segments. … … … … … … … … … … 2 | ||||
| 2. | (1b) Siphons fusing at the nodes between segments, either over a short or a long distance. … … … … … … … … … … 3 | ||||
| 3. | (2a) Subperipheral cortex dense, consisting of moderately swollen utricles with a constriction at their base. Nodal siphons adhering in groups and usually communicating with each other through minute pores. Thalli erect. … H. melanesica of section Rhipsalis | ||||
| 4. | (2b) Subperipheral cortex thin, consisting of cylindrical utricles (neither swollen, nor constricted). Diameter and length of subperipheral utricles increasing towards the medulla. Adherence of nodal siphons absent or weak. No pores connecting the neighbouring nodal siphons. Thalli erect or pendant. … … … … section Micronesicae | ||||
| 5. | (3a) Complete fusion of siphons at the node: fused siphons continue into the subsequent segment as a single, thicker siphon. … … 4 | ||||
| 6. | (3b) Nodal siphon fusion over a short distance (once to twice the siphon diameter). … … 8 | ||||
| 7. | (4a) Siphons below the node relatively narrow and frequently branching. Subnodal branches numerous, entangling and fusing towards the node, resulting in difficult observation of the fusion pattern. Segments weakly to moderately calcified. Uncorticated rim present along the distal perimeter of the segment. Daughter segments arising from any point along this rim. Thalli erect.… … … … … … … section Halimeda | ||||
| 8. | (4b) Siphons not branching more frequently below the node than elsewhere in the medullary. Entanglement of subnodal siphons absent or weak, resulting in easy observation of the fusion pattern. … … . 5 | ||||
| 9. | (5a) Uncorticated belt extending along the distal part of the segment perimeter. Daughter segments emerging anywhere along this belt. Thalli erect or partially sprawling. … … … … … … … … … … H. bikinensis | ||||
| 10. | (5b) No uncorticated belt along the distal part of the segment perimeter. Daughter segments emerging from isolated pits or from small, slightly elongate uncorticated regions along the segment perimeter. Thalli erect, sprawling or pendant. … … … … … … 6 | ||||
| 11. | (6a) Segments flattened, paper-thin, moderately calcified. Segments emerging from anywhere along the distal part of the perimeter of the parent segment. … … … … … … … … … … H. hummii of section Halimeda | ||||
| 12. | (6b) Segments thicker than 0.6 mm, either flattened or globose to tear-shaped, and strongly calcified. … … … … … … … 7 | ||||
| 13. | (7a) Segments flattened or globose to tear-shaped. Daughter segments of flattened segments emerging from large pits along the distal segment perimeter; daughter segments of globose to tear-shaped segments arising from (generally three) reduced pits spread over the top region of the segment. … … … . section Pseudo-opuntia | ||||
| 14. | (7b) Segments flattened. Daughter segments emerging anywhere along the uncorticated belt along the distal part of the segment perimeter. … … … … … … H. bikinensis | ||||
| 15. | (8a) Nodal siphons generally fusing into a single unit. Nodal siphons adhering to all their neighbours and communicating with them by means of large pores. Occasionally, siphons fusing in large groups (more than five siphons) rather than into a single unit. Thallus erect and often possessing a bulbous holdfast. Segments in the basal zone of the thallus usually agglutinating into stipe- and/or fan-like structures. Segments moderately to strongly calcified. Pores occasionally minute rather than large; in this case nodal siphons adhering in groups and thallus holdfast felt-like. … … … … … … … … … … … … section Rhipsalis | ||||
| 16. | (8b) Siphons fusing in twos, threes, or occasionally in small groups (less than five siphons). Thalli erect, pending or sprawling. Attachment to the substratum by means of rhizoid tufts or a non-bulbous, felty holdfast. Segments in the basal thallus region not agglutinating to a stipe- and/or fan-like structure. … … … … … … … … … … 9 | ||||
| 17. | (9a) Incomplete (short) fusions and complete fusions co-occur. Mature thalli smaller than 5 cm. … … H. hummii of section Halimeda | ||||
| 18. | (9b) All fusions incomplete. Mature thalli larger than 5 cm. … … … … … section Opuntia | ||||
Perspectives
A revision of Halimeda species is needed, given the existence of paraphyletic species, of cryptic diversity hidden within a single perceived species (e.g. H. minima) and of cognate pairs, genetically distant species that have converged morphologically (Kooistra et al., Citation2002). Hillis-Colinvaux (Citation1980) had access only to morphological character states; she had no analysis of independent data to determine which morphological features were homologous. For instance, she could not identify all the cognates reported by Kooistra et al. (Citation2002). Although she noticed slight morphological differences between what she perceived as the same species in different geographical regions [see Colinvaux (Citation1969) on H. copiosa – H. hederacea and Hillis-Colinvaux (Citation1980) on Mediterranean and western Atlantic H. tuna], she could not evaluate the meaning of these differences because the characters also varied within geographical regions. Many species in her most recent monograph (Hillis-Colinvaux, Citation1980) are thus biphyletic entities. They may, in fact, differ between one another morphologically, either in as yet unexplored characters or in characters that are now considered variable. A new monograph ought, apart from proper illustrations of anatomical details, to incorporate a thorough evaluation of measurable features associated with medullary, cortical and gametangial siphon anatomy and segment morphology. Such an approach may uncover currently overlooked differences among species and sections; differences that will not only facilitate distinction of cognates and other look-alikes but will also permit more sound comparison with the morphology of fossil Halimeda.
Acknowledgements
We thank M. Coffroth, P. Colinvaux, E. Coppejans, O. Dargent, O. De Clerck, G. De Smedt, R. Haroun, I. Hendriks, L. Hillis, L. Kirkendale, F. Leliaert, L. Liao, J. Maté, A. N'Yeurt, C. Payri, G. Procaccini, W.F. Prud'homme van Reine, T. Schils and B. Wysor for sample collection, E. Coppejans, P. Baas, W.F. Prud'homme van Reine and M.G. Wynne for facilitating access to herbarium specimens from the GENT, L and MICH herbaria, P. Vanai (Environmental Service of Wallis and Futuna) for facilitating fieldwork on Uvea Island, and P. Goetghebeur for his help on nomenclature. We thank E. Coppejans for his valuable comments on the manuscript. H.V. is indebted to the Bijzonder Onderzoeksfonds (Ghent University) for grant 011D9101 and to the Fund for Scientific Research Flanders for research project grant 3G002496.
References
References
- Agardh JG 1887 Till Algernes Systematik, nya bidrag, femte afdelningen Lunds Universitets års-Skrift, Afdeln. Mathem. Naturvet
- Askenasy E 1888 Algen In Die Forschungsreise S.M.S. Gazelle Th. 4 Bot., Berlin
- Ballantine , DL . 1982 . Halimeda hummii sp. nov., Halimeda cryptica v. acerifolia var. nov. (Caulerpales, Chlorophyta), and additional records of Halimeda species from Puerto Rico . J. Phycol , 18 : 86 – 91 .
- Bandeira-Pedrosa ME Pereira SMB Oliveira EC 2001 Taxonomy and distribution of the genus Halimeda Lamouroux (Bryopsidales, Chlorophyta) in the Brazilian coast In Supplemental abstracts in Programme 7th Int. Phycol Congress 72 Aristotle Univ. of Thessaloniki, Greece
- Barton ES 1901 The genus Halimeda Monographs of the Siboga Expedition 60 Leiden
- Borowitzka , MA and Larkum , ADW . 1977 . Calcification in the green alga Halimeda. V. An ultrastructure study of the thallus development . J. Phycol , 13 : 6 – 16 .
- Braga , JC , Martin , JM and Riding , R . 1996 . Internal structure of segment reefs: Halimeda algal mounds in the Mediterranean Miocene . Geology , 24 : 35 – 38 .
- Clifton , KE . 1997 . Mass spawning by green algae on coral reefs . Science , 275 : 1116 – 1118 .
- Clifton , KE and Clifton , LM . 1999 . The phenology of sexual reproduction by green algae (Bryopsidales) on Caribbean coral reefs . J. Phycol , 35 : 24 – 34 .
- Colinvaux , L and Hillis . 1969 . Halimeda copiosa and Halimeda hederacea . J. Phycol , 5 : 88
- De Toni JB 1889 Sylloge Algarum Vol. I Sect. I Chlorophyceae
- Dong , M and Tseng , CK . 1980 . Studies on some marine green algae from the Xisha Islands, Guandong Province, China. II . Stud. Mar. Sinica , 17 : 1 – 10 .
- Drew , EA . 1995 . Diversity of the green algal genus Halimeda in the Chagos Archipelago, central Indian Ocean . Aquat. Bot , 52 : 143 – 150 .
- Drew , EA and Abel , KM . 1988 . Studies on Halimeda. II: reproduction, particularly the seasonality of gametangia formation, in a number of species from the Great Barrier Reef Province . Coral Reefs , 6 : 207 – 218 .
- Gepp ES 1904 The sporangia of Halimeda. J. Bot., Lond 42 193 197
- Goloboff , PA . 1993 . Estimating character weights during tree search . Cladistics , 9 : 83 – 91 .
- Graham , EA . 1975 . Fruiting in Halimeda (order Siphonales) 1. Halimeda cryptica Colinvaux and Graham . Bull. Mar. Sci , 25 : 130 – 133 .
- Hay , ME , Paul , VJ , Lewis , SM , Gustafson , K , Tucker , J and Trindell , RN . 1988 . Can tropical seaweeds reduce herbivory by growing at night? Diel patterns of growth, nitrogen content, herbivory, and chemical versus morphological defenses . Oecologia , 75 : 233 – 245 .
- Hillis , LW . 1959 . A revision of the genus Halimeda (order Siphonales) . Publ. Institute Marine Science , 6 : 321 – 403 .
- Hillis , LW . 2000 . Phylogeny of Halimeda (Bryopsidales): linking paleontological, morphological and molecular data . Acta Palaeontol. Romaniae, Cluj-Napoca , 2 : 183 – 189 .
- Hillis , LW , Engman , JA and Kooistra , WHCF . 1998 . Morphological and molecular phylogenies of Halimeda (Chlorophyta, Bryopsidales) identify three evolutionary lineages . J. Phycol , 34 : 669 – 681 .
- Hillis-Colinvaux , L . 1980 . Ecology and taxonomy of Halimeda: primary producer of coral reefs . Adv. Mar. Biol , 17 : 1 – 327 .
- Hillis-Colinvaux , L . 1988 . Calcareous green algae in the reefs of the Indian Ocean . Biol. Soc. Wash. Bull , 8 : 14 – 18 .
- Kamura , S . 1966 . On the sexual reproduction of two species of Halimeda (Chlorophyta) . Bull. of Arts Science, Univ. Ryukyus, Math. and Nat. Sci , 9 : 302 – 313 .
- Kooistra , WHCF . 2002 . Molecular phylogenies of Udoteaceae (Bryopsidales, Chlorophyta) reveal non-monophyly for Udotea, Penicillus and Chlorodesmis . Phycologia , 41 : 453 – 462 .
- Kooistra , WHCF , Coppejans , EGG and Payri , C . 2002 . Molecular systematics, historical ecology and phylogeography of Halimeda (Bryopsidales) . Mol. Phylogen. Evol , 24 : 121 – 138 .
- Kraft , GT . 2001 . Marine and estuarine benthic algae (Chlorophyta) of Lord Howe Island, South-western Pacific . Aust. Syst. Bot , 13 : 509 – 648 .
- Lamouroux , JVF . 1812 . Extrait d'un mémoire sur la classification des polypes coralligènes non entièrement pierreux . Nouv. Bull. Sci. Soc. Phil , 3 : 181 – 188 .
- Littler DS Littler MM 2000 Caribbean reef plants: an identification guide to the reef plants of the Caribbean, Bahamas, Florida and Gulf of Mexico Offshore graphics Inc., Washington D.C
- Meinesz , A . 1980 . Connaissances actuelles et contribution à l'étude de la reproduction et du cycle des Udotéacées (Caulerpales, Chlorophytes) . Phycologia , 19 : 110 – 138 .
- Noble , JN . 1986 . Halimeda magnidisca (Caulerpales, Chlorophyta), a new species from the Great Barrier Reef, Australia . Phycologia , 25 : 331 – 339 .
- Pinkel , R . 1983 . Doppler sonar observations of internal waves: wave-field structure . J. Phys. Oceanogr , 13 : 804 – 815 .
- Posada , D and Crandall , KA . 1998 . Modeltest: testing the model of DNA substitution . Bioinformatics , 14 : 817 – 818 .
- Schlagintweit F Ebli O 1998 Halimeda paucimedullaryis n. sp. and Oroseina pletzachensis n. sp., two new calcareous algae from the Upper Cretaceous of the Northern Calcareous Alps (Gosau Group, Austria), followed by remarks on Dissocladella ? pyriformis Schlagintweit, 1991 Revue Paléobiol Genève 17 361 371
- Swofford DL 2002 PAUP* Phylogenetic Analysis Using Parsimony (* and other methods) Version 4.0b10 Sinauer Associates Inc., Sunderland, Massachusetts
- Taylor WR 1950 Plants of Bikini and other northern Marshall Islands Univ. of Michigan Press, Ann Arbor, Michigan
- Tsuda , RT and Kamura , S . 1991 . Floristics and geographic distribution of Halimeda (Chlorophyta) in the Ryukyu Islands . Jpn. J. Phycol , 39 : 57 – 76 .
- Tsuda , RT and Wray , RO . 1977 . Bibliography of marine benthic algae in Micronesia . Micronesica , 13 : 85 – 120 .
- Valet , G . 1966 . Sur une espèce rare et une nouvelle espèce d'Halimeda de Mélanésie . Rev. Gén. Bot , 73 : 680 – 685 .
- Walters , LJ and Smith , CM . 1994 . Rapid rhizoid production in Halimeda discoidea Decaisne (Chlorophyta, Caulerpales) fragments: a mechanism for survival after separation from adult thalli . J. Exp. Mar. Biol. Ecol , 175 : 105 – 120 .
- Walters , LJ , Smith , CM , Coyer , JA , Hunter , CL , Beach , KS and Vroom , PS . 2002 . Asexual propagation in the coral reef macroalga Halimeda (Chlorophyta, Bryopsidales): production, dispersal and attachment of small fragments . J. Exp. Mar. Biol. Ecol , 278 : 47 – 65 .