Abstract
A systematic reassessment of the sole representative of the South African genus Phyllymenia, P. belangeri, indicates a lack of clear-cut diagnostic characters to separate it from Grateloupia. The morphology of the auxiliary cell ampullae, widely regarded as a key generic character in the Halymeniaceae, is almost identical in both genera. The autapomorphic character that has traditionally separated Phyllymenia from Grateloupia is the formation of pronounced lateral cytoplasmic protuberances on inner cortical cells. However, the establishment of protuberances is correlated with the presence of large intercellular spaces between neighbouring cortical cell files, coupled with narrow intercalary cortical cells. Thus, in order to establish secondary pit connections to more distant neighbouring cells, cortical cell protuberances may have become more prominent in P. belangeri than in species of Grateloupia with smaller intercellular spaces. Phylogenetic analyses of chloroplast-encoded rbcL sequences for a dataset including four representatives of P. belangeri and 32 other species, currently placed in Grateloupia or Prionitis, resolve (with high support) P. belangeri as a sister taxon of G. longifolia from South Africa. Based on morphological and molecular evidence, it is therefore proposed that Phyllymenia be reduced to a synonym of Grateloupia.
Introduction
Generic delimitation within the red algal family Halymeniaceae is in a state of flux (Womersley & Lewis, Citation1994). Approximately 21 genera have traditionally been characterized on the basis of various gross morphological characters relating to thallus size, form, texture, and degree of branching (e.g. Kylin, Citation1956; Chiang, Citation1970; Womersley & Lewis, Citation1994). However, recent studies combining chloroplast-encoded rbcL sequence data with traditional morphological observations have resulted in several taxonomic changes. Pachymeniopsis Yamada and Prionitis J. Agardh have been merged into Grateloupia C. Agardh (Kawaguchi, Citation1997; Wang et al., Citation2001), while Polyopes J. Agardh now encompasses Sinkoreana H.B. Lee, J.A. Lewis, Kraft & I.K. Lee and several species previously classified in Carpopeltis Schmitz (Kawaguchi et al., Citation2002). Uncertainty about the taxonomic position of several genera and numerous species still remains. The morphology and systematic position of one of these genera, the monotypic Phyllymenia J. Agardh, is treated in the present study.
Phyllymenia was based on a single species, Phyllymenia hieroglyphica J. Agardh (1848), described from Table Bay near Cape Town, South Africa. Soon after however, J. Agardh (Citation1851) relegated Phyllymenia to a section within the genus Iridaea Bory. Apparently unaware of Phyllymenia, Schmitz (Citation1896) erected the genus Cyrtymenia in which he placed Cyrtymenia hieroglyphica (J. Agardh) Schmitz and Cyrtymenia cornea (Kützing) Schmitz. Phyllymenia was resurrected by Setchell & Gardner (Citation1936), who showed that Iridaea belangeri Bory (Citation1834) was identical to Agardh's P. hieroglyphica. In addition, C. cornea was transferred to Phyllymenia by Setchell & Gardner (Citation1936), but later moved to Pachymenia J. Agardh by Chiang (Citation1970), based on cortical filament, carpogonial branch and auxiliary cell ampullary features. Two other species have been attributed to Phyllymenia, or its synonym Cyrtymenia, namely Cyrtymenia somalensis (Hauck) De Toni based on Grateloupia somalensis Hauck, and Cyrtymenia sparsa Okamura from Japan. Chiang (Citation1970) considered C. sparsa a true Grateloupia and made the appropriate combination, Grateloupia sparsa (Okamura) Chiang, a decision that remains widely accepted to date (Yoshida et al., Citation1995; Yoshida, Citation1998; Kawaguchi et al., Citation2002). The systematic position of C. somalensis remains unclear. The species was never transferred to Phyllymenia and is listed as G. somalensis in the Catalogue of Indian Ocean Algae (Silva et al., Citation1996).
The basis upon which Phyllymenia and Grateloupia are separated has always been vague. When first described (Agardh, Citation1848), Phyllymenia was not compared to any other genus. Agardh (Citation1849) later commented briefly on its similarities with Grateloupia and Iridaea. Apart from stating that generic boundaries within the family are poorly defined, Schmitz (Citation1896) and Schmitz & Hauptfleisch (Citation1897) identified a number of gross morphological characters that would distinguish Phyllymenia from Grateloupia, Aeodes J. Agardh and Pachymenia, including an irregularly foliose and perforated thallus, and sporangia confined to cavities in the corrugated thallus surface. Later studies (Chiang, Citation1970) demonstrated clear differences between Aeodes and Pachymenia on the one hand, and Grateloupia and Phyllymenia on the other hand, primarily based on the degree of branching of the auxiliary cell ampullae. Unlike Aeodes and Pachymenia, which possess Aeodes-type auxiliary cell ampullae, characterized by profusely branched ampullary filaments, Grateloupia and Phyllymenia possess Grateloupia-type auxiliary cell ampullae, said to consist of a primary filament bearing two-to-three simple secondary ampullary filaments (Chiang, Citation1970). This distinction between genera with an Aeodes-type ampulla (Aeodes, Pachymenia, Polyopes, and Dermocorynus P.L. Crouan & H.M. Crouan pro parte) and genera with a Grateloupia-type ampulla (Grateloupia, Phyllymenia, Prionitis and Yonagunia Kawaguichi & Masuda) conforms to current phylogenies based on rbcL sequences (Fredericq et al., Citation1996; Wang et al., Citation2001; Gavio & Fredericq, Citation2002; Kawaguchi et al., Citation2002, Citation2004; Hommersand & Fredericq, Citation2003). However, doubt remains about the taxonomic position of Phyllymenia (Wang et al., Citation2001), a genus distinguished from Grateloupia only by a few characters of overall thallus morphology (Schmitz & Hauptfleisch, Citation1897; Kylin, Citation1956) and the presence of protuberances on the inner cortical cells (Chiang, Citation1970). In this study the status of Phyllymenia is reassessed on the basis of morphological and gene sequence data, using a dataset comprising species currently placed in Grateloupia or Prionitis. Grateloupia somalensis and G. sparsa, both at one time attributed to Phyllymenia, are also included in the analysis.
Materials and methods
Morphological analysis
Morphological observations were made on specimens preserved in a 5% formalin-seawater solution. Whole-mount and sectioned material was stained either with aniline-blue and mounted in Karo® syrup, or stained with Wittmann's aceto-iron-hematoxylin-chloral-hydrate, and mounted in Hoyer's medium as described in Hommersand et al. (Citation1992). Drawings were made with a camera lucida and photographs were taken with an Olympus DP50 digital camera mounted on a Leitz Diaplan compound microscope or Leica Wild M10 stereo microscope. Herbarium abbreviations follow Holmgren et al. (Citation1990).
Molecular analyses
Samples for molecular analyses () were desiccated in the field in silica gel (Chase & Hillis, Citation1991). Total genomic DNA was extracted using either a Chelex® 100 resin-based protocol (Goff & Moon, Citation1993), a CTAB extraction method modified from Doyle & Doyle (Citation1987), or a DNAeasy Plant Minikit (Qiagen, Valencia, CA). The gene selected for amplification was chloroplast-encoded rbcL; primers used for amplification and PCR conditions were derived from Wang et al. (Citation2000) or Gavio & Fredericq (Citation2002). The PCR product was treated with shrimp alkaline phosphatase (1 U/µl, Amersham E70092Y) and exonuclease I (20 U/µl, Epicentre Technologies X40505K) for 15 min at 37°C, followed by 15 min at 80°C to inactivate the enzymes. This material was then used for cycle sequencing without any further purification, using the ABI Prism BigDye V 2.0 Terminator Cycle Sequencing kit. Sequencing was performed using a Perkin Elmer ABI Prism 377 automated DNA sequencer or a ABI 3100 Prism Genetic Analyzer (PE Applied Biosystems, Foster City, CA) following previously described protocols (Gavio & Fredericq, Citation2002, Citation2003; Mateo-Cid et al., Citation2005).
Table 1. List of species used in rbcL analysis with accession numbers
Generated sequences were aligned manually in MacClade 4.0 (Maddison & Maddison, Citation2000) and exported for phylogenetic analysis in PAUP 4.0b10 (Swofford, Citation2002) for Maximum Parsimony (MP) and Maximum Likelihood analyses (ML), and MrBayes 3.0 (Huelsenbeck & Ronquist, Citation2001) for Bayesian Inference (BI). Due to missing data at the 5′ and 3′ end of many rbcL sequences, the first 107 and last 102 sites of the 1,467-bp gene were excluded from the analysis, leaving a truncated dataset of 1,259 bp for the analyses.
In the MP analysis, all characters and character changes were weighted equally. Heuristic searches, consisting of 5,000 replicates of random sequence addition, were performed with TBR and MULTREES activated. MP bootstrap analysis consisted of 1,500 replications of full heuristic searches. Prior to ML analysis a hierarchical likelihood ratio test (hLRT, a = 0.05) was performed in Modeltest 3.06 (Posada & Crandall, Citation1998) to select the substitution model best fitting the data set. The parameters of the selected model were then fixed and used to analyse the data sets under maximum likelihood using a heuristic search with 100 replicates of random sequence additions and TBR. ML bootstrap analyses consisted of 100 replications of 10 random sequence additions, limiting the amount of rearrangements to 10,000 or 1 h.
Nucleotide substitution models for Bayesian Inference were calculated using MrModeltest (Nylander, Citation2002). Posterior probabilities were calculated using a Metropolis-coupled Markov chain Monte Carlo approach with sampling according to the Metropolis–Hastings algorithm. The analysis used four chains, one cold and three incrementally heated. A single run consisted of 1 million generations that were sampled every 100th tree. Likelihood values reached a stable value after 10,000–20,000 generations. To ensure that we only included trees after the chain had reached a stable value, the burn-in was fixed at 100,000 generations, which produced 9000 sampled trees and corresponding posterior probability distributions.
Results
Type collections and nomenclature
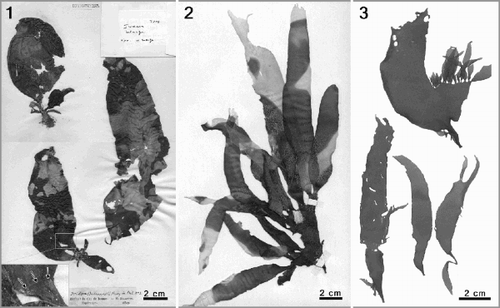
Phyllymenia is typified by P. hieroglyphica J. Agardh (), treated as a synonym of P. belangeri since Setchell & Gardner (Citation1936). The type of P. belangeri (Iridaea belangeri Bory) is housed in the Bornet & Thuret Herbarium (PC TA14583). However, the sheet carries three specimens representing three different species from two red algal families (). The voucher on the top left corresponds to a young specimen of Gigartina polycarpa (Kützing) Setchell & Gardner (Gigartinaceae); the specimen on the bottom left represents an interesting collage; the basal part is Sarcothalia stiriata (Turner) Leister (Gigartinaceae) and the distal blade belongs to P. belangeri ( insert). As the specimen on the right represents a female gametophyte of P. belangeri, it is designated as the lectotype in accordance with ICBN Art. 9.12 (Greuter et al., Citation2000). Although the external morphology of the lectotype is not fully representative of P. belangeri, the corrugated thallus surface and presence of gonimoblasts in diagnostic ampullae provide unequivocal evidence of its identity.
Figs 1–3. Phyllymenia types and external morphology. . Type sheet of Phyllymenia belangeri (Herb. Bornet & Thuret, PC TA14583). Top left– young specimen of Gigartina polycarpa; bottom left–basal part is Sarcothalia stiriata and distal blade represents P. belangeri (insert); right–female gametophyte of P. belangeri. . Lectotype of Phyllymenia hieroglyphica (LD 22831). . Recently collected specimens from the Western Cape Province, South Africa (GENT TS425) to show habit.
Morphology
Habit and vegetative morphology
A crustose holdfast supports a short, subterete stipe bearing one-to-several erect, dark-red to purplish blades, up to 30 cm in length. The foliose thallus ranges from simple, cuneate (, bottom) to lacerate blades bearing numerous proliferations that are restricted to the margins (, top). Young blades are typically elongate and slightly curved to one side, 2–3 cm wide, flexible and with a typical corrugated surface. Older blades, 400–500 µm thick in the median and basal thallus parts, may show a few to several irregular perforations along the ridges (, ), and become somewhat cartilaginous near the base and the margins.
Figs 4–10. Phyllymenia vegetative morphology. . Detail of the characteristic corrugated surface with irregular perforations (arrowheads). . Cross-section showing gradual transition between the cortex and medullary layer. . Longitudinal section through a tetrasporic thallus showing predominantly periclinal arrangement of the medullary filaments. . Cross-section through the basal part of the thallus showing a dense medulla with numerous rhizoidal filaments. , . Detail of cortex with anticlinal rows of dichotomously branched filaments. . Inner cortical cells with lateral protuberances establishing secondary pit connections.
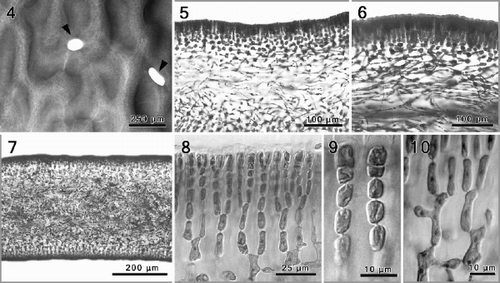
The thallus is multiaxial and consists of a filiform medulla and a cellular cortex. The medulla, comprising about half the thallus thickness (ca. 200–250 µm), consists of narrow filaments of elongate cells that run predominantly parallel to the plane of growth, as seen in longitudinal sections (, ). The medullary filaments are therefore laxly interwoven in a predominantly periclinal arrangement and diverge from the points of branching at narrowly oblique angles (). The terminal portions of contiguous medullary cells are typically bulbous (). Shorter and narrower secondary medullary filaments, each up to three segments long, are cut off from primary medullary cells and grow in all directions, with the terminal cells establishing secondary pit connections to medullary and inner cortical cells a short distance away. While relatively few secondary filaments are produced in the upper thallus parts, many short secondary medullary filaments are cut off in the lowermost blade portions and the stipe, filling the intercellular spaces. The result is a dense network of rhizoidal filaments that become thick-walled ().
The inner cortex, the transition zone between the filiform medulla and the regularly shaped and spaced cells of the cortex, comprises a mixture of irregularly shaped cells. Inner cortical cells are rounded to stellate, depending on the number of pit connections, coupled with the degree of cell stretching or expansion to accommodate thallus growth. The cortex consists of straight rows of unbranched and sparingly dichotomously branched cell files, each 7–10 cells long (, ). Secondary pit connections linking cells of neighbouring cell files are absent in the outermost 5 cell layers (). Cortical cells are always elongate-cylindrical (, ). Pronounced lateral cytoplasmic protuberances are initiated from lower intercalary cortical cells. Cortical cells that are not linked by a secondary pit connection typically remain uninucleate (, ), whereas they become multinucleate () upon fusion with a conjunctor cell that was itself cut off from a contiguous cell's protrusion. The number of nuclei per cell is proportional to the number of secondary pit connections. The intercellular spaces between neighbouring cortical cell files are large (, ).
Reproductive morphology
Reproductive structures are scattered over the fronds, except in the basal parts. Gametophytes are presumably dioecious. Carpogonial branches and auxiliary cells are located in separate, rather straight, simple ampullae, situated in the cortex–medulla transition zone. Carpogonial branch ampullae consist of a primary ampullary filament, most commonly 7–10 cells long, and two to three secondary, unbranched filaments (). Typically the first and at least one cell adjacent to the supporting cell bear secondary filaments, comprising 7–12 ovoid to cylindrical cells. The carpogonial branch is two-celled and the hypogynous cell bears a short lateral filament, 3–4 cells long. The carpogonium itself is difficult to observe (perhaps senescent in most cases). Auxiliary cell ampullae () are similar in appearance to the carpogonial ampullae. Auxiliary cells are situated between two or three straight cell files, the ampullary filaments, and are easily distinguished from the surrounding ampullary cells by their larger size (10–15 µm long) and oval shape. Each auxiliary cell produces a single distal gonimoblast initial, which divides distally to form several gonimolobes, all of which continue to divide forming a compact, globose cluster of cells (, ).
Although the early post-fertilization stages, including the formation of connecting filaments, have not been observed, the terminal portions of incoming connecting filaments were seen fused at the base of auxiliary cells, and the outgoing connecting filaments were observed as well on several occasions (). Gonimoblast development is concomitant with the progressive cutting off of small laterals by the intercalary ampullary cells, resulting in regularly branched candelabra-like ampullae that weakly envelop the developing carposporophyte (, ). Pit connections between the auxiliary cell and contiguous ampullary cells, and among the suprabasal ampullary cells themselves, become dislodged and progressively break down, resulting in open cytoplasmic channels among these cells (, ). The result is an irregular fusion cell product that follows the contour of the incorporated ampullary cells. At no point do fusions become established between gonimoblasts and vegetative cells. Ampullary cells initially expand in size and stain darkly, but subsequently lose their cytoplasm and flatten, forming a thin involucre of ampullary filaments that converges towards the surface in a distinct ostiole above the carposporophyte. Mature carposporophytes are pear-shaped to spherical, 400–750 µm wide (). At maturity, all gonimoblast cells become transformed into isodiametric to angular carposporangia, 12–18 µm across.
Figs 11–16. Phyllymenia reproductive morphology. . A maturing cystocarp surrounded by involucral filaments, deeply embedded in the inner cortical layers. . Squashed carpogonial ampulla, with an 8-celled primary filament from which secondary filaments have developed from the first and fifth cell (black arrows), the hypogenous cell (hy) bears a 4-celled filament (white arrow). . A narrowly flask-shaped auxiliary cell ampulla with three, simple secondary filaments. , . Successive stages of gonimoblast development, and formation of involucral filaments; note connecting filaments (cf) attached to the auxiliary cell (ac). . Detail of cruciately divided tetrasporangium and tetrasporangial initials (arrowhead) within modified outer cortical cells.
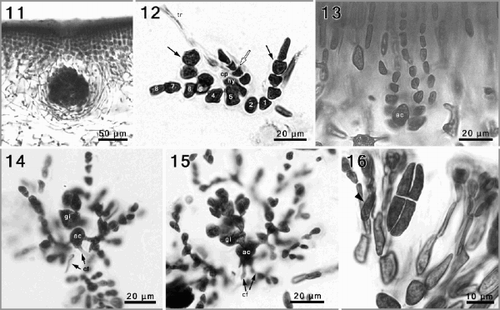
Tetrasporangial initials are cut off laterally from intercalary cortical cells located 4–5 cell layers below the surface (, arrow head). Mature tetrasporangia are cruciately divided and narrowly cylindrical (8–14 × 35–60 µm). Cells of cortical filaments that cut off the tetrasporangial initials and the cell immediately distal to them are typically more elongate than the same cells in ordinary vegetative filaments (). Spermatangia were not observed.
rbcL sequence analysis
No insertions or deletions were found in the rbcL sequences, permitting unambiguous alignment of all sequences. Four P. belangeri sequences were compared to those of 32 species currently placed in Grateloupia or Prionitis. Halymenia durvillei Bory, Halymenia floresia (Clemente) C. Agardh and Cryptonemia luxurians (C. Agardh) J. Agardh were used as outgroup taxa. Of 1,259 bp included in the dataset, 852 are constant and 407 vary, of which 304 are parsimony informative. Excluding outgroup taxa, interspecific pairwise distances (uncorrected p-distance) varied by 2.4–12.7%. In cases where more than one sample of the same species was sequenced from a single geographic region, intraspecific sequence divergence was typically very low. The sequences of P. belangeri (4 samples) and Grateloupia longifolia Kylin (3 samples), collected at two and three localities, respectively, along the west coast of South Africa had identical sequences. The two sequences of Grateloupia filicina (Lamouroux) C. Agardh from Italy diverged by only 2 bp (0.2%). In those instances where sequences were compared from geographically isolated populations of the same ‘species', i.e., G. dichotoma from the NE Atlantic coast of Spain and Brazil, and G. filicina from the Mediterranean Sea and the Indian Ocean, the taxa were only distantly related. There was a 104 bp (7.7%) sequence divergence for the G. dichotoma isolates and 76 bp (5.7%) sequence divergence for G. filicina.
The MP analysis () resulted in two equally most parsimonious trees of 1,163 steps (tree length), which differed only in the position of some clades with little or no bootstrap support, i.e. the position of G. somalensis and G. versicolor J. Agardh. In one of the trees the position of these two species was unresolved, forming a polytomy with the P. belangeri–Grateloupia phuquocensis Tanaka & Pham-Hoàng clade. For the ML analysis a General Time Reversible model with Invariable sites and Gamma distribution (GTR + I + G) was selected with the following parameters: assumed nucleotide frequencies A=0.3090, C=0.1434, G=0.2187, T=0.3289; substitution rate matrix with A–C substitutions = 1.0000; A–G = 4.7926, A–T = 0.7050, C–G = 0.70500, C–T = 11.6427, G–T = 1.0000; proportion of sites presumed to be invariable = 0.5735; rates for variable sites assumed to follow a gamma distribution with shape parameter = 1.1869; number of rate categories = 4. A heuristic search with these settings produced a single tree (−ln L = 4853.64116) which differed only in the position of some clades with little or no bootstrap support from the MP trees: the position of the G. somalensis–G. versicolor clade, the relative positions of Grateloupia patens (Okamura) Kawaguchi and Grateloupia schmitziana Okamura. The overall majority rule consensus tree of the Bayesian Inference is fully resolved, except for the position of Grateloupia acuminata Holmes, Grateloupia asiatica Kawaguchi & Wang, G. schmitziana and the Grateloupia lanceolata (Okamura) Kawaguchi -Prionitis lyalii Harvey clade, which was also poorly resolved in MP and ML analyses.
Fig. 17. Phylogram of one out of two most parsimonious trees (Length = 1163) inferred from rbcL sequence data. Bootstrap percentages of MP (top) and ML (middle) as well as posterior probabilities of BI (bottom) are indicated for each node if higher than 50%.
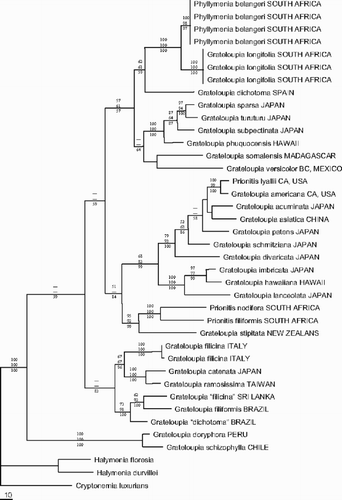
Although most of the terminal and subterminal clades are supported by high bootstrap values or posterior probabilities in all analyses, the backbone of the Grateloupia phylogeny received only little to moderate support. MP, ML and BI analyses are unequivocal about the taxonomic position of P. belangeri. It was resolved as a sister species to G. longifolia with 100% bootstrap and posterior probability support.
Discussion
The generic classification of the Halymeniaceae is primarily based on a combination of characteristics of the auxiliary cell ampullae and external morphology. Chiang (Citation1970) discerned five different types of auxiliary cell ampullae based on the degree of branching of the primary, secondary and, if present, tertiary or higher order filaments. The simplest type, the Grateloupia type, in which the primary filaments branch, producing two to three unbranched secondary ampullary filaments, was reported for Dermocorynus (pro parte, see Guiry & Maggs, Citation1982), Grateloupia (except Grateloupia intestinalis (Hooker & Harvey) Setchell ex Parkinson, see Kraft, Citation1977), Pachymeniopsis, Phyllymenia and Prionitis. Comparative sequence analysis of rbcL strongly supports the overall morphology of the auxiliary cell ampullae as a stable character at the generic level; consequently, both Pachymeniopsis and Prionitis have been merged into Grateloupia (Kawaguchi, Citation1997; Wang et al., Citation2001).
Previously accepted diagnostic characters at the generic level, such as general thallus outline, texture and thickness of the cortical layer, do not reflect the phylogeny of the Halymeniaceae with Grateloupia-type auxiliary cell ampullae (Wang et al., Citation2001; this study). As for Phyllymenia, the main diagnostic characters included a corrugated surface, sometimes with irregular perforations, and inner cortical cells forming lateral protuberances. Surface morphology and thallus texture are of little taxonomic value in Grateloupia. Merging Prionitis into Grateloupia, Wang et al. (Citation2001) discussed the value of thallus texture, arguing that the traditional distinction between lubricous Grateloupia and cartilaginous Prionitis is contradicted by several species. Chiang (Citation1970) stated that protuberances on the middle and inner cortical cells are not encountered in Grateloupia. However, the establishment of such protuberances is correlated with the presence of large intercellular spaces between neighbouring cortical cell files, coupled with intercalary cortical cells that remain elongate. In order to establish secondary pit connections to distantly removed neighbouring cells, cortical cell protuberances may have evolved to become more prominent in P. belangeri than in species of Grateloupia with narrower intercellular spaces. Furthermore, P. belangeri typically grows in the subtidal zone of coasts renowned for considerable swells and pounding waves (Branch et al., Citation1994), and the presence of large protuberances may be linked to improved cohesiveness of algae in wave-swept habitats (see Pueschel, Citation1988). Grateloupia longifolia (presumably the closest relative of P. belangeri) grows in lower energy environments and lacks similar protuberances on the inner cortical cells (Stegenga et al., Citation1997, as G. doryphora; pers. obs.), although it has numerous secondary pit connections.
A character that has not received much attention is the morphology of the cortical filaments associated with tetrasporangium formation, which may differ markedly within a genus. In P. belangeri, Grateloupia asiatica Kawaguchi & Wang and Grateloupia subpectinata Holmes (Kawaguchi et al., Citation2001; Faye et al., Citation2004; this study) cells within cortical filaments associated with tetrasporangia become markedly elongate and resemble the nemathecia of Polyopes (Kawaguchi et al., Citation2003). Cortical filaments remain undifferentiated in both the generitype, G. filicina, and in Grateloupia turuturu Yamada. This character may require further attention.
On the basis of both molecular and morphological characters we conclude that Phyllymenia does not merit generic status and that the monotypic genus should be treated as a synonym of Grateloupia. Therefore, the following new combination is proposed:
Grateloupia belangeri (Bory) comb. nov.
BASIONYM: Iridaea belangeri Bory in Bélanger, Voyage aux Indes-Orientales: 160, pl. 15, , 1834.
HOMOTYPIC SYNONYM: Phyllymenia belangeri (Bory) Setchell & Gardner, Citation1936: 473.
HETEROTYPIC SYNONYMS: Phyllymenia hieroglyphica J. Agardh, 1848: 47; Grateloupia hieroglyphica (J. Agardh) J. Agardh, 1851: 183; Cyrtymenia hieroglyphica (J. Agardh) Schmitz in Schmitz & Hauptfleisch, Citation1897: 511.
The molecular analysis () is also unambiguous about the status of G. somalensis, which Chiang (Citation1970) considered of uncertain status. De Toni (Citation1905) transferred it to Cyrtymenia (a heterotypic synonym of Phyllymenia), but neither Setchell & Gardner (Citation1936) nor Kylin (Citation1956) placed it in Phyllymenia. Based on a specimen collected in Madagascar, we conclude that G. somalensis belongs in Grateloupia. Although its relationship to other species in the genus is not fully resolved (sister to G. versicolor but lacking support), G. somalensis undoubtedly belongs in Grateloupia. As suggested by Wang et al. (Citation2001) careful morphological investigation of the female reproductive structures is necessary before taxa are transferred to different genera. Therefore, pending critical study of vegetative and reproductive development, the Prionitis species included in this study have not been transferred to Grateloupia.
In conclusion, the ongoing merger of several genera into Grateloupia (Prionitis pro parte, Pachymeniopsis, Phyllymenia) is allowing a reassessment of generic concepts in the Halymeniaceae, and of the evolutionary and biogeographic patterns that may have contributed to their diversity and worldwide distribution.
Acknowledgements
Funding for this project was provided by the International Scientific and Technological Cooperation (BIL98/84) between Ghent University and the University of Cape Town and FWO Research Project (3G002496), and by NSF grant DEB-315995 to SF. ODC is indebted to the Fund for Scientific Research-Flanders (FWO-Flanders) for a Postdoctoral Researcher grant, and to the University of Cape Town for the Smuts Memorial Postdoctoral Fellowship in 2000. We offer our appreciation to Andy Vierstraete for running the ML bootstrap analysis on his Linux server. Collectors who sent vouchers and silica dried samples are gratefully acknowledged: R.J. Anderson, I. Barbara, M. Cormaci, J.M. Huisman, M.H. Hommersand, and C. Rodriguez-Prieto. The authors also wish to express their gratitude towards Bruno de Reviers of the Museum National d' Histoire Naturelle in Paris for his help in retrieving the type of Phyllymenia belangeri.
References
References
- Agardh , JG . 1848 . Om de Kapska artena af slägtet Iridaea . K. Svenska Vet. Akad. Förh. , 5 : 46 – 49 .
- Agardh , JG . 1849 . Öfver de Capska arterna af slägtet Iridaea . K. Svenska Vet. Akad. Handl. , 1847 : 81 – 97 .
- Agardh JG 1851 Species Genera et Ordines Algarum 2 C.W.K Gleerup Lund
- Bory De Saint-Vincent JBGM 1834 Hydrophytes, Hydrophytae In Voyage aux Indes-Orientales pendant les années 1825, 1826, 1827, 1828 et 1829 Bélanger, C. editor 159 178 pls. XV, XVI. Botanique Cryptogamie Paris
- Branch GM Griffiths C L Branch ML Beckley LE 1994 Two Oceans, a Guide to the Marine Life of Southern Africa, David Philip Publishers Cape Town
- Chase , MW and Hillis , HH . 1991 . Silica-gel – an ideal material for field preservation of leaf samples for DNA studies . Taxon , 40 : 215 – 220 .
- Chiang , YM . 1970 . Morphological studies of red algae of the family Cryptonemiaceae . Univ. Calif. Publ. Bot. , 58 : 1 – 95 .
- De Toni GB 1905 Sylloge Algarum. Vol. IV. Florideae. Sectio IV Patavii [Padova]
- Doyle , JJ and Doyle , JL . 1987 . A rapid DNA isolation procedure for small quantities of fresh leaf tissue . Phytochem. Bull. , 19 : 11 – 15 .
- Faye , EJ , Wang , HW , Kawaguchi , S , Shimada , S and Masuda , M . 2004 . Reinstatement of Grateloupia subpectinata (Rhodophyta, Halymeniaceae) based on morphology and rbcL sequences . Phycol. Res. , 52 : 59 – 69 .
- Fredericq S Hommersand MH Freshwater DW 1996 The molecular systematics of some agar- and carrageenan-containing marine red algae based on rbcL sequence analysis Proceedings XVth Int. Seaweed Symp Hydrobiologia 326/327 125 235
- Gavio , B and Fredericq , S . 2002 . Grateloupia turuturu (Halymeniaceae, Rhodophyta) is the correct name of the non-native species in the Atlantic known as Grateloupia doryphora . Eur. J. Phycol. , 37 : 349 – 359 .
- Gavio , B and Fredericq , S . 2003 . Botryocladia caraibica (Rhodymeniaceae, Rhodymeniales), a new species from the Caribbean . Cryptogamie Algologie , 24 : 93 – 106 .
- Goff , LJ and Moon , DA . 1993 . PCR Amplification of nuclear and plastid genes from algal herbarium specimens and algal spores . J. Phycol. , 29 : 381 – 384 .
- Greuter W McNeill J Barrie FR Burdet HM Demoulin V Filgueiras TS Nicolson DH Silva PC Skog JE Trehane P Turland NJ Hawksworth DL 2000 International Code of Botanical Nomenclature (St Louis Code) Koeltz Scientific Books, Königstein [Regnum Vegetabile 138.]
- Guiry , MD and Maggs , CA . 1982 . The morphology and life history of Dermocorynus montagnei Crouan frat (Halymeniaceae; Rhodophyta) from Ireland . Br. Phycol. J. , 17 : 215 – 228 .
- Holmgren PK Holmgren NH Barnett LC editors 1990 Index Herbariorum Part I. The Herbaria of the World 8th ed. New York Botanical Gardens New York
- Hommersand MH Fredericq S 2003 Biogeography of the red seaweeds of the South African west coast: a molecular approach Proc. XVIIth Int. Seaweed Symp. 325 336 Oxford University Press Oxford
- Hommersand , MH , Fredericq , S and Cabioch , J . 1992 . Developmental morphology of Gigartina pistillata (Gigartinaceae, Rhodophyta) . Phycologia , 31 : 300 – 325 .
- Huelsenbeck , JP and Ronquist , FR . 2001 . MR BAYES: Bayesian inference of phylogeny . Bioinformatics , 17 : 754 – 755 .
- Kawaguchi , S . 1997 . Taxonomic notes on the Halymeniaceae (Gigartinales, Rhodophyta) from Japan III. Synonymization of Pachymeniopsis Yamada in Kawabata with Grateloupia C. Agardh. . Phycol. Res. , 45 : 9 – 21 .
- Kawaguchi , S , Wang , HW , Horiguchi , T , Sartoni , G and Masuda , M . 2001 . A comparative study of the red alga Grateloupia filicina (Halymeniaceae) from the northwestern Pacific and Mediterranean with the description of Grateloupia asiatica sp. nov. . J. Phycol. , 37 : 433 – 442 .
- Kawaguchi , S , Wang , HW , Horiguchi , T , Lewis , JA and Masuda , M . 2002 . Rejection and transfer of some species of Carpopeltis and Sinkoreana to Polyopes (Rhodophyta, Halymeniaceae) . Phycologia , 41 : 619 – 635 .
- Kawaguchi , S , Shimada , S , Wang , HW , Faye , ET and Masuda , M . 2003 . Polyopes tosaensis Kawaguchi & Masuda, sp. nov. (Halymeniaceae, Rhodophyta) from Japan . Eur. J. Phycol. , 38 : 315 – 324 .
- Kawaguchi , S , Shimada , S , Wang , HW and Masuda , M . 2004 . The new genus Yonagunia Kawaguchi et Masuda (Halymeniaceae, Rhodophyta), based on Y. tenuifolia Kawaguchi et Masuda sp. nov. from southern Japan and including Y. formosona (Okamura) Kawaguchi et Masuda comb. nov. from Southeast Asia . J. Phycol. , 40 : 180 – 192 .
- Kraft , GT . 1977 . The morphology of Grateloupia intestinalis from New Zealand, with some thoughts on generic criteria within the Cryptonemiaceae (Rhodophyta) . Phycologia , 16 : 43 – 51 .
- Kylin H 1956 Die Gattungen der Rhodophyceen, C.W.K Gleerups Lund Sweden
- Maddison DR Maddison WP 2000 MacClade 4: Analysis of Phylogeny and Character Evolution, version 4.0, Sinauer Associates Sunderland MA
- Mateo-Cid , LE , Mendoza-Gonzales , AC , Gavio , B and Fredericq , S . 2005 . Grateloupia huertana sp. nov. (Halymeniaceae, Rhodophyta): a peculiar new prostrate species from tropical Pacific Mexico . Phycologia , 44 : 4 – 16 .
- Nylander JAA 2002 MrModeltest v1.0b. Program distributed by the author. Department of Systematic Zoology, Uppsala University
- Posada , D and Crandall , KA . 1998 . MODELTEST: testing the model of DNA substitution . Bioinformatics , 14 : 817 – 818 .
- Pueschel , CM . 1988 . Secondary pit connections in Hildenbrandia (Rhodophyta, Hildenbrandiales) . Br. Phycol. J. , 23 : 25 – 32 .
- Schmitz , F . 1896 . Kleinere Beiträge zur Kenntniss der Florideen VI . Nuova Notarisia , 7 : 1 – 22 .
- Schmitz F Hauptfleisch P 1897 Grateloupiaceae Die Natürlichen Pflanzenfamilien Engler, A. & Prantl K., editors 508 514 Engelmann Leipzig Germany
- Setchell , WA and Gardner , NL . 1936 . Iridiophycus gen. nov. and its representation in South America . Proc. Natl. Acad. Sci. , 22 : 469 – 473 .
- Silva , PC , Basson , PW and Moe , RL . 1996 . Catalogue of the benthic marine algae of the Indian Ocean . Univ. Calif. Publ. Bot. , 79 : 1 – 1256 .
- Stegenga , H , Bolton , JJ and Anderson , RJ . 1997 . Seaweeds of the South African west coast . Contributions from the Bolus Herbarium , 18 : 1 – 655 .
- Swofford DL 2002 PAUP*: Phylogenetic Analysis Using Parsimony (and other methods), Sinauer Associates Sunderland MA
- Wang , HW , Kawaguchi , S , Horiguchi , T and Masuda , M . 2000 . Reinstatement of Grateloupia catenata (Rhodophyta, Halymeniaceae) on the basis of morphology and rbcL sequences . Phycologia , 39 : 228 – 237 .
- Wang , HW , Kawaguchi , S , Horiguchi , T and Masuda , M . 2001 . A morphological and molecular assessment of the genus Prionitis J. Agardh (Halymeniaceae, Rhodophyta) . Phycol. Res. , 49 : 251 – 261 .
- Womersley HBS Lewis JA 1994 Family Halymeniaceae Bory 1828: 158 The Marine Benthic Flora of Southern Australia: Rhodophyta. Part IIIA Womersley, H.B.S. editor 167 218 Australian Biological Research Study Canberra
- Yoshida T 1998 Marine Algae of Japan, Uchida Rokahuba Publishing Tokyo Japan
- Yoshida , T , Yoshinaga , K and Nakajima , Y . 1995 . Check list of marine algae of Japan . Jpn. J. Phycol. , 43 : 115 – 171 .