Abstract
This study clarifies the fine structure of the vegetative and initial valves of Achnanthes yaquinensis and briefly compares them to other Achnanthes species. It also elucidates the structure of the perizonium, based on auxospore development in short-term cultures. The araphid valve has marginal ridges and terminal spines that allow connecting valves to form a chain. The terminal spines develop from the rapheless sternum. The complete cingulum consists of 3–5 split bands with two rows of areolae. These features can be used to discriminate species within the genus. Sexual reproduction is isogamous with two mother cells producing two auxospores, which are enclosed in mucilage. The perizonium develops on one side of an auxospore only, comprising one large central longitudinal band and four closed bands. There are no transverse perizonial bands. The raphid valve of the initial cell forms first, underneath the longitudinal perizonium, followed by the araphid valve, which is not covered by any perizonial bands. The araphid valve of the initial cells lacks a marginal spine, and the rapheless sternum lies more centrally than in the vegetative cell. The relationship of the genus Achnanthes to other monoraphid diatoms is discussed briefly.
Introduction
The genus Achnanthes was established by Bory (1822) to include monoraphid, heterovalvar, epiphytic diatoms that are flexed in girdle view. More than 500 species of Achnanthes sensu lato have been recorded (Van Landingham, Citation1967), occurring in marine, fresh and brackish waters. Recently, the genus has received considerable attention and has been subdivided into several genera, including Achnanthidium Kützing (re-established by Round et al., Citation1990), Psammothidium Bukhtiyarova & Round (Bukhtiyarova & Round, Citation1996), Karayevia Round & Bukhtiyarova, Kolbesia Round & Bukhtiyarova, Rossithidium Round & Bukhtiyarova (Round & Bukhtiyarova, Citation1996), and Pogoneis Round & Basson (Round & Basson, Citation1997), all of which occur in freshwater environments. However, most members of Achnanthes sensu stricto occur in marine and brackish waters and can be distinguished from freshwater Achnanthes by conspicuous cribrate areolae in their valves and girdle bands.
Achnanthes yaquinensis was described by McIntire & Reimer (Citation1974) from marine waters. These authors (McIntire & Reimer, Citation1974) documented some of the morphology of this taxon using light (LM) and scanning electron microscopy (SEM). However, they did not present detailed observations of the fine structure of the species. As part of a larger study of the genus Achnanthes (Toyoda et al., Citation2003, Citation2005 a, Citation b ; Toyoda & Williams, Citation2004), we used different microscopical techniques to clarify the morphology of this taxon, and to establish its relationships to other species in the genus.
Auxosporulation and sexual reproduction have been observed in Achnanthes brevipes var. intermedia (Kützing) Cleve, Achnanthes longipes Agardh, Achnanthes subconstricta (Meister) Toyoda and Achnanthes subssesilis Kützing (Karsten, Citation1899; Idei & Chihara, Citation1991; Mizuno, Citation1994; Chepurnov & Roschin, Citation1995; Chepurnov & Mann, Citation1999; Sabbe et al., Citation2004). These reports primarily describe sexual reproductive behaviour, rather than the fine structure of the auxospores and initial cells. Species of Achnanthes are isogamous, two zygotes (auxospores) being formed from four monoecious gametes, produced by two mother cells. Karsten (Citation1899) illustrated auxospores of A. longipes and A. brevipes, and provided an illustration of the perizonium, while von Stosch (Citation1982) noted that A. longipes produced only longitudinal perizonial bands, with no transverse perizonial bands. Idei (Citation1993) published LM illustrations of sexual reproduction in A. brevipes var. intermedia in which a particular configuration of the mother cells is required to produce gametes. The valve of one cell must touch the girdle of another cell to induce the two gametes to emerge from each cell, pairing occurring outside the cells. Two auxospores are formed, followed by the perizonium and initial valve. However, the fine structure of the perizonium of Achnanthes has not been illustrated using EM. This study presents new information on the morphology of the vegetative valves, initial cells and perizonium of A. yaquinensis from Japan using SEM, TEM and a Focused Ion Beam (FIB) system.
Materials and methods
Achnanthes yaquinensis was obtained from three localities on the coast of Japan (). Material is housed in the Phycological Laboratory, Tokyo University of Marine Science & Technology.
Table 1. Information on the collection sites and habitat of material of Achnanthes yaquinensis examined for this study
Living cells were first observed using LM and Confocal Laser Scanning Microscopy (CLSM: OLYMPUS-FLUOVIEW BX50) to determine plastid morphology using the autofluorescence of plastids under ultraviolet light. Fresh material was then prepared for LM examination using the bleaching method to prevent the frustules from breaking apart (Nagumo & Kobayasi, Citation1990). Because Achnanthes is heterovalvar, to avoid the possibility of mis-identification based on single valves, the raphid (RV) and araphid (ARV) valves of the same frustule were examined using a ‘double-faced slide’, made by mounting samples in between two coverslips in ‘MGK-S’ (Matsunami Glass Industry, Ltd., Osaka, Japan). Initial cells of A. yaquinensis were obtained after short term culture (ca. 4 days) at 25°C, 14 h light: 10 h dark, 33.5 psu salinity in modified 1/2 PES (Provasoli, Citation1968), after stressing the cultures by subjecting them to 2 days in darkness.
For EM, a drop of sodium hypochlorite solution with 5–10% chlorine was added to wet samples on a slide, and several individuals, suitable for observation by SEM and TEM, were selected using a capillary pipette under LM. After selection, a drop of distilled water was placed on another glass slide, and the diatoms were transferred to this slide to remove the hypochlorite solution. Each specimen was finally transferred to a coverslip for EM. An SEM (HITACHI-S4000), TEM (HITACHI-S9000) and a FIB system (HITACHI-3000FB) were used. The latter allowed cross sections of initial cells to be obtained by slicing the cell with an ion beam (Suzuki et al., Citation2001 a, Citation b ). With FIB, any section of a cell can be easily observed and more structural information can be gained.
Valve terminology follows Anonymous (Citation1975), Ross et al. (Citation1979) and Cox & Ross (Citation1981), with additional terms from Toyoda et al. (Citation2003).
Observations
Structure of vegetative cells
Individual cells are panduriform in valve view, flexed in girdle view, with a convex ARV and concave RV, the latter closer to the substratum. Each cell contains two large chloroplasts, separated by the median transapical plane, each plastid with a central pyrenoid (–). Cells usually form chain-like colonies () attached to a substratum, often delicate filamentous seaweeds or similar material. The chain of cells is attached by a mucilage stalk, which is excreted from the terminal raphe fissure on the lowermost RV.
Figs 1–3. LMs of living cells showing chloroplasts. . Girdle view of single cell showing two large plastids, one on either side of the median transapical plane, each with a rounded pyrenoid. . CLSM of individual in showing autofluorescence of the two plastids. . Colony of nine cells. Colonies are usually epiphytic on filamentous algae and attached by a stalk secreted by the lowermost cell.
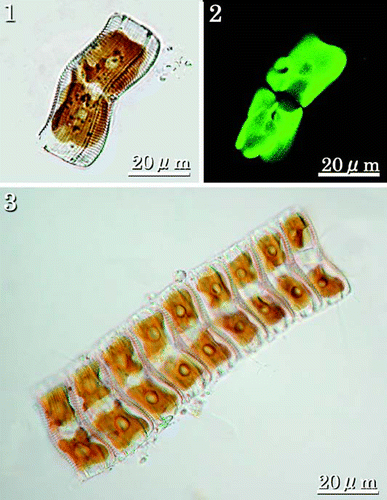
Valves are 34–104 µm in length, gently constricted at the centre, 10–21 µm maximum width (, ). They have single rows of areolae between each pair of transverse virgae. There are 8–9 transapical virgae (often thickened and referred to as costae) in 10 µm on the RV and 8–8.5 on the ARV. In LM, the central area of the RV is expanded into a narrow, linear, thickened stauros, reaching the valve margins (–). The raphe is filiform and extends the length of the valve. The polar raphe fissure deflection is not evident in LM. There are terminal orbiculi at both ends of the ARV (, ).
Figs 4–7. LMs of cleaned vegetative frustules showing valve and girdle morphology. –. RV (a) and ARV (b) of the same individuals. shows a small cell at the lower end of the size range. shows a large cell near the upper end of the size range. Figs 6–7. Girdle views showing the convex ARV, and the concave RV. Scale bar applies to all figures.
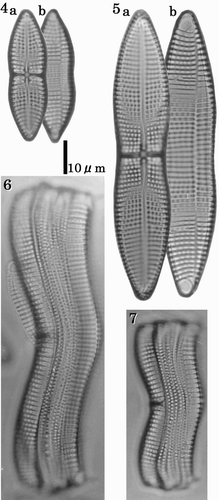
In SEM, the valve surfaces are gently rounded (). Each raphe slit has a distinct central pore, which is slightly deflected to one side of the valve, while the terminal fissures are deflected in the opposite direction to the central fissures (, ). Internal views of the RV show that the stauros and costae extend from the raphe sternum and protrude internally (–). The raphe fissure is almost straight but slightly expanded at the centre; the terminal fissures turn slightly in the opposite direction and end in small helictoglossae (, ). The ARV has two terminal spines and marginal ridges (). The latter have smooth edges (), while the terminal spines extend from the rapheless sternum. The terminal orbiculi are occluded by large cribrate plates (). In internal view, the transapical costae of the ARV are thicker than the sternum (). Areolae are occluded by complex cribra, often supported by five pegs and slightly below the external valve surface (, ). The terminal orbiculi are raised internally (). Each girdle band is open at one end, and has areolae occluded by cribra. The valvocopula has two rows of areolae, one rounded, and the other rectangular (–).
Figs 8–21. SEMs of vegetative cells. . External view of the RV showing filiform raphe fissure. . External view of RV centre showing the transverse stauros. . Terminal raphe fissure, curved slightly in the opposite direction to the central pores. . Internal view of the RV with valvocopula; costae are raised above the inner surface of the valve. . Internal view of cribra. . Internal view of centre of valve showing the thickened stauros. . Internal view of the terminal raphe fissure, which curves in the opposite direction to the external terminal raphe fissure. . External view of the ARV with marginal ridges and terminal spines. . Detail showing smooth edge of a marginal ridge. . Terminal spine and terminal orbiculus. Terminal spine emerges from the rapheless sternum. . Internal view of the ARV. . External view of cribra. . Internal view of cribra. Note that cribra are usually supported at 4 or 5 points. . Internal view of terminal orbiculus showing the occluding cribrum.
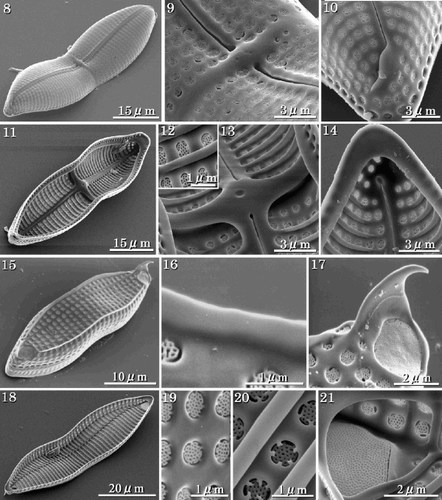
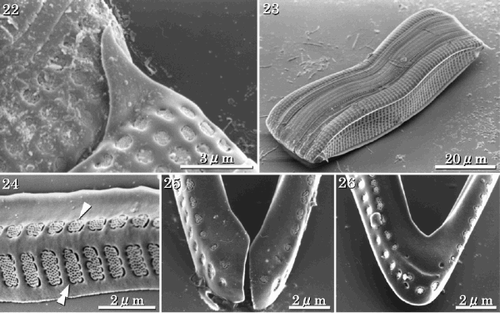
Figs 22–26. SEMs of vegetative frustules. . Enlarged view of the connection between adjacent individuals in apical terminal view. The terminal spine is used in aligning the colony. . Girdle view. . Valvocopula of the RV with two rows of areolae, one with roundish quadrangular areolae (arrowhead), the other with rectangular areolae (double-arrowhead). . Open ends of a girdle band. . Opposite closed end of the band shown in .
Auxospore and initial cell structure and formation
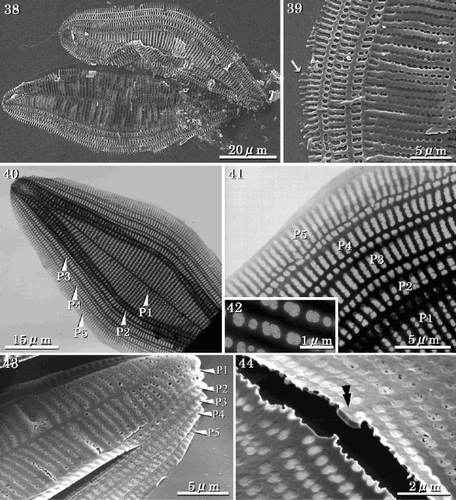
Achnanthes yaquinensis is isogamous. Two gametes from each of two mother cells fuse to produce two auxospores (, , , ). Usually, the auxospores expand within the RV adjacent to one mother cell to become about three times the size of the mother cell (–). Initially the auxospore and initial cell surfaces are coated with large quantities of mucilage (). A perizonium is formed on the concave side only of each auxospore (, ). An initial ARV is then formed on the outer side (), followed by the RV under the perizonium, and finally the girdle bands. Thus, the RV of the initial cell is covered with longitudinal perizonial bands (, , ), which are similar in shape to the vegetative valve. However, the ARV of the initial cell often differs in shape from the usual vegetative valves. The valve face is more rounded and the sternum develops along the central axis of the valve (, ). The areolae are similar in shape to those of the ARV of vegetative valves (). In the initial valve, the terminal ridge is sometimes absent () and marginal ridges are lacking (). Normal frustules are produced after two cell divisions. The two post-initial cells have either the epivalve or the hypovalve of the initial cell and a complementary vegetative valve.
Figs 27–29. LMs of auxospores and initial cells. Arrowheads indicate mother frustules. . Auxospores attached to one of the mother frustules. . Perizonia of pre-initial cells that have not formed RVs, ARVs or girdles. . Initial cells. Note that these become about three times the size of the mother cell.

Figs 30–37. SEMs of pre-initial and initial cells. . Pre-initial cells. . Immediately after formation, initial valve enveloped by large amounts of mucilage. . RV of the initial cell showing the RV covered with a longitudinal perizonium. . Internal view of the longitudinal perizonium. . External view of ARV of an initial cell with a rounded valve face, orbiculi and terminal spines. . Portion of initial ARV showing rapheless sternum and cribra. . Terminal orbiculus. Orbiculi of initial cells are similar to those in the vegetative valves, however they often lack terminal spines. . Internal view of ARV of the initial cell. The rapheless sternum lies along the centre of the valve.
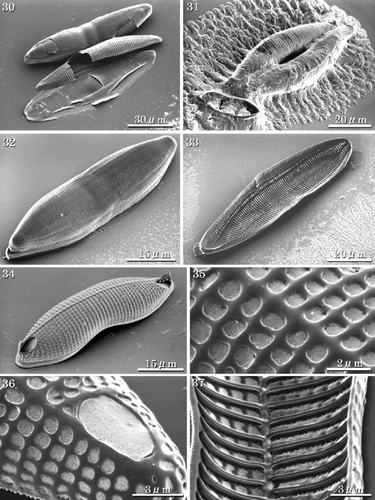
Figs 38–44. SEMs and TEMs showing perizonial structure. . SEM of early stage perizonia showing concave large perizonial bands. . Detail of , arrow indicating the growing edge of the perizonial band. . TEM of the fully formed perizonium composed of one large longitudinal band and four closed bands. . Detail of showing rows of areolae in the closed bands. . Detail of showing areolae occluded by cribra. . SEM of longitudinal perizonium. . Detail of the centre of the perizonium, with a break across the longitudinal band revealing a sternum-like thickening (double arrowhead). Abbreviations: P1: central longitudinal large band; P2: second closed band; P3: third closed band; P4: fourth closed band; P5: the final closed band.
Structure of the perizonium
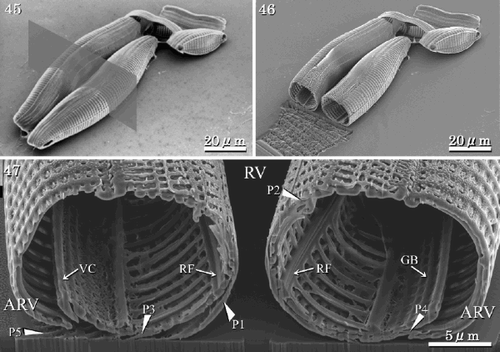
The perizonium covers only the RV of the initial cell. It consists of five silicified components, one central longitudinal perizonial band flanked by four secondary closed perizonial bands (, , , ). The earliest observed stage in the development of the perizonium is the formation of the central longitudinal band (, ), after which four closed bands are formed around and under the first one. The closed bands lie on both sides of the central longitudinal band, each element overlapped by its more central neighbour (, , ). They are comparatively thin, silicified structures. The two perizonia grow in such a way that they are juxtaposed to each other (, ). The central longitudinal band has thin longitudinal and transverse ribs, and distinct small areolae occluded by cribra (). The transverse ribs of the secondary closed bands develop from the longitudinal axis and the areolae develop between the transverse ribs (≡ virgae) as in the vegetative valve (). A slightly thicker longitudinal rib is seen at the centre of the central band, where the raphe fissure of the vegetative valve would lie (). The areolae are almost circular in the central band, while areolae in the closed bands are either circular or rectangular (, ). The closed perizonial bands may also develop as lateral outgrowths from a longitudinal axis (). No transverse perizonial bands or scales were observed during any of the stages from auxosporulation to initial cell formation.
Figs 45–47. Structure of initial cells (SEM and FIB). . Initial cells before sectioning. Grey rectangle denotes location of cross section. . Initial cells after sectioning. . Section of initial valves showing the RVs, ARVs, girdle bands and longitudinal perizonium. Abbreviations: P1: central longitudinal perizonial band; P2–P5: closed perizonial bands; RF: raphe fissure; VC: valvocopula; GB: girdle bands.
Discussion
This study clarifies several morphological details of the vegetative and initial cells, and auxospore of Achnanthes yaquinensis. This species resembles Achnanthes subconstricta (Meister) Toyoda in some respects: the valves are panduriform, the ARV has terminal orbiculi, and the frustules form chains of cells (cf. Toyoda et al., Citation2003). However, the taxa can easily be distinguished by the number of rows of areolae between the costae. Achnanthes yaquinensis has a single row while A. subconstricta has more than three. In addition, A. subconstricta usually has six marginal spines that develop from the marginal ridge, while A. yaquinensis has only two terminal spines, one at each end of the valve, which develop from the rapheless sternum. These structures assist in the formation of chains, where opposed valve faces connect. They are also taxonomically informative valve features. For instance, Achnanthes inflata Grunow and Achnanthes javanica var. javanica Grunow have marginal ridges, but both these species lack terminal and marginal spines (Le Cohu, Citation1989; Toyoda et al., Citation2003). In spite of its very similar shape in LM, A. brevipes var. intermedia has neither marginal or terminal ridges, nor marginal or terminal spines. The latter can form only short chains, usually comprising two or three cells (Toyoda & Williams, Citation2004). Achnanthes yaquinensis and A. subconstricta form long chains, cell attachment being aided by the spines or ridges on their ARVs. However, A. longipes can form a long colony attached by a long mucilage stalk without any spines or ridges. Achnanthes angustata Grunow forms contrastingly characteristic chains: cells are attached at their apices with mucilage pads, without spines or ridges (e.g., Blunn & Evans, Citation1981; Daniel et al., Citation1987; Kützing, Citation1844; Round et al., Citation1990). The type of colony formation is therefore useful for identification.
Achnanthes yaquinensis has two large plastids per cell, one on either side of the median transapical plane, like almost all other species of Achnanthes sensu stricto, except A. longipes, which has many small plastids (Cleve, Citation1894, Citation1895; Round et al., Citation1990, p. 502, fig. b), and A. inflata, which has two U-shaped plastids in valve view, one at each end of the cell (Toyoda, unpubl.). The structure of the plastid has not been used to discriminate between Achnanthes and other monoraphid genera, because there is insufficient information on the number, shape and arrangement of the plastids in many species. However, there are some useful data on several species of Achnanthes. Cox (Citation1981, Citation1987) and Mann (Citation1988) noted that number and structure of the plastids are important and relevant to the taxonomy of diatoms. We agree and hence suggest that the plastids of more species should be examined, because of the variety in their number, shape and disposition.
The primary differences between Achnanthes sensu stricto and the other species in Achnanthes sensu lato are the structure of the areolae and the presence of areolae on the girdle bands (Round et al., Citation1990; Bukhtiyarova & Round, Citation1996; Round & Bukhtiyarova, Citation1996). In Achnanthes sensu stricto the areolae are occluded by cribra on the RV, the ARV and bands (, ), while the other achnanthoid species lack cribra and their poroid areolae are often occluded by hymenes, while girdle bands are non-porous. In A. yaquinensis, the valvocopula has two rows of different shaped areolae, round and elongate, while the other bands are also open and have two or more rows of areolae. The shape of the areola usually varies between species in this genus. For instance, A. longipes also has different shaped areolae for the two rows on the valvocopula, A. javanica var. javanica has small round areolae in two rows on the valvocopula and A. subconstricta has three or more rows on each girdle band (Novarino, Citation1992; Toyoda et al., Citation2003).
Bukhtiyarova & Round (Citation1996) noted that several elements of the raphe system (e.g. Round & Bukhtiyarova Citation1996, figs 1a–m) are useful for discriminating between species. We suggest that these are also important for elucidating the relationship between Achnanthes sensu stricto and Achnanthidium, and other achnanthoid genera. In A. yaquinensis, the raphe is simple and lies along the centre of the valve. The external fissure has a simple central pore and the polar fissures curve in the same direction at both ends of the RV. This is common to many species of Achnanthes sensu stricto. On the other hand, species of the genus Psammothidium usually have slightly sigmoid raphe fissures (Bukhtiyarova & Round Citation1996). Stefano & Marino (Citation2003) used features of the raphe system to discriminate between closely related genera within the Cocconeidaceae and as partial justification for the establishment of a new genus, Amphicocconeis Stefano & Marino.
The main differences in the morphology of the vegetative and initial cells in A. yaquinensis are seen in the ARV. While the vegetative cell has both marginal ridges and terminal spines, the initial cell lacks both marginal ridges and (usually) terminal spines. Furthermore, the position of the rapheless sternum in the initial valve differs from that in the vegetative valve. Lateral movement of the silica deposition vesicle, seen in normal vegetative reproduction to form the rapheless sternum (Boyle et al., 1984), does not occur. We also suggest that the RV of the initial valve is almost identical to that of the vegetative valve because it is protected by the longitudinal perizonial bands during formation.
Karsten (Citation1899) described auxospores from A. longipes, A. brevipes and A. subsessilis. They are isogamous, as in almost all pennate diatoms except Rhabdonema Kützing and Grammatophora Ehrenberg, which are oogamous (Geitler Citation1969). Karsten (Citation1899) illustrated perizonia of A. brevipes and A. subsessilis, in which he was able to recognise longitudinal perizonial bands. He (Karsten, Citation1899) also illustrated transverse perizonial bands in A. longipes, although von Stosch (Citation1982) did not find any transverse perizonial bands in A. longipes, showing illustrations of longitudinal perizonial bands only. In some araphid diatoms, e.g., Fragilariforma virescens (Ralfs) Williams & Round, auxospores do not form any transverse perizonial bands (Williams, Citation2001).
A perizonium protects the naked auxospore during the formation of the initial frustule, while also determining the maximum width and length of the cells. Transverse perizonial bands are especially important, as these determine the maximum width of the valve, which shows less variation than maximum length. There are differences in the form and arrangement of perizonial bands. For example, in auxospores of Rhoicosphenia curvata (Kützing) Grunow and Caloneis Cleve, transverse perizonial bands overlay the initial valve, and the auxospores have both a central primary transverse band and split perizonial bands (Mann Citation1982, Citation1994). Whether transverse perizonial bands have disappeared during evolution is an interesting and important question for diatom systematics. In line with current opinion, we are confident that monoraphid diatoms are derived from biraphid diatoms, testified by raphe traces in the rapheless sternum (Andrews, Citation1981; Mayama & Kobayasi, Citation1989). It is possible that during its evolution from a biraphid ancestor Achnanthes also lost transverse perizonial bands. However, it is not clear why Achnanthes does not form transverse perizonial bands, and its systematic significance remains enigmatic. Williams (Citation2001) suggests that irregular shapes of post-auxospore cells in F. virescens are ‘permitted’ because the lack of transverse perizonial bands removes constraints on cell shape. Achnanthes cells are, ab initio, markedly flexed, perhaps also linked to the lack of transverse perizonial bands. It would be interesting to study perizonial structure in other flexed raphid genera.
Knowledge of auxosporulation in diatoms is expanding with recently published figures (Kaczmarska et al., Citation2000; Kobayashi et al., Citation2001; Schmid & Crawford, Citation2001; Sato et al., Citation2004), which show several morphological features of the auxospore. However, we need more information on auxosporulation in other species, which will provide additional data for taxonomic and systematic studies on Achnanthes sensu stricto and other achnanthoid genera.
Acknowledgements
We are grateful to Dr. David M. Williams and Dr. Eileen J. Cox, The Natural History Museum, United Kingdom for reading, commenting on, and refining this manuscript. We thank Mr. Shinya Sato, Alfred-Wegener-Institute for Polar and Marine Research, Germany, for providing some samples. We also thank Mr. Masanari Furiki, Ms. Masako Nishimura and Ms. Kaori Ichikawa, HITACHI Science Systems, Ltd. for kindly making sections of auxospores of Achnanthes yaquinensis using a Focused-Ion-Beam system (S-3000FB, HITACHI), and Ms Yuki Nakada, Queen Mary & Westfield College, University of London, for improving the English.
This work was supported in part by Grant-in-Aid no. 15570087 from the Ministry of Education, Science, Sports and Culture, Japan.
References
References
- Andrews , GW . 1981 . Achnanthes linkei and origin of monoraphid diatoms . Bacillaria , 4 : 29 – 40 .
- Anonymous . 1975 . Proposals for a standardization of diatom terminology and diagnoses . Nova Hedwigia, Beih. , 53 : 323 – 354 .
- Blunn , GW and Evans , LV . 1981 . Microscopical observations on Achnanthes subsessilis,with particular reference to stalk formation . Bot. Mar. , 24 : 193 – 199 .
- Bory de Saint Vincent JBM 1822 Dictionnaire Classique d’Historie Naturelle 1 Paris
- Boyle , JA , Pickett-Heaps , JD and Czarnecki , DB . 1984 . Valve morphogenesis in the pennate diatom Achnanthes coarctata . J. Phycol. , 20 : 563 – 576 .
- Bukhtiyarova , L and Round , FE . 1996 . Revision of the genus Achnanthes sensu lato. Psammothidiuma new genus based on A. marginulatum . Diatom Res. , 11 : 1 – 30 .
- Chepurnov , VA and Roschin , AM . 1995 . Inbreeding influence on sexual reproduction of Achnanthes longipes Ag. (Bacillariophyta) . Diatom Res. , 10 : 21 – 29 .
- Chepurnov , VA and Mann , DG . 1999 . Variation in the sexual behaviour of Achnanthes longipes (Bacillariophyta). Inbred monoecious lineages . Eur. J. Phycol. , 34 : 1 – 11 .
- Cleve , PT . 1894 . Synopsis of the naviculoid diatoms . K. Svenska Vetenskaps-Akad. Handl. , 26 ( 2 ) : 1 – 194 .
- Cleve , PT . 1895 . Synopsis of the naviculoid diatoms . K. Svenska Vetenskaps-Akad. Handl. , 27 ( 3 ) : 1 – 219 .
- Cox EJ 1981 The use of chloroplasts and other feature of the living cell in the taxonomy of naviculoid diatoms Proceedings of the 6th Symposium on Recent and Fossil Diatoms (Ross, R., editor) 115 132 O. Koeltz, Koenigstein
- Cox , EJ . 1987 . Placoneis Mereschkowsky: the re-evaluation of a diatom genus originally characterized by its chloroplast type . Diatom Res. , 2 : 145 – 157 .
- Cox EJ Ross R 1981 The striae of pennate diatoms Proceedings of the 6th Symposium on Recent and Fossil Diatoms (Ross, R. editor) 267 278 O. Koeltz, Koenigstein
- Daniel , GF , Chamberlain , AHL and Jones , EBG . 1987 . Cytochemical and electron microscopical observation on the adhesive materials of marine fouling diatoms . Br. Phycol. J. , 22 : 101 – 118 .
- Geitler , L . 1969 . Comparative studies on the behavior of allogamous pennate diatoms in auxospore formation . Am. J. Bot. , 56 : 718 – 722 .
- Idei M 1993 [Achnanthes brevipes C. Agardh var. intermedia (Kützing) Cleve.] An Illustrated Atlas of the Life History of Algae. Vol. 3. Unicellular and Flagellated Algae (Hori, T. editor) 260 261 Uchida Rokakuho Tokyo (in Japanese)
- Idei M Chihara M 1991 Sexual reproduction and systematic of diatoms Heredity 45 39 45 (In Japanese)
- Karsten , G . 1899 . Die Diatomeen der Kieler Bucht . Wiss. Meeresunters Abt. Kiel, N.F. , 4 : 17 – 205 .
- Kaczmarska , I , Bates , SS , Ehrman , JM and LéGer , G . 2000 . Fine structure of the gamete, auxospore and initial cell in the pennate diatom Pseudo-nitzschia multiseries (Bacillariophyta) . Nova Hedwigia , 71 : 337 – 357 .
- Kobayashi A Osada K Nagumo T Tanaka J 2001 An auxospore of Arachnoidiscus ornatus Ehrenberg Proceedings of the 16th International Diatom Symposium (Economou-Amilli, A. editor) 197 204 University of Athens Greece
- Kützing FT 1844 Die kieselschaligen Bacillarien oder Diatomeen Nordhausen
- Le Cohu , R . 1989 . Morphologie des valves et évolution du cingulum chez Achnanthes inflata (Bacillariophyceae) . Ann. Limnol. , 25 : 39 – 45 .
- Mann , DG . 1982 . Structure, life history and systematics of Rhoicosphenia (Bacillariophyta). Auxospore formation and perizonium structure of Rh. curvata . J. Phycol. , 18 : 264 – 274 .
- Mann DG 1988 Towards a revision of the raphid diatoms Proceedings of the 10th International Diatom Symposium (Simola, H. editor) 23 35
- Mann DG 1994 The origins of shape and form in diatoms: the interplay between morphogenetic studies and systematics Shape and Form in Plants and Fungi (Ingram, D.S., Hudson, A.J. editors) 17 38 Academic Press London
- Mayama , S and Kobayasi , H . 1989 . Sequential valve development in the monoraphid diatom Achnanthes minutissima var.saprophila . Diatom Res. , 4 : 111 – 117 .
- McIntire , CD and Reimer , CW . 1974 . Some marine and brackish-water Achnanthes from Yaquina Estuary, Oregon (U.S.A.) . Bot. Mar. , 17 : 164 – 175 .
- Mizuno , M . 1994 . Sexual reproduction and auxospore formation in Achnanthes javanica f. subconstricta . Diatom Res. , 9 : 133 – 141 .
- Nagumo , T and Kobayasi , H . 1990 . The bleaching method for gently loosening and cleaning a single diatom frustule . Diatom , 5 : 45 – 50 .
- Novarino , G . 1992 . Some observations on the girdle of Achnanthes longipes . Diatom Res. , 7 : 281 – 292 .
- Provasoli L 1968 Media and products for the cultivation of marine algae Cultures and Collections of Algae (Watanabe, A., Hattori, A. editors) 63 75 Japanese Society of Plant Physiologists Tokyo
- Ross , R , Cox , EJ , Karayeva , NI , Mann , DG , Paddock , TBB , Simonsen , R and Sims , PA . 1979 . An amended terminology for the siliceous components of the diatom cell . Nova Hedwigia, Beih. , 64 : 513 – 533 .
- Round , FE and Bukhtiyarova , L . 1996 . Four new genera based on Achnanthes (Achnanthidium) together with a re-definition of Achnanthidium . Diatom Res. , 11 : 345 – 361 .
- Round , FE and Basson , PW . 1997 . A new monoraphid diatom genus (Pogoneis) from Bahrain and the transfer of previously described species A. hungarica and A. taeniata to new genera . Diatom Res. , 12 : 71 – 81 .
- Round FE Crawford RM Mann DG 1990 The Diatoms. Biology & Morphology of the Genera., Cambridge University Press Cambridge
- Sabbe , K , Chepurnov , VA , Vyverman , W and Mann , DG . 2004 . Apomixis in Achnanthes (Bacillariophyceae); development of a model system for diatom reproductive biology . Eur. J. Phycol. , 39 : 327 – 341 .
- Sato , S , Nagumo , T and Tanaka , J . 2004 . Auxospore formation and the morphology of the initial cell of the marine araphid diatom Gephyria media (Bacillariophyceae) . J. Phycol. , 40 : 684 – 691 .
- Schmid , AM and Crawford , RM . 2001 . Ellerbeckis arenaria (Bacillariophyceae): formation of auxospores and initial cells . Eur. J. Phycol. , 36 : 307 – 320 .
- Stefano , MD and Marino , D . 2003 . Morphology and taxonomy of Amphicocconeis gen. nov. (Achnanthales, Bacillariophyceae, Bacillariophyta) with considerations on its relationship to other monoraphid diatom genera . Eur. J. Phycol. , 38 : 361 – 370 .
- Suzuki , H , Tanaka , J and Nagumo , T . 2001a . Morphology of the marine diatom Cocconeis pseudomarginata Gregory var. intermedia Grunow . Diatom Res. , 16 : 93 – 102 .
- Suzuki , H , Ooishi , Y , Dan , Y and Nagumo , T . 2001b . Observations of microalgal cross sections with a Focused-Ion-Beam (FIB) system . Jap. J. Phycol. (Sorui) , 49 : 7 – 10 .
- Toyoda , K and Williams , DM . 2004 . Description of Achnanthes Bory (Bacillariophyceae) based on Kützing's type slides and materials I: New morphological information on Achnanthes brevipes var. intermedia (Kütz.) Cleve . Diatom , 20 : 159 – 166 .
- Toyoda , K , Nagumo , T , Osada , K and Tanaka , J . 2003 . Morphological investigations of Achnanthes javanica Grunow and A. subconstricta (Meister) comb. nov . Diatom Res. , 18 : 365 – 375 .
- Toyoda , K , Nagumo , T , Tanaka , J and Williams , DM . 2005a . Taxonomy and fine structure of marine diatomAchnanthes grunowii nom. nov . Jap. J. Phycol. , 80 : 1 – 8 .
- Toyoda K Cox EJ Sims PA Williams DM 2005b The typification of Achnanthes Bory based on Echinella stipitata Lyngbye, with an account of the morphology and fine structure of Lyngbye's species Diatom Res 20 in press
- van Landingham SL 1967 Catalogue of the Fossil and Recent Genera and Species of Diatoms and their Synonyms. Part. I. Achnanthoceras through Bacillaria, J. Cramer Lehre
- von Stosch , HA . 1982 . On auxospore envelopes in diatoms . Bacillaria , 5 : 127 – 156 .
- Williams DM 2001 Comments on the structure of ‘post-auxospore’ valves of Fragilariforma virescens Lange–Bertalot Festschrift, Studies on diatoms (Jahn, R., Kociolek, J.P., Witkowski, A. & Compère, P. editors) 103 117 A.R.G. Gantner Germany