Abstract
The presence or absence of gamete fusion in the dioecious and isogamous brown alga Scytosiphon lomentaria (Scytosiphonaceae, Phaeophyceae) was investigated for thalli collected from three localities, Oshoro, Asari and Muroran in Hokkaido, northern Japan. Both sexual and asexual thalli were found in each locality. These sexual (35 samples) and asexual thalli (15 samples) were used to establish unialgal cultures. ITS2 sequences were determined in all cultures, the rbcL-spacer-S in 14, and cox3 in 25 cultures. Thirteen haplotypes (A1–4, B, C1–4, D, E1–3) were found in ITS2 sequences (241–252 bp). Ten haplotypes (A1–4, B, C1–4 and D) were found in sexual samples: haplotypes A and B were found in Oshoro, C and D in Asari, and C in Muroran. Haplotypes E1–3 were found in asexual samples. Sequence divergence values (including gap information) were less than 4.03% among sexual samples (A, B, C and D types), 0.38–0.77% among asexual samples (E types) and 4.39–5.70% between sexual and asexual samples. In the mitochondrial cox3 gene region (543 bp), six haplotypes (K1–3, L, M and N) were obtained. Cox3-K types were found in samples of ITS2-A types, cox3-L type in ITS2-B type, cox3-M type in ITS2-C and D types, and cox3-N type in ITS2-E types. For cox3 sequences, nucleotide differences were 0.18–4.42% among sexual samples (K, L and M types), but 8.66–10.31% between sexual and asexual samples (N type). In partial rbcL (174 bp)-spacer (188 bp)-partial rbcS (90 bp) sequences, six haplotypes (R–W) were found although there were 1–4 bp nucleotide differences among these haplotypes. R, U and W types were found in sexual samples, and S, T and V types in asexual samples. Results of the sequence analyses suggest that our presumed asexual thalli are genetically different from sexual thalli and may be derived from populations that lack sexual reproduction.
Introduction
Scytosiphon lomentaria (Lyngbye) Link (Scytosiphonaceae, Phaeophyceae) is widely distributed in cold and temperate waters. It is dioecious and isogamous gamete fusion has been reported (Tatewaki, Citation1966; Nakamura & Tatewaki, Citation1975; Kogame, Citation1998). However, thalli that release asexual zoids have also been observed (Kristiansen & Pedersen, Citation1979; Parente et al., Citation2003), and when crossed, these zoids will not fuse, indicating that they are asexual. Such asexual zoids are morphologically indistinguishable from isogametes, and observations of gamete fusion are the only practical way to distinguish asexual zoids from gametes.
In two scytosiphonacean algae from Australia, an unidentified species of Scytosiphon C. Agardh and Colpomenia peregrina (Sauvageau) Hamel, it has been reported that thalli produce gametes or zoids that do not show sexual fusion, and that gametogenesis is rare and may be a transient, seasonal phenomenon (Clayton, Citation1980, Citation1981). In cultures of the brown algae Stilophora tenella (Esper) P.C. Silva (= Stilophora rhizodes (C. Agardh) J. Agardh, nom. illeg.) and Spermatochnus paradoxus (Roth) Kützing gametophytes produce asexual zoids at higher temperatures and gametes at lower temperatures (Müller, Citation1981; Peters & Müller, Citation1986; Peters, Citation1987). Gametogenesis is probably controlled by temperature in these cases. Age might also be a factor regulating gametogenesis in Scytosiphon (Clayton, Citation1981). It is possible, therefore, that thalli of S. lomentaria might release gametes or asexual zoids depending on seasonal environmental conditions or age (hypothesis 1). On the other hand, it is possible that thalli releasing asexual zoids are derived from populations lacking sexual reproduction (hypothesis 2). However, reports of asexual populations in brown algae are relatively sparse. One reason for this may be that identification of asexual populations is difficult in brown algae: the absence of sexual fusion in the laboratory is not absolute evidence of asexual populations since gametogenesis might be missed owing to transient sexual reproduction or unfavourable conditions, as mentioned above (Clayton, Citation1982, Citation1988; Innes & Yarish, Citation1984).
Although identification of asexual populations by culture studies may be difficult in Scytosiphon, genetic population analysis would facilitate their identification since genetic differences are expected between sexual and asexual populations (Innes & Yarish, Citation1984; Pearson & Murray, Citation1997). Intraspecific variation in DNA sequences has been reported for macroalgae in the ITS regions of nrRNA genes, a chloroplast-encoded Rubisco spacer and mitochondrial genes (Peters et al., Citation1997; Kamiya et al., Citation1999; Serrão et al., Citation1999; Zuccarello et al., Citation1999; Famá et al., Citation2000; Coyer et al., Citation2002; Gabrielsen et al., Citation2002; Zuccarello & West, Citation2002). In our preliminary survey of S. lomentaria, ITS2 sequences differed between gamete-releasing and asexual zoid-releasing thalli, suggesting that the asexual zoid-releasing thalli may be derived from asexual populations. In this study, genetic relationships between gamete-releasing and asexual zoid-releasing thalli were investigated further using DNA sequences of the mitochondrial cox3 and the partial rbcL-spacer-partial rbcS region, as well as the ITS2 region, to identify asexual populations in S. lomentaria.
Materials and methods
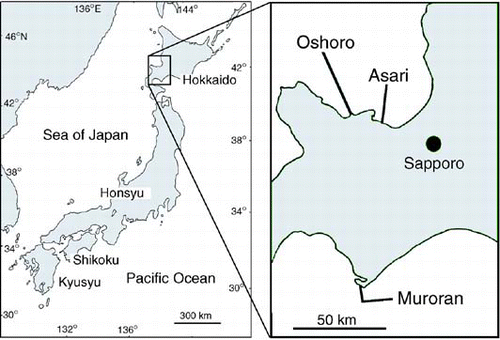
Thalli were collected in Oshoro (43°13′N, 140°52′E), Asari (43°10′N, 141°05′E) and Muroran (42°11′N, 140°47′E), Hokkaido, Japan (, ). Parts of samples were dried as voucher herbarium specimens and are deposited in the Herbarium of the Graduate School of Science, Hokkaido University (SAP 094825-094833). Mature individual thalli (10–21 thalli per collection) were placed in a plastic Petri dish (90 × 20 mm) containing PESI medium (Tatewaki, Citation1966), and the dishes were incubated at 10°C under a 16:8 h light:dark regime (30–50 µmol photons m−2 s−1). In the morning, zoids were released from the thalli, and thalli were checked by smell for the presence of a sexual pheromone possibly indicating female thalli. Final sex determination was carried out by observing gamete behaviour during sexual fusion: a settled female gamete is surrounded by several male gametes, which adhere to the female gamete using their anterior flagella, after which one fuses with the female (Nakamura & Tatewaki, Citation1975; Kogame, Citation1998). Thalli for which sexual fusion was not observed were assumed to be asexual. Unialgal cultures were established, by isolating gametes or asexual zoids, for the individual thalli whose sex (female, male and asexual) had been determined. Cultures were maintained in plastic Petri dishes (90 × 20 mm) containing PESI medium at 10°C or 15°C under the light conditions above.
Table 1. Localities, sample codes and haplotypes for the ITS2, cox3 and partial rbcL-spacer-partial rbcS (rbcL-sp-S) regions
Total DNA was extracted from cultured thalli and purified as described previously (Kogame et al., Citation1999). The purified DNA was used as template DNA in a polymerase chain reaction (PCR) to amplify the ITS2 region, the partial rbcL-spacer-partial rbcS (rbcL-sp-S) region and the mitochondrion-encoded cox3 (cytochrome oxidase subunit 3) gene. The pairs of primers used for PCR were: 5.8SBF (5′-CGATGAAGAACGCAGCGAAATGCGAT-3′; Goff et al., Citation1994; for forward) and 25BR2 (5′-TCCTCCGCTTAGTATATGCTTAA-3′; Kogame & Masuda, Citation2001; for reverse) for ITS2; rbcL3F (5′-CAGGTGCTACAGCTAACCGTGT-3′; Kogame & Masuda, Citation2001; for forward) and RSPR (5′-AATAAAGGAAGACCCCATAATTCCCA-3′; Kogame et al., Citation1999; for reverse) for the rbcL-sp-S region; CAF4A (5′-ATGTTTACTTGGTGRA GRGA-3′; this study; for forward) and CAR4A (5′-CCCCACCARTAWATNGTNAG-3′; this study; for reverse) for the cox3 gene. PCR and sequencing were carried out as described by Kogame et al. (Citation1999) except that the annealing temperature was 45°C for PCR of the cox3.
Sequences were aligned by eye using PAUP*4.0b (Swofford, Citation2001). In the ITS2 analyses, gaps were counted as single events regardless of gap length (Peters et al., Citation1997), and a gap matrix was added to the end of the alignment. Nucleotide differences among haplotypes were calculated with the ‘pair-wise distances’ command in PAUP. Phylogenetic analyses were performed using maximum-parsimony (MP) and neighbour-joining (NJ) methods in PAUP*4.0b. MP trees were constructed by heuristic search with step-wise addition and TBR branch swapping. NJ trees were constructed using Kimura 2-parameter distance (Kimura, Citation1980) for cox3 and rbcL-sp-S analyses. Since this distance is not appropriate for gap characters, a standard distance was selected for ITS2 analyses. A bootstrap analysis was performed with 1000 replicates.
Results
Observations of morphology and gamete fusion
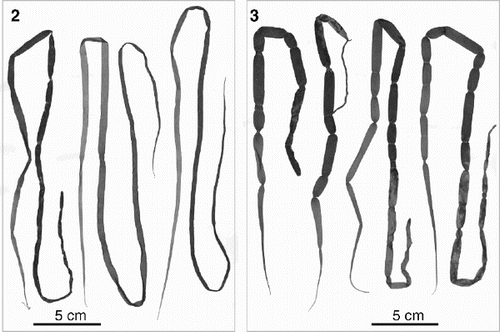
Both sexual and asexual thalli were found at Oshoro, Asari and Muroran. Morphological features of the thalli were identical to those described by Kogame (Citation1998). At Muroran and Asari, sexual thalli () were complanate and rarely slightly constricted, whereas asexual thalli () were cylindrical and strongly constricted. At Oshoro, both sexual and asexual thalli were cylindrical and constricted, and were difficult to distinguish from each other. Sexual and asexual thalli grew together at Asari and Muroran. At Oshoro, sexual thalli grew more abundantly in wave-protected areas.
Figs 2–3. Habit of Scytosiphon lomentaria collected from Muroran, Hokkaido, Japan on 10 March 2000. . Sexual thalli (SAP 094827). . Asexual thalli (SAP 094829).
The process of gamete fusion was as previously described by Kogame (Citation1998). Asexual zoids usually showed negative phototaxis and seemed to settle more rapidly than female gametes. Sexual pheromone was not detected by smell in dishes of asexual thalli. Discoid thalli with tufts of branched filaments developed in cultures of both gametes and asexual zoids. Erect thalli sometimes formed on the tufts.
DNA sequence analyses
Fifty samples were used for DNA sequence analyses: 25 samples from Oshoro, 9 from Asari and 16 from Muroran. The ITS2 sequences were determined for all samples, and cox3 for 25 samples, and rbcL-sp-S for 14 samples (). The length of the ITS2 sequences ranged from 241 to 252 bp with many gaps in the alignment (). Thirteen total haplotypes were found for this region: A1–4, B, C1–4, D, E1–3 (, ). Ten (A1–4, B, C1–4, D) only occurred in sexual samples. Haplotypes A and B were found from Oshoro, C and D from Asari, and C from Muroran (). Haplotypes E1–3 were only found in asexual samples. Sequence divergence (including gap information; ) was less than 4.03% among sexual samples (A, B, C and D types), 0.38–0.77% among asexual samples (E types) and 4.39–5.70% between sexual and asexual samples. The midpoint-rooted NJ () and MP (31 most parsimonious trees) trees based on the ITS2 sequences showed that sexual (A, B, C and D types) and asexual samples (E types) grouped with each other with 99% (NJ) and 100% (MP) bootstrap values. The clade of A1–4 types was also relatively highly supported with 99% (NJ) and 81% (MP) bootstrap values.
Fig. 4. A sub-alignment of thirteen ITS2 haplotypes (A1–4, B, C1–4, D, E1–3) of Scytosiphon lomentaria from Hokkaido, Japan. A2: the sequence of Os-7iv98-8-fe (accession AB094189), B: Os-7iv98-1-fe (accession AB094190), C1: As-11v99-6-ma (accession AB094191), D: As-11v99-7-fe (accession AB094192), E2: As-11v99-1-ax (accession AB094193). Gaps were counted as single events regardless of gap length, and a gap matrix is added to the end of the alignment.
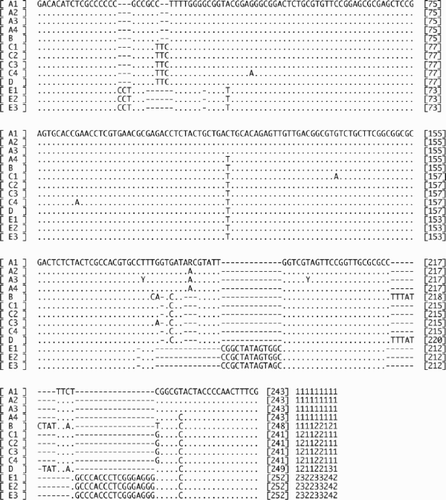
Fig. 5. A midpoint-rooted neighbour-joining tree inferred from sequences of thirteen ITS2 haplotypes in Scytosiphon lomentaria from Hokkaido, Japan. Bootstrap values indicate the percentage (neighbour-joining tree/most-parsimonious tree) based on 1000 replicates.
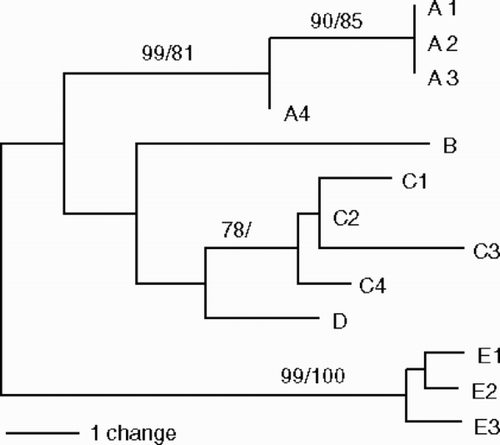
Table 2. Pair-wise distances among ITS2-haplotype sequences in Scytosiphon lomentaria from Hokkaido, Japan. Gaps were treated as single events regardless of gap length. Below diagonal: total character differences. Above diagonal: mean character differences (adjusted for missing data)
The length of the alignment of cox3 sequences was 543 bp. Six haplotypes (K1–3, L, M and N) were found (, ). Except for one substitution, all nucleotide differences came from substitutions at the third nucleotide of codons (synonymous substitution). Cox3-K types were found in samples of ITS2-A types, cox3-L in ITS2-B, cox3-M in ITS2-C and D, and cox3-N in ITS2-E. In comparisons of cox3 sequences, nucleotide differences () were 0.18–4.42% among sexual thalli (K, L and M types), and 8.66–10.31% between sexual thalli and asexual thalli (N type). Midpoint-rooted NJ () and MP (single most parsimonious tree) trees were inferred based on cox3 sequences and showed the same topology and similar bootstrap values in the major clades. The major clades of each cox3 haplotype were supported by high bootstrap values (>97%), and sexual and asexual samples were clearly separated.
Fig. 6. An alignment of six cox3 haplotypes (K1–3, L, M and N) of Scytosiphon lomentaria from Hokkaido, Japan. K1: the sequence of Os-7iv98-6-ma (accession AB094194), L: Os-7iv98-1-fe (accession AB094195), M: As-11v99-6-ma (accession AB094196), N: Os-7iv98-23-ax (accession AB094197).
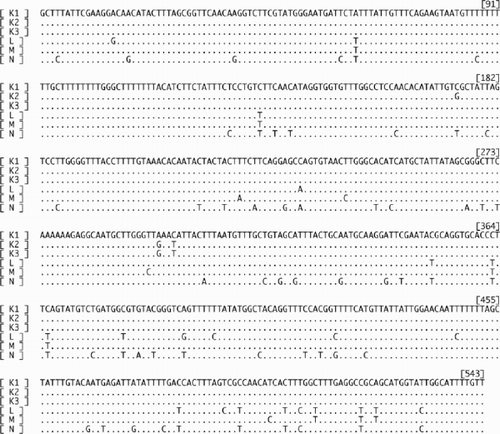
Fig. 7. A midpoint-rooted neighbour-joining tree inferred from cox3 sequences in Scytosiphon lomentaria from Hokkaido, Japan. Bootstrap values indicate the percentage (neighbour-joining tree/most-parsimonious tree) based on 1000 replicates. Haplotypes of cox3 and ITS2, and sexual or asexual populations are indicated.
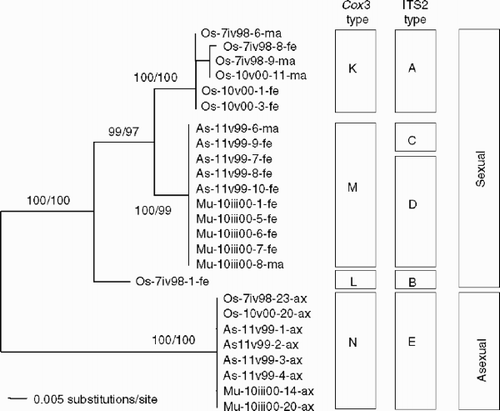
Table 3. Pair-wise distances among cox3-haplotype sequences in Scytosiphon lomentaria from Hokkaido, Japan. Below diagonal: total character differences. Above diagonal: mean character differences
In the partial rbcL (174 bp)-spacer (188 bp)-partial rbcS (90 bp) sequences, six haplotypes (R, S, T, U, V and W) were found (, ). There were four variable sites in the partial rbcL, one in the spacer but no variable sites in the partial rbcS. The nucleotide differences among the haplotypes were only 1–4 bp (0.22–0.89%). The R, U and W types were found in sexual samples, and S, T and V types in asexual samples. However, these haplotypes did not correspond with localities as clearly as the ITS and cox3 haplotypes did. The MP and NJ analyses of rbcL-sp-S haplotypes resulted in low resolution, showing no high bootstrap values in any clades (data not shown), due to small sequence differences (1–4 bp).
Table 4. Haplotypes (R–W) of the partial rbcL (174 bp)-rbc spacer (188 bp) region in Scytosiphon lomentaria from Hokkaido, Japan
Discussion
Sexual and asexual thalli differed in their ITS2 and cox3 DNA sequences, even in sympatric areas, and were clearly separated in our phylogenetic analyses. Furthermore, rbcL-sp-S haplotypes corresponded to sexual and asexual thalli. These results indicate that gene flow between the sexual and asexual thalli was not detected in our study; however, further genetic analyses of many samples may be needed to confirm a complete absence of gene flow. However, our results suggest that: (1) recombination by means of sexual reproduction is rare or absent between our sexual and asexual thalli; (2) sexual and asexual thalli are genetically different. Therefore, we suggest that the presumed asexual thalli were probably derived from asexual populations lacking a sexual cycle. This conclusion agrees with hypothesis 2 postulated above. On the other hand, it could not be determined whether or not sexual populations produce asexual zoids in other seasons. Although genetically sexual thalli producing asexual zoids were not detected in this study, sampling throughout the growing season would be needed to reveal this. Therefore, hypothesis 1 cannot be completely rejected for the sexual populations investigated.
The haplotypes of the rbcL-sp-S region showed lower resolution than those of ITS2 and cox3: rbcL-sp-S haplotypes did not correspond to localities, and reliable molecular trees of the rbcL-sp-S haplotypes were not obtained owing to very low nucleotide differences (1–4 bp). The sequence divergence of the rbcL-sp-S haplotypes (0.22–0.89%) was much lower than those of ITS2 and cox3 haplotypes (0–5.70% and 0.18–10.31%, respectively). However, there was sequence variation of the rbcL-sp-S region in samples with the same cox3 haplotype (M and N types). These results suggest that substitution rates may be higher in the variable sites detected in the rbcL-sp-S region than those in the cox3 region. There were only 1–4 bp nucleotide differences among the rbcL-sp-S haplotypes so the lower resolution of the rbcL-sp-S haplotypes may also be due to parallelism. Careful consideration of the significance of nucleotide differences of a few base substitutions is required when analysing genetic relationships.
Morphological differences between sexual and asexual thalli in S. lomentaria have been observed at Muroran by M. Tatewaki and T. Motomura (pers. comm.): sexual thalli were complanate and non-constricted, whereas asexual thalli were cylindrical and constricted. Such morphological differences were also observed at Muroran and Asari in this study. The morphological differences may be genetically determined since sexual and asexual thalli differed in their ITS2 and cox3 sequences. Sexual thalli from Oshoro were cylindrical and slightly constricted, and were different from those from Muroran and Asari. Since the sexual thalli from Oshoro also differed in ITS2 and cox3 haplotypes from those from Muroran and Asari, the morphological differences might be due to genetic differences and not environmental conditions such as temperature and wave action.
The absence of sexual stages in populations of various algae suggests the widespread existence of asexual populations in algae. Female-dominant populations of Cutleria cylindrica Okamura have been reported from Japan, female gametes of which have low fertilization rates (Kitayama et al., Citation1992). Cultures from such populations have a direct-type life history without sporophytic generations. Genetic evidence for predominantly asexual reproduction has been demonstrated in populations of Ulva linza Linnaeus from Long Island Sound: the patterns of genetic variation at enzyme loci were significantly different from that expected for populations with predominantly sexual reproduction (Innes & Yarish, Citation1984: as Enteromorpha). Two types of asexual populations have been reported in Ulva prolifera O.F. Müller from Japan, distinguished from sexual populations by having direct-type life histories without meiosis, and asexual zoids that differ from gametes and asexual zoids of sexual thalli in size and DNA content (Hiraoka et al., Citation2003: as Enteromorpha). However, ITS1 and 2 sequences did not correlate with life histories in these populations (Hiraoka et al., Citation2003). In the red algal genera Mastocarpus and Ahnfeltiopsis, apomictic, direct-type life histories, without tetrasporophytic generations, were investigated in the laboratory and the field (Polanshek & West, Citation1977: as Gigartina; DeCew & West, Citation1981: as Gymnogongrus; West et al., Citation1978: as Gigartina; Masuda et al., Citation1984, Citation1987: as Gigartina; Zupan & West, Citation1988). Populations showing direct-type life histories may be derived from sexual populations that have lost the capacity for sexual reproduction (Masuda et al., Citation1984). In collections of Dasya ocellata (Grateloup) Harvey from the British Isles only tetrasporophytes have been observed, and a direct tetraspore-to-tetrasporophyte life history was reported, chromosome counts demonstrating the lack of meiosis in tetrasporangia (Maggs, Citation1998). A sexual life history only occurs in southern populations of this species (Maggs, Citation1998). However, several authors have mentioned that the presence of a direct-type life history may not be strong evidence for the complete lack of sexual reproduction since transient sexual reproduction may occur (Polanshek & West, Citation1977; Clayton, Citation1982, Citation1988; Innes & Yarish, Citation1984). Genetic investigations are required to reveal relationships between sexual and such asexual-like thalli.
Intra-population or intra-individual polymorphism of ITS sequences has been described for other macroalgae (e.g., Caulerpa, Famá et al., Citation2000; Chordaria, Kim & Kawai, Citation2002), and we detected intra-population polymorphism in ITS2, cox3 and rbcL-sp-S regions. It has been reported that such intraspecific variation is correlated with geographical population patterns in many macroalgal species (Pillmann et al., Citation1997; Gabrielsen et al., Citation2002; Zuccarello & West, Citation2002). Our ITS2 and cox3 haplotypes occurred in particular localities, although these were all in a small geographical area. The sexual populations of Asari (on the coast of the Sea of Japan) and Muroran (on the Pacific coast) showed similar ITS2 (C and D) and the same cox3 (M) haplotypes, indicating that they are closely related genetically. The ITS2 and cox3 haplotypes may be widely distributed along the coasts of Hokkaido Island. The haplotype and morphological uniformity of asexual populations of S. lomentaria strongly suggests that asexual thalli might be distributed along the coasts of Hokkaido independent of any differentiation and/or geographical extension of sexual thalli.
Three sexual lineages were found in this study, and each had a specific combination of the ITS2 and the cox3 haplotypes: ITS2 A–cox3 K, ITS2 C or D-cox3 M and ITS2 B–cox3 L. If sexual recombination occurs randomly between these lineages, the nuclear ITS2 haplotypes should be randomly associated with the mitochondrial cox3 haplotypes. Based on the particular combinations of haplotypes, the existence of reproductively isolated cryptic species is suspected for the sexual lineages. However, our data are insufficient to demonstrate unequivocally the existence of cryptic species. The populations from Asari and Muroran only showed the ITS2 C or D–cox3 M pair, no sexual recombination between different haplotype pairs can be detected in these populations. Although two pairs (ITS2 A–cox3K and ITS2 B–cox3 L) were detected at Oshoro, only one ITS2 B–cox3 L sample was found. Based on the phylogenetic results, the asexual lineage could belong to a different species from the three sexual lineages; the asexual samples might represent a population of a different species that also includes sexual populations. Further comprehensive studies of S. lomentaria populations are needed to clarify these issues.
Although complete mitochondrial genomes were reported in two brown algae (Oudot-le Secq et al., Citation2001, Citation2002), phylogenetic and population studies using mitochondrial genes are still scarce, and intraspecific variations are little known (Coyer et al., Citation2002). In red algae, intraspecific and intra-population variations have been reported in the intergenic region between the mitochondrial cox2 and cox3, and these may lead to a better understanding of the populations (Zuccarello et al., Citation1999; Gabrielsen et al., Citation2002; Zuccarello & West, Citation2002). Our study has shown that the mitochondrial cox3 is very useful for investigations of intraspecific relationships in brown algae too.
Acknowledgements
We wish to thank Prof. M.D. Guiry and Dr F. Rindi for their critical reading of the manuscript. We are grateful to staff of the Muroran Marine Station, Hokkaido University and to Mr K. Shinta, Oshoro Marine Biological Station, Hokkaido University, for their assistance in collecting material. We also thank Dr M. Tatewaki and Dr T. Motomura for their comments on morphological differences between sexual and asexual thalli in Scytosiphon lomentaria at Muroran, and Mr Y. Kato for his assistance in DNA sequencing. This work was partly supported by the Grant-in-Aid for Scientific Research provided by the Ministry of Education, Science, Sports and Culture, Japan (14540639).
References
References
- Clayton , MN . 1980 . Sexual reproduction–a rare occurrence in the life history of the complanate form of Scytosiphon (Scytosiphonaceae, Phaeophyta) from southern Australia . Br. Phycol. J. , 15 : 105 – 118 .
- Clayton , MN . 1981 . Observations on the factors controlling the reproduction of two common species of brown algae, Colpomenia peregrina and Scytosiphon sp. (Scytosiphonaceae), in Victoria . Proc. Royal Soc. Victoria , 92 : 113 – 118 .
- Clayton , MN . 1982 . Life history studies in the Ectocarpales (Phaeophyta): contributions toward the understanding of evolutionary processes . Bot. Mar. , 25 : 111 – 116 .
- Clayton , MN . 1988 . Evolution and life histories of brown algae . Bot. Mar. , 31 : 379 – 387 .
- Coyer , JA , Peters , AF , Hoarau , G , Stam , WT and Olsen , JL . 2002 . Inheritance patterns of ITS1, chloroplasts and mitochondria in artificial hybrids of the seaweeds Fucus serratus and F. evanescens (Phaeophyceae) . Eur. J. Phycol. , 37 : 173 – 178 .
- DeCew , TC and West , JA . 1981 . Life histories in the Phyllophoraceae (Rhodophyta: Gigartinales) from the Pacific coast of north America. I. Gymnogongrus linearis and G. leptophyllus . J. Phycol. , 17 : 240 – 250 .
- Famá , P , Olsen , JL , Stam , WT and Procaccini , G . 2000 . High levels of intra- and inter-individual polymorphism in the rDNA ITS1 of Caulerpa racemosa (Chlorophyta) . Eur. J. Phycol. , 35 : 349 – 356 .
- Gabrielsen , TM , Brochmann , C and Rueness , J . 2002 . The Baltic Sea as a model system for studying postglacial colonization and ecological differentiation, exemplified by the red alga . Ceramium tenuicorne. Mol. Ecol. , 11 : 2083 – 2095 .
- Goff , LJ , Moon , DA and Coleman , AW . 1994 . Molecular delineation of species and species relationships in the red algal agarophytes Gracilariopsis and Gracilaria (Gracilariales) . J. Phycol. , 30 : 521 – 537 .
- Hiraoka , M , Dan , A , Shimada , S , Hagihira , M , Migita , M and Ohno , M . 2003 . Different life histories of Enteromorpha prolifera (Ulvales, Chlorophyta) from four rivers on Shikoku Island, Japan . Phycologia , 42 : 275 – 284 .
- Innes , DJ and Yarish , C . 1984 . Genetic evidence for the occurrence of asexual reproduction in populations of Enteromorpha linza (L.) J. Ag. (Chlorophyta, Ulvales) from Long Island Sound . Phycologia , 23 : 311 – 320 .
- Kamiya , M , Tanaka , J , King , RJ , West , JA , Zuccarello , GC and Kawai , H . 1999 . Reproductive and genetic distinction between broad and narrow entities of Caloglossa continua (Delesseriaceae, Rhodophyta) . Phycologia , 38 : 356 – 367 .
- Kim , S-H and Kawai , H . 2002 . Taxonomic revision of Chordaria flagelliformis (Chordariales, Phaeophyceae) including novel use of the intragenic spacer region of rDNA for phylogenetic analysis . Phycologia , 41 : 328 – 339 .
- Kimura , M . 1980 . A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences . J. Mol. Evol. , 16 : 111 – 120 .
- Kitayama , T , Kawai , H and Yoshida , T . 1992 . Dominance of female gametophytes in field populations of Cutleria cylindrica (Cutleriales, Phaeophyceae) in the Tsugaru Strait, Japan . Phycologia , 31 : 449 – 461 .
- Kogame , K . 1998 . A taxonomic study of Japanese Scytosiphon (Scytosiphonales, Phaeophyceae), including two new species . Phycol. Res. , 46 : 39 – 56 .
- Kogame , K and Masuda , M . 2001 . Crustose sporophytes of Colpomenia bullosa (Scytosiphonaceae, Phaeophyceae) in nature . Crypt., Algol. , 22 : 201 – 208 .
- Kogame , K , Horiguchi , T and Masuda , M . 1999 . Phylogeny of the order Scytosiphonales (Phaeophyceae) based on DNA sequences of rbcL, partial rbcS and partial LSU nrDNA . Phycologia , 38 : 496 – 502 .
- Kristiansen , A and Pedersen , PM . 1979 . Studies on life-history and seasonal-variation of Scytosiphon lomentaria (Fucophyceae, Scytosiphonales) in Denmark . Bot. Tidsskr. , 74 : 31 – 56 .
- Maggs , CA . 1998 . Life history variation in Dasya ocellata (Dasyaceae, Rhodophyta) . Phycologia , 37 : 100 – 105 .
- Masuda , M , West , JA , Ohno , Y and Kurogi , M . 1984 . Comparative reproductive patterns in culture of different Gigartina subgenus Mastocarpus and Petrocelis populations from northern Japan . Bot. Mag., Tokyo , 97 : 107 – 125 .
- Masuda , M , West , JA and Kurogi , M . 1987 . Life history studies in culture of a Mastocarpus species (Rhodophyta) from central Japan . J. Fac. Sci., Hokkaido Univ. Series V (Botany) , 14 : 11 – 38 .
- Müller , DG . 1981 . Culture studies on reproduction of Spermatochnus paradoxus (Phaeophyceae, Chordariales) . J. Phycol. , 17 : 384 – 389 .
- Nakamura , Y and Tatewaki , M . 1975 . The life history of some species of Scytosiphonales . Sci. Pap. Inst. Algol. Res., Fac. Sci., Hokkaido Univ. , 6 : 57 – 93 .
- Oudot-le Secq , M-P , Fontaine , J-M , Rousvoal , S , Kloareg , B and Loiseaux-de Goër , S . 2001 . The complete sequence of a brown algal mitochondrial genome, the Ectocarpale Pylaiella littoralis (L.) Kjellm . J. Mol. Evol. , 53 : 80 – 88 .
- Oudot-le Secq , M-P , Kloareg , B and Loiseaux-de Goër , S . 2002 . The mitochondrial genome of the brown alga Laminaria digitata: a comparative analysis . Eur. J. Phycol. , 37 : 163 – 172 .
- Parente , MI , Neto , AI and Fletcher , RL . 2003 . Morphology and life history of Scytosiphon lomentaria (Scytosiphonaceae, Phaeophyceae) from the Azores . J. Phycol. , 39 : 353 – 359 .
- Pearson , EA and Murray , SN . 1997 . Patterns of reproduction, genetic diversity, and genetic differentiation in California populations of the geniculate coralline alga Lithothrix aspergillum (Rhodophyta) . J. Phycol. , 33 : 753 – 763 .
- Peters AF 1987 Reproduction and sexuality in the Chordariales (Phaeophyceae). A review of culture studies Progress in Phycological Research Vol. 5 (Round, F.E. & Chapman, D.J., editors) 223 263 Biopress Bristol
- Peters , AF and Müller , DG . 1986 . Sexual reproduction of Stilophora rhizodes (Phaeophyceae, Chordariales) in culture . Br. Phycol. J. , 21 : 417 – 423 .
- Peters , AF , van Oppen , MJH , Wiencke , C , Stam , WT and Olsen , JL . 1997 . Phylogeny and historical ecology of the Desmarestiaceae (Phaeophyceae) support a Southern Hemisphere origin . J. Phycol. , 33 : 294 – 309 .
- Pillmann , A , Woolcott , GW , Olsen , JL , Stam , WT and King , RJ . 1997 . Inter- and intraspecific genetic variation in Caulerpa (Chlorophyta) based on nuclear rDNA ITS sequences . Eur. J. Phycol. , 32 : 379 – 386 .
- Polanshek , AR and West , JA . 1977 . Culture and hybridization studies on Gigartina papillata (Rhodophyta) . J. Phycol. , 13 : 141 – 149 .
- Serrão , E , Alice , LA and Brawley , SH . 1999 . Evolution of the Fucaceae (Phaeophyceae) inferred from nrDNA-ITS . J. Phycol. , 35 : 382 – 394 .
- Swofford D 2001 PAUP*. Phylogenetic analysis Using Parsimony (*and Other Methods), Version 4, Sinauer Associates Sunderland Massachusetts
- Tatewaki , M . 1966 . Formation of a crustaceous sporophyte with unilocular sporangia in . Scytosiphon lomentaria. Phycologia , 6 : 62 – 66 .
- West , JA , Polanshek , AR and Shevlin , DE . 1978 . Field and culture studies on Gigartina agardhii (Rhodophyta) . J. Phycol. , 14 : 416 – 426 .
- Zuccarello , GC and West , JA . 2002 . Phylogeography of the Bostrychia calliptera–B. pinnata complex (Rhodomelaceae, Rhodophyta) and divergence rates based on nuclear, mitochondrial and plastid DNA markers . Phycologia , 41 : 49 – 60 .
- Zuccarello , GC , Burger , G , West , JA and King , RJ . 1999 . A mitochondrial marker for red algal intraspecific relationships . Mol. Ecol. , 8 : 1443 – 1447 .
- Zupan , JR and West , JA . 1988 . Geographic variation in the life history of Mastocarpus papillatus (Rhodophyta) . J. Phycol. , 24 : 223 – 229 .