Abstract
A new sand-dwelling dinoflagellate, Pileidinium ciceropse Tamura et Horiguchi gen. et sp. nov., is described from a sandy beach on Mecherchar Island, Republic of Palau, using light and electron microscopy. The cell is laterally compressed and consists of a small epitheca and a large hypotheca. It has a yellow-brown chloroplast and a spherical pyrenoid. This dinoflagellate possesses ornamented thecal plates and an unusual thecal plate arrangement of 1′, 5′′, 4c, 4s, 5′′′, 1′′′′. The cingulum is incomplete and does not encircle the entire cell. Transmission electron microscopy reveals that the cell possesses typical dinoflagellate organelles. Phylogenetic analysis based on SSU rDNA did not demonstrate a consistent phylogenetic position of Pileidinium ciceropse within the Dinophyceae and therefore affinities with existing families could not be determined.
Introduction
Since the early taxonomic studies on benthic dinoflagellates by E. C. Herdman (Citation1922, Citation1924 a, Citation b), many reports of benthic dinoflagellates have been published (e.g. Balech, Citation1956; Dragesco, 1965; Horiguchi & Chihara, Citation1983, Citation1987; Saunders & Dodge, Citation1984; Larsen, Citation1985; Dodge & Lewis, Citation1986; Faust, Citation1994; Horiguchi & Pienaar, Citation1994; Horiguchi, Citation1995; Holmes, Citation1998; Hoppenrath & Elbrächter, Citation1998; Hoppenrath Citation2000 a, Citation b , Citation c ; Ten-Hage et al., Citation2000, Toriumi et al., Citation2002, Yohimatsu et al., 2004). Many benthic dinoflagellates are laterally compressed and have a girdle located relatively high in the upper part of the cell. Interestingly, many of the armoured sand-dwelling dinoflagellates exhibit a unique thecal plate arrangement, e.g., Adenoides Balech, Amphidiniella Horiguchi, Amphidiniopsis Woloszynska, Cabra Murray et Patterson, Plagiodinium Faust et Balech, Planodinium Saunders et Dodge, Roscoffia Balech, Sabulodinium Saunders et Dodge and Thecadinium Kofoid et Skogsberg. Although many benthic armoured dinoflagellate genera have been characterized by scanning electron microscopy, there is still limited information on their diversity and phylogenetic relationships.
During the course of our studies of marine sand-dwelling dinoflagellates in tropical regions, we found a new armoured dinoflagellate possessing a laterally compressed body and a unique thecal plate arrangement in sand samples taken in Palau. The morphology of our dinoflagellate differs from any hitherto described, armoured dinoflagellate. In this paper, we describe the morphology and ultrastructure of this new dinoflagellate. In addition to the morphological investigations, we tried to establish its phylogenetic position based on molecular data.
Materials and methods
Sand samples were collected from a sandy beach on Mecherchar Island, Republic of Palau, on 22nd May 2002. A spoonful of each sample was added to a plastic beaker containing IMK Medium for Marine Microalgae (Daigo, Tokyo, Japan). The plastic beakers were placed in a culture cabinet at 25°C, under about 50 µmol m−2 s−1 illumination in a 14:10 h light:dark light regime. Cells were isolated from the enriched sand samples using a micropipette. Subsequently, a clonal culture (25TM2, Laboratory of Phycology, Graduate School of Science, Hokkaido University) was established and maintained under the conditions described above.
Light microscopy
Cells were observed using an Olympus BX-50 microscope equipped with Nomarski interference optics (Olympus Co. Ltd., Tokyo, Japan). We used a glass slide with a frame of vinyl adhesive tape and a cover slip (Horiguchi et al., Citation2000) to observe life cycle stages. To study the thecal plates, cells were stained with 0.01% Fluorescent Brightener 28 (Sigma, UK) and examined using a Nikon Optiphoto-2 fluorescence microscope (Nikon, Tokyo, Japan).
Electron microscopy
For SEM observations, individually isolated cells were placed into 1% osmium tetroxide solution on a poly-L-lysine-coated glass plate for 10 min, rinsed in distilled water and dehydrated through an ethanol series (15 min in each step of 30%, 50%, 70%, 90% and 95%, and 1 h in absolute ethanol). Subsequently the dehydrated cells were dried in a critical point dryer HCP-1 (Hitachi, Tokyo, Japan) and sputter-coated with platinum-palladium. The observations were made using a Hitachi S-2250N (Hitachi, Tokyo, Japan) scanning electron microscope.
For transmission electron microscopy, cells were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer at room temperature for 2 h. Buffer-rinsed specimens (0.2 M sodium cacodylate buffer, pH 7.0) were post-fixed in 2% OsO4 with 10% sodium potassium ferricyanate at room temperature for 2 h. After dehydration through an acetone series, specimens were embedded using Spurr's resin (TAAB Laboratories Equipment Ltd., England) and sectioned. Sections were collected on formvar-coated copper grids, and were double stained with 2% uranyl acetate for 10 min and with lead citrate for 13 min at room temperature. Observation and photography were performed using a JEM-100S (Jeol LTD, Tokyo, Japan) transmission electron microscope.
DNA extraction, PCR amplification and sequencing
Total genomic DNA was extracted from the clonal culture strain using the benzyl chloride method of Zhu et al. (Citation1993). The amplification primers, PCR protocol and sequencing protocol to obtain almost the complete 18S rDNA used in this study were as described by Takano & Horiguchi (Citation2004), except that we used SR5 5′-ACTACGGCTTTTTAACTGC-3′ and SR8 5′-GGATTGACAGATTGAGAGCT-3′ instead of SR5TAK and SR8TAK, respectively. We sequenced both the forward and reverse strands.
Sequence analysis
The SSU rDNA sequences were aligned manually, based on its secondary structure, available from the European Ribosomal rRNA database server (http://www.psb.ugent.be/rRNA/ssu/index.html). Sequences of 28 additional alveolate taxa were included in the alignment (). We used Noctiluca scintillans as an outgroup. Distance, maximum parsimony (MP) and maximum likelihood (ML) analyses were performed with PAUP version 4.0b10 (Swofford, Citation2002). The distance matrix was calculated using the Kimura two-parameter method (Kimura, Citation1980) and the tree was constructed using the neighbour joining (NJ) method (Saitou & Nei, Citation1987). In the MP analysis, an heuristic search option with the random addition of sequence (1000 replicates) and the tree-bisection branch swapping algorithm (TBR) were used for tree searching. All characters were weighted equally. To explore which model of sequence evolution for ML best fits the data, the program Modeltest version 3.04 (Posada & Crandall, Citation1998) was used. This employs the hierarchical likelihood ratio test (hLRT). The model selected for the ML analysis by hLRT for the data set was TrN + I + G. In ML analysis, the heuristic search was performed with a TBR branch-swapping algorithm, and the NJ tree obtained above was used as the starting tree. The parameters in this analysis were: assumed nucleotide frequencies A = 0.2611, C = 0.1974, G = 0.2561 and T = 0.2854; substitution rate matrix with AC = 1, AG = 3.0819, AT = 1, CT = 7.1261 and GT = 1; proportion of sites assumed to be invariable = 0.3645; rates for variable sites assumed to follow a gamma distribution with shape parameter = 0.661; and number of rate categories = 4. Bootstrap analyses with 1000 replications of data set (Felsenstein, Citation1985) for NJ and MP were calculated to estimate statistical reliability.
Table 1. List of dinoflagellates taxa included in the SSU rDNA phylogenetic analysis
Results
Pileidinium ciceropse Tamura et Horiguchi, gen. et sp. nov. ( – ).
Figs 1–2. Light micrographs of Pileidinium ciceropse, gen. et sp. nov. . Lateral view. The epitheca is pointed toward the dorsal side. . Ventral view. Abbreviations: N; nucleus, Py; pyrenoid.
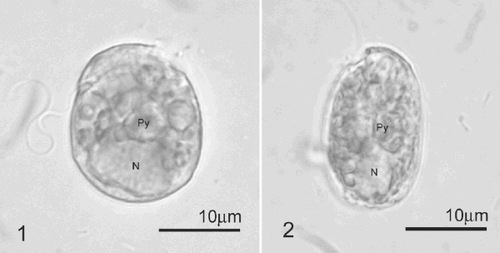
Figs 3–10. SEM images of Pileidinium ciceropse, gen. et sp. nov. . Left lateral view. . Right lateral view. The cell consists of a small epitheca and the large hypotheca. . Dorsal view. . Ventral view. . Apical view. A part of the epitheca is connected with the hypotheca. . Antapical view. . Apical and left lateral view. . Apical and right lateral view. Cingulum is incomplete. The 4′′ and 5′′ plates of the epitheca are connected to the 4′′′ plate of the hypotheca. Arrowhead indicates a circular pore. Scale bars represent 10 µm.

Figs 11–16. SEM and fluorescence microscopy of Pileidinium ciceropse, gen. et sp. nov. . SEM of sulcal plates. . Fluorescence microscopy of part of the epitheca, sulcus and 1′′′ plate. Arrow indicates a circular pore in the epitheca. . Fluorescence microscopy of part of the epitheca and cingulum. . Fluorescence microscopy of sulcal plates except ls. . Fluorescence microscopy of part of the epitheca, cingulum and 3′′′ plate. . Fluorescence microscopy of part of the epitheca and cingulum. Arrow indicates a circular pore.
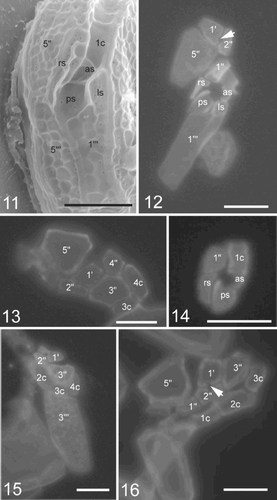
Figs 17–20. TEM of Pileidinium ciceropse, gen. et sp. nov.. . Longitudinal section through the cell. . Longitudinal profile of trichocyst. . Mitochondria with tubular cristae. . Accumulation body. Abbreviations: N; dinokaryotic nucleus, Py; pyrenoid, G; Golgi body, C; chloroplast, m; mitochondria, Ac; accumulation body, Pu; pusule.
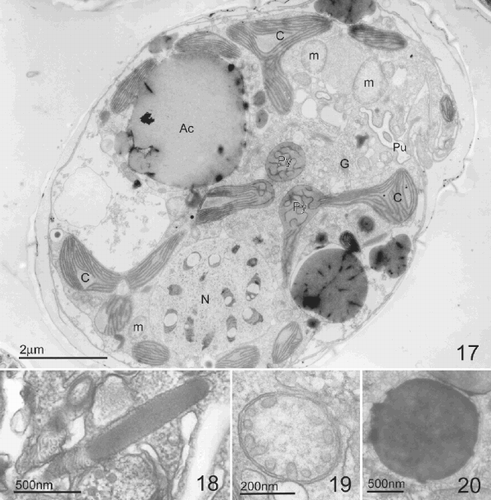
Figs 21–27. TEM of Pileidinium ciceropse, gen. et sp. nov. . Longitudinal section through the cell. Pyrenoid is surrounded by starch grains. . Detail of chloroplast. . High magnification of chloroplast. Arrowheads indicate the triple membranes of the chloroplast boundary. . Sausage-shaped vesicles. . Polyvesicular body. . Vesicles in which filaments radiate in all directions. . Hair containing vesicles. Abbreviations: Py; pyrenoid, G; Golgi body, C; chloroplast, m; mitochondria, s; starch grain.
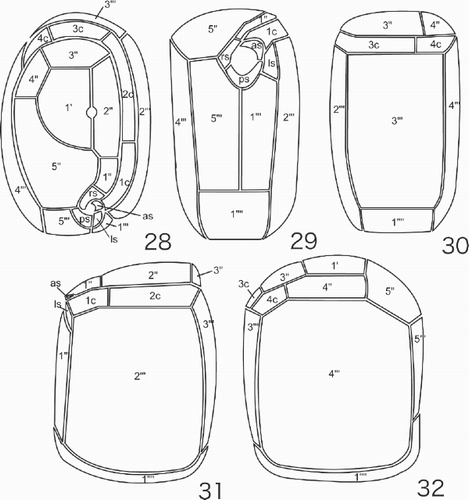
Figs 28–32. Line drawings of the thecal plate arrangement in Pileidinium ciceropse, gen. et sp. nov. . Apical view. . Ventral view. . Dorsal view. . Left lateral view. . Right lateral view.
Pileidinium Tamura et Horiguchi gen. nov. DIAGNOSIS: Cellula armata, ex epitheca parva et hypotheca magna constans, compressaque et cingulo incompleto. Formula scutelli: 1′, 5′′, 4c, 4s, 5′′′, 1′′′′. Flagellatum photosyntheticum.
Cell armoured, consisting of small epitheca and large hypotheca, laterally compressed. Cingulum incomplete; thecal plate formula 1′, 5′′, 4c, 4s, 5′′′, 1′′′′. Photosynthetic flagellate.
TYPE SPECIES: Pileidinium ciceropse Tamura et Horiguchi.
ETYMOLOGY: Based on the Latin word Pileus, meaning a skullcap of felt. This describes the shape of a hooded coat, resembling the shape of the epitheca–hypotheca connection.
Pileidinium ciceropse Tamura et Horiguchi, sp. nov.
DIAGNOSIS: Cellula compressa aspectua ventrale elliptica, et aspectu laterale trapezoideus; 14.0–26.0 µm longa, 10.0–14.0 µm lata, 14.0–20.0 µm profundus (inter dorsum et venter). Laminae 4′′ et 5′′ laminis 4′′′ et 5′′′ consociatae. Epitheca operculiformis, in cursum dorsalem deminuta; nucleus ovalis, in regione postica situs; chloroplastus luteibrunneus; pyrenoides sphaerica, per vaginam amyli obtectaque in parte antica hypoconi sitaque; sine stigmate. Flagellatum marinum, arenariumque.
Cell laterally flattened, elliptical in ventral view and trapezoidal in lateral view; 14.0–26.0 µm long, 10.0–14.0 µm wide, 14.0–20.0 µm deep (between the dorsal and ventral surfaces). Plates 4′′ and 5′′ connected to plates 4′′′ and 5′′′. Epitheca lid-like, tapering in a dorsal direction; nucleus oval, situated in the posterior region; chloroplast yellow-brown; pyrenoid spherical, covered by a layer of starch and situated in the anterior part of the hypocone; without an eyespot. Marine, sand-dwelling flagellate.
HOLOTYPE: An embedded specimen in epoxy resin has been deposited in the herbarium of the Graduate School of Science, Hokkaido University as SAP 097818 (Isotype, SAP 097819).
TYPE LOCALITY: Mechelcher Island (7°13′ N, 134°38′ E), Republic of Palau.
ETYMOLOGY: ‘Ciceropse’ indicates its chick pea-like shape and colour.
The cells are laterally flattened, with an elliptical profile in ventral view and a near-trapezoidal profile in lateral view, (–). The cell is 14.0–26.0 µm long (average: 20.28 ± 2.46, n = 50), 10.0–14.0 µm wide (average: 11.92 ± 1.34, n = 50) and 14.0–20.0 µm (average: 15.36 ± 1.43, n = 50) deep. The epitheca in lateral view is lid-like shaped, with the opening directed towards the ventral side (). The height of the epitheca is about one-seventh the length of the cell. The hypotheca is roughly trapezoidal with rounded upper and lower sides in lateral view, which widen slightly from the ventral to the dorsal side.
The nucleus is ovoid and situated in the bottom of the hypotheca (). The pyrenoid is spherical, surrounded by small starch granules and situated at the centre of the hypotheca (–). The pusule is spherical, lying in the upper part of the ventral side of the hypotheca. The chloroplast is yellow-brown in colour and its lobes radiate from the central pyrenoid (). No eyespot was observed. An accumulation body is sometimes recognizable.
The scanning electron and fluorescence microscopical observations revealed that the thecal plates are delicate and superficially ornamented with fine reticulations (–). Like a hooded coat, part of the epitheca is directly connected to the hypotheca on the dorsal and right lateral side, without any cingulum. The cingulum is incomplete, encircling two-thirds of the cell (, , , , ). The thecal plate formula is 1′, 5′′, 4c, 4s, 5′′′, 1′′′′ (–, –). The first apical plate (1′) is situated at the centre of the epitheca, surrounded by plates 2′′, 3′′, 4′′ and 5′′ (–, ). A circular pore is located between plates 1′ and 2′′ (, , , ). The 5′′ plate is large, bordered by plates 4′′′ and 5′′′ of the hypotheca (, , , , ), while the 4′′ plate is situated between plates 3′′ and 5′′, bordered by plates 4c and 4′′′ (, , ).
The sulcus, which is composed of four small sulcal plates, is very short and narrow (, , , ). A small, crescent-shaped, anterior sulcal plate (as) abuts plate 1c (, , , , ). A large, smooth-surfaced, posterior sulcal plate (ps), shaped like an inverted triangle is located between plates 1′′′ and 5′′′ (, , , , ). This plate is directed toward the cingular plates and is slightly curved along its longitudinal axis (, , , ). The right sulcal plate (rs) lies across the boundary between plates 5′′ and 5′′′ (, , , , ). The left sulcal plate (ls) abuts plates 1′′′, 2′′′ and 1c (, , , ).
The cingulum is composed of four plates, the 4c plate forming the end of the cingulum, bordered by the 3′′and 4′′ plates on one side and by the 3′′′ and 4′′′ plates on the other (, , , , , –).
In the hypotheca, the lateral 2′′′ and 4′′′ plates are large and form the broad faces of the hypotheca (, , –). The rectangular 3′′′ plate is moderately sized, situated between plates 2′′′ and 4′′′ (, , –). The 1′′′ and 5′′′ plates together form the shape of a human lung. The medium-sized, rectangular 1′′′′ plate is slightly-curved, situated at the bottom of the cell, bordered by plates 1′′′, 2′′′, 3′′′′, 4′′′ and 5′′′ (, , , –).
At the ultrastructural level, Pileidinium ciceropse shows typical dinoflagellate features: i.e., a dinokaryon (), a dino-chloroplast (, –) with a pyrenoid (, ), mitochondria with tubular cristae (, , ), trichocysts (), a Golgi body (, ), a pusule () and starch grains (). The chloroplast is single and its lobes radiate from a central pyrenoid towards the periphery of the cell (, ). The chloroplast envelope comprises three membranes, and contains lamellae, each comprising three stacked thylakoids (, ). The pyrenoid, whose matrix is traversed by thylakoids, is located at the centre of the cell and is surrounded by starch grains (). Golgi bodies are found in the area surrounding the pyrenoid (, ). Various kinds of vesicles whose function is unclear are present. These include sausage-shaped vesicles (), polyvesicular bodies (), vesicles in which filaments radiate in all directions (). Hair containing vesicles are also found. They appear to be identical with ‘a flagellar hair-containing vesicle’ described by Dodge (Citation1973) ().
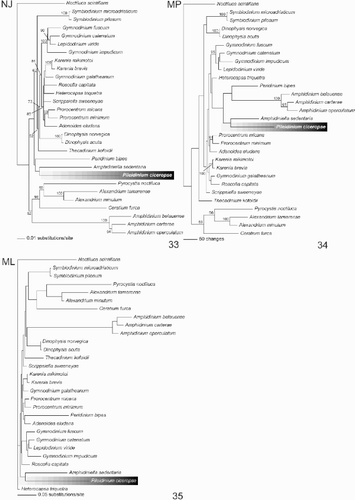
The result of the NJ, MP and ML analyses of the SSU rDNA are shown in –. All trees recovered from the different analyses revealed that Pileidinium ciceropse grouped with Amphidiniella sedentaria, although the bootstrap value was low (<50%). In the NJ tree, P. ciceropse, A. sedentaria and Peridinium bipes formed a clade, but support for this relationship was also weak ( In the MP tree, a clade consisting of P. ciceropse and A. sedentaria formed a sister group to the clade including the Amphidinium-clade and P. bipes. However, once again this relationship was poorly supported (). In the ML tree, P. ciceropse formed a clade with A. sedentaria, which was clearly separated from other taxa ().
Figs 32–35. Phylogenetic trees constructed from SSU rDNA gene sequences. . Distance tree using neighbor joining method based on Kimura's two-parameter distance. The position of Pileidinium ciceropse is highlighted. Numbers at the nodes indicate the bootstrap values (1000 replicates). . MP tree in a heuristic search, showing the phylogenetic position of Pileidinium ciceropse. The numbers at the nodes indicate the bootstrap values (1000 replicates). . ML tree highlighting the phylogenetic position of Pileidinium ciceropse.
Discussion
Many benthic armoured dinoflagellates share distinctive morphological features. These include laterally- or dorsiventrally-compressed cells and a girdle in the upper part of the cell. Furthermore, most of them possess rather unusual thecal plate arrangements compared to planktonic forms, e.g., Adenoides, Cabra, Thecadinium, Sablodinium. The new dinoflagellate described in this paper conforms to this typical benthic armoured form of dinoflagellates.
The plate arrangement of P. ciceropse is unique. The tabulation of P. ciceropse was determined with some difficulty using the Kofoid tabulation system. The cingulum was used as a ‘landmark’ and plates that directly touched the cingulum were designated as either precingular or postcingular plates. The 5′′ plate was unique in that it does not directly abut the cingulum, but seems to belong to the same equatorial series as the 1′′ to 4′′ plates. We therefore interpreted it as part of the precingular series. We found an obscure pore between the 1′ and 2′′ plates (, , ). At this stage, we are not certain whether this pore is homologous with the apical or ventral pore of other dinoflagellates.
There are many benthic armoured dinoflagellates with laterally-compressed bodies, with a small epitheca and a large hypotheca, e.g., Amphidiniopsis, Cabra, Roscoffia, Thecadinium, Thecadiniposis. The benthic Plagiodinium belizeanum Faust et Balech shows the greatest similarity to P. ciceropse in cell shape and thecal plate arrangement (Faust & Balech, Citation1993). Plagiodinium belizeanum has a small, cap-like epitheca, is ventrally flattened and possesses a plate pattern of Po, 5′, 0′′, 5c, 5s, 5′′′, 1′′′′. It has a very similar hypothecal plate arrangement, comprising six plates: large lateral 2′′′ and 4′′′ plates, narrow ventral 1′′′ and 5′′′ plates, dorsal 3′′′ plate and convex antapical 1′′′′ plate. However, the epithecal, cingular and sulcal plate arrangements differ from those of P. ciceropse. Plagiodinium belizeanum lacks precingular plates in the epitheca but has a complete cingulum. Pileidinium ciceropse, on the other hand, possesses an apical plate, five precingular epithecal plates and an incomplete cingulum.
Plagiodinium belizeanum is thought to be related to Thecadinium (Faust & Balech, Citation1993). Within the genus Thecadinium, T. kofoidii (Herdman) Larsen and T. neopetasatum Saunders et Dodge are most similar to P. ciceropse each having the hypotheca mainly covered by two large lateral plates. Thecadinium neopetasatum resembles T. kofoidii in shape, but is slightly larger and more rounded (Saunders & Dodge, Citation1984; Hoppenrath, Citation2000 a). The plate formula of T. kofoidii is P, 3′, 1a, 4′′, 5 (6?) c, 5s, 4′′′, 1′′′′, and that of T. neopetasatum is P, 3′, 1a, 6′′, 6c, ?s, 5′′′, 1′′′′ (Hoppenrath, Citation2000 a). The thecal plate arrangements of both Thecadinium species differ from that of P. ciceropse. Although Thecadinium dragescoi Balech has an incomplete cingulum, its thecal plate arrangement differs from that of P. ciceropse. Because of the presence of an ‘x’ plate and two antapical intercalary plates, the sulcal architecture and its cell shape, the allocation of T. dragescoi to Thecadinium is probably incorrect (Hoppenrath, Citation2000 a; Hoppenrath et al., Citation2004).
Cabra matta Murray et Patterson also has an incomplete cingulum (Murray & Patterson, Citation2004), although C. matta and P. ciceropse share some features, e.g., small epitheca and large hypotheca with two large thecal plates (2′′′ and 4′′′), their epithecal thecal plate arrangement and cingula clearly differ. Furthermore, C. matta differs from P. ciceropse in lacking a chloroplast.
Adenoides eludens (Herdman) Balech (Balech, Citation1956) is similar to P. ciceropse in having a small epitheca and a large hypotheca. According to Hoppenrath et al. (Citation2003), A. eludens has the plate formula, Po, 4′, 0′′, 6c, 4 (5)s, 5′′′, 5 (3)p, 1′′′′ (2′′′′), and is oval to round, slightly flattened in the lateral plane, possesses a small depressed epitheca and an undisplaced cingulum that completely encircles the cell body. Pileidinium ciceropse, on the other hand, is trapezoidal in shape with a bulging epitheca and an incomplete cingulum.
Amphidiniella sedentaria Horiguchi (Horiguchi, Citation1995) is a minute benthic species. In our phylogenetic analysis it formed a clade with P. ciceropse and the two might be closely related. Morphologically, however, A. sedentaria is distinguished from P. ciceropse by its dorsiventrally compressed shape and thecal plate arrangement, Po, 4′, 1a, 7′′, 5c, 4s, 6′′′, 2′′′′ (Horiguchi, Citation1995). It should be pointed out that the bootstrap value for the Amphidiniella and P. ciceropse clade is extremely low and therefore, a close relationship between these species is, at present, uncertain.
Some ultrastructural studies of benthic dinoflagellates are available at present. For the taxa described above, ultrastructural studies are available for A. eludens and A. sedentaria. Adenoides eludens possesses single stalked pyrenoids, as in Peridiniella catenata (Levander) Balech (Hansen & Moestrup, Citation1998; Hoppenrath et al., Citation2003). The pyrenoid matrix of A. sedentaria is occasionally penetrated by cytoplasmic tubules (Horiguchi, Citation1995), while the pyrenoid of P. ciceropse is single, lying at the cell centre and invaded by thylakoids. Ultrastructural studies on benthic dinoflagellates remain limited and too little is available for comparison. More research on this subject is badly needed.
The full complement of features (cellular, ultrastructural and thecal plate arrangement) of P. ciceropse does not correspond with any other known species and we therefore propose that it is a new species. Based on its distinct cell shape, cellular features and thecal plate arrangement, it is evident that the organism described here cannot be assigned to any existing genera, and we believe that it is therefore appropriate to establish a new genus, with Pileidinium ciceropse as the type species.
Because of their unusual thecal plate arrangements, it is difficult to assign some sand-dwelling dinoflagellates to existing suprageneric taxa, including Adenoides, Amphidiniella, Roscoffia, Sabulodinium, Planodinium and Cabra. Hoppenrath et al. (Citation2003) mentioned that the features of Adenoides resemble the Gonyaulacales rather than the Peridiniales. In a phylogenetic study, however, Adenoides was not related to any of the gonyaulacaleans (Saldarriaga et al., Citation2001) although it is included in the Gymnodiniales–Prorocentrales–Peridiniales complex (GPP-complex) (Saldarriaga et al., Citation2003). Horiguchi (Citation1995) suggested that the genus Amphidiniella might belong to the Gonyaulacales, but our molecular study did not support this. These examples indicate that, because of the highly modified thecal plate arrangements, it is quite difficult to determine phylogenetic affinities using morphological data only, and that it is essential to include molecular phylogenetic analyses. Although we were able to sequence the SSU rDNA of P. ciceropse, it was not possible to demonstrate its precise phylogenetic position within the Dinophyceae due to poor resolution at the base of the branches. To overcome this problem, analyses including other genes and more taxa are needed.
Acknowledgements
Our sincere thanks go to Professor Yoshiaki Hara, who gave us an opportunity to participate in the Palau expedition. The authors wish to thank Dr Stuart D. Sym for reading the manuscript. This work was partly supported by a 21st Century Center of Excellence (COE) Program on ‘‘Neo-Science of Natural History” (Program Leader: Hisatake Okada) at Hokkaido University financed by Ministry of Education, Culture, Sports, Science and Technology (MEXT). The work was also financed by Grant-in-Aid (12NP0102, 16370039) from the MEXT.
References
References
- Balech , E . 1956 . Étude des Dinoflagellés du sable de Roscoff . Rev. algol. (N. Ser.) , 2 : 29 – 252 .
- Dodge JD 1973 The Fine Structure of Algal Cells, Academic Press London New York
- Dodge , JD and Lewis , J . 1986 . A further SEM study of armoured sand-dwelling marine dinoflagellates . Protistologica , 22 : 221 – 230 .
- Dragesco , J . 1965 . Étude cytologique de quelques flagellés mésopsammiques . Cah. Biol. Mar. , 6 : 83 – 115 .
- Faust , MA . 1994 . Three new benthic species of Prorocentrum (Dinophyceae) from Carrie Bow Cay, Belize: P. sabulosum sp. nov.P. sculptile sp. nov., and P. arenarium sp. nov. . J. Phycol. , 30 : 755 – 763 .
- Faust , MA and Balech , E . 1993 . A further SEM study of marine benthic dinoflagellates from a Mangrove Island, Twin Cays, Belize, including Plagiodinium belizeanum gen. et sp. nov. . J. Phycol. , 29 : 826 – 832 .
- Felsenstein , J . 1985 . Confidence limits on phylogenes: an approach using the bootstrap . Evolution , 39 : 783 – 791 .
- Hansen , G and Moestrup , Ø. 1998 . Light and electron microscopical observations on Peridiniella catenata (Dinophyceae) . Eur. J. Phycol. , 33 : 293 – 305 .
- Herdman , EC . 1922 . Notes on dinoflagellates and other organisms causing discolouration of the sand at Port Erin . Trans. Liverpool Biol. Soc. , 36 : 15 – 30 .
- Herdman , EC . 1924a . Notes on dinoflagellates and other organisms causing discolouration of the sand at Port Erin. . Trans. Liverpool Biol. Soc. , 38 : 58 – 63 .
- Herdman , EC . 1924b . Notes on dinoflagellates and other organisms causing discolouration of the sand at Port Erin . Trans. Liverpool Biol. Soc. , 38 : 75 – 84 .
- Holmes , MJ . 1998 . Gambierdiscus yasumotoi sp. nov. (Dinophyceae). A toxic benthic dinoflagellate from Southeastern Asia . J. Phycol. , 34 : 661 – 668 .
- Hoppenrath , M . 2000a . Morphology and taxonomy of the marine sand-dwelling genus Thecadinium (Dinophyceae) with the description of two new species from the North German Wadden sea . Phycologia. , 39 : 96 – 108 .
- Hoppenrath , M . 2000b . Morphology and taxonomy of six marine sand-dwelling Amphidiniopsis species (Dinophyceae, Peridiniales), four of them new, from the German Bight, North Sea . Phycologia , 39 : 482 – 497 .
- Hoppenrath , M . 2000c . Morphology and taxonomy of Synophysis (Dinophyceae, Dinophysiales) including two new marine sand-dwelling species from the North German Wadden Sea . Eur. J. Phycol. , 35 : 153 – 162 .
- Hoppenrath , M and Elbrächter , M . 1998 . Roscoffia capitata (Dinophyceae) refound: notes on morphology and biology . Phycologia , 37 : 450 – 457 .
- Hoppenrath , M , Schweikert , M and Elbrächter , M . 2003 . Morphological reinvestigation and characterization of the marine, sand-dwelling dinoflagellate Adenoides eludens (Dinophyceae) . Eur. J. Phycol. , 38 : 385 – 394 .
- Hoppenrath , M , Saldarriaga , JF , Schweikert , M , Elbrächter , M and Taylor , FJR . 2004 . Description of Thecadinium mucosum sp. nov. (Dinophyceae), a new sand-dwelling marine dinoflagellate, and an emended description of Thecadinium inclinatum Balech . J. Phycol. , 40 : 946 – 961 .
- Horiguchi , T . 1995 . Amphidiniella sedentaria gen. et sp. nov. (Dinophyceae), a new sand-dwelling dinoflagellate from Japan . Phycol. Res. , 43 : 93 – 99 .
- Horiguchi , T and Chihara , M . 1983 . Stylodinium littorale a new marine dinococcalean alga (Pyrrophyta) . Phycologia , 22 : 23 – 28 .
- Horiguchi , T and Chihara , M . 1987 . Spiniferodinium galeiforme a new genus and species of benthic dinoflagellates (Phytodiniales, Pyrrophyta) from Japan . Phycologia , 26 : 478 – 487 .
- Horiguchi , T and Pienaar , RN . 1994 . Ultrastructure of a new marine sand-dwelling dinoflagellate Gymnodinium quadrilobatum sp. nov. (Dinophyceae) with special reference to its endosymbiotic alga . Eur. J. Phycol. , 29 : 237 – 245 .
- Horiguchi , T , Yoshizawa-Ebata , J and Nakayama , T . 2000 . Halostylodinium arenarium, gen et sp nov (Dinophyceae), a coccoid sand-dwelling dinoflagellate from subtropical Japan . J. Phycol. , 36 : 960 – 971 .
- Kimura , M . 1980 . A simple method for estimating rate of base substitutions through comparative studies of nucleotide sequences . J. Mol. Evol. , 16 : 111 – 120 .
- Larsen , J . 1985 . Algal studies of the Danish Wadden Sea II. A taxonomic study of psammobious dinoflagellates . Opera Botanica , 79 : 14 – 37 .
- Murray , S and Patterson , DJ . 2004 . Cabra matta, gen nov., sp. nov., a new benthic, heterotrophic dinoflagellate . Eur. J. Phycol. , 39 : 229 – 234 .
- Posada , MA and Crandall , KA . 1998 . Modeltest: testing the model of DNA substitution . Bioinformatics , 14 : 817 – 818 .
- Saitou , N and Nei , M . 1987 . The neighbor-joining method for reconstructing phylogenetic trees . Mol. Biol. Evol. , 4 : 406 – 425 .
- Saldarriaga , JF , Taylor , FJR , Keeling , PJ and Cavalier-Smith , T . 2001 . Dinoflagellate nuclear SSU rDNA phylogeny suggests multiple plastid losses and replacements . J. Mol. Evol. , 53 : 204 – 213 .
- Saldarriaga , JF , Leander , BS , Taylor , FJR and Keeling , PJ . 2003 . Lessardia elongata gen. et sp. nov. (Dinoflagellata, Peridiniales, Podolampaceae) and the taxonomic position of the genus . Roscoffia. J. Phycol. , 39 : 368 – 378 .
- Saunders RD Dodge JD 1984 An SEM study and taxonomic revision of some armoured sand–dwelling dinoflagellates Protistologica XX, fasc. 2 271 283
- Swofford DL 2002 PAUP* – Phylogenetic analysis using parsimony (* and other methods) Version 4. Sinauer Associates Sunderland Massachusetts
- Takano , Y and Horiguchi , T . 2004 . Surface ultrastructure and molecular phylogenetics of four unarmoured heterotrophic dinoflagellates, including the type species of the genus Gyrodinium (Dinophyceae) . Phycol. Res. , 52 : 107 – 116 .
- Ten-Hage , L , Turquet , J , Quod , JP and Couté , A . 2000 . Coolia areolata sp. nov. (Dinophyceae), a new sand-dwelling dinoflagellate from the southern Indian Ocean . Phycologia , 39 : 377 – 383 .
- Toriumi , S , Yoshimathu , S and Dodge , JD . 2002 . Amphidiniopsis uroensis sp. nov. and Amphidiniopsis pectinaria sp. nov. (Dinophyceae): two new benthic dinoflagellates from Japan . Phycol. Res. , 50 : 115 – 124 .
- Yoshimatsu , S , Toriumi , S and Dodge , JD . 2004 . Morphology and taxonomy of five marine sand-dwelling Thecadinium species (Dinophyceae) from Japan, including four new species: Thecadinium arenarium sp. nov.Thecadinium ovatum sp. nov.Thecadinium striatum sp. nov. and Thecadinium yashimaense sp. nov. . Phycol. Res. , 52 : 211 – 223 .
- Zhu , H , Qu , F and Zhu , L-H . 1993 . Isolation of genomic DNA from plants, fungi and bacteria using benzylchloride . Nucl. Acids. Res. , 21 : 5279 – 5280 .