Abstract
High densities of diatoms were found attached to sand grains down to half a metre below the sediment surface (6.5 g chl a m−2) on an exposed beach (Lough Neagh, N. Ireland). However, seasonal variation of biomass was low (11–21 µg chl a g−1 dry sand). Sand movement was the main controlling factor but silica and nitrate limitation also contributed during summer months. Laboratory assays showed that nutrients were derived mostly from the overlying water. Two species dominated but with very different strategies for coping in this abrasive environment. Cymbellonitzschia diluviana was small, low growing and aggregated in depressions whereas the larger, non-motile and stalked Martyana martyi was dispersed over the most exposed surfaces. Their contrasting distributions are related to their different structure and modes of cell division. However, one adaptation common to both species was the ability to form resting cells. We investigated the environmental factors controlling the formation and recovery of resting cells using both chloroplast shape and fluorescence indicators. Both species showed a gradual change in and out of the resting state, which enabled them to compromise between the need to conserve energy when buried but recover rapidly when brought back to the surface by wave action and water level changes.
Introduction
Sandy shores are exposed habitats characterized by an unstable substrate. In 1965, Round pointed out how little was known about the algal communities that live on sand. His report was followed by a number of studies describing problems of attachment, production and burial in both freshwater (Moss & Round, Citation1967; Harper & Harper, Citation1967; Harper, Citation1969; Hickman & Round, Citation1970; Moss, Citation1977) and marine sands (Meadows & Anderson, Citation1968; Steele & Baird, Citation1968; Steele & Munro, Citation1970; Cadee & Hegeman, Citation1974). Since then, more has been learnt about species composition (e.g. Kingston et al., Citation1979; Stevenson & Stoermer, Citation1981; Edlund & Stoermer, 1996), life cycles (Jewson & Lowry, Citation1993) and aspects of colonization, especially in running waters (Pringle & Bowers, Citation1984; Krejci & Lowe, Citation1986; Miller et al., Citation1987). However, we still know relatively little about the ecology of individual species and their specialized adaptations for living on sand.
A key feature of the psammic habitat is the force of wave action and its impact on sediment movement. One of the few interdisciplinary studies to investigate this was initiated by Steele & Baird (Citation1968) and Steele et al. (Citation1970). They found viable cells buried to a depth of 0.2 m below the sand surface in a Scottish sea loch but were unable to explain fully why there was negligible pigment degradation. A similar situation was found in a large lake, Lough Neagh, where high concentrations of live cells were found attached to sand grains down to half a metre below the sand surface (Jewson & Briggs, Citation1993). A distinctive feature of this habitat was the presence of multiple longshore sand bars formed by the interaction of wave action and water level changes (Carter & Balsillie, Citation1983; Carter & Carter, Citation1983; Carter, Citation1993). So, one aim of this study was to investigate the significance of sediment movements in causing the unusual depth distribution of epipsammon. Nutrient availability was also investigated, because Happey-Wood & Priddle (Citation1984) had found that growth of benthic diatoms in organic sediments coincided with the ‘minimum’ concentrations of silica in the overlying water. They concluded that diatoms were obtaining silica, and probably other nutrients, from the sediment or interstitial water. While this may be true in organic or fine sediments, it is not known if it applies to sandy habitats, which are more disturbed and in which cells live attached to grains that are often lifted into the water as a wave passes.
A second aim was to investigate the specialized adaptations required to survive and flourish in such an unstable, abrasive environment. For this study, two diatom species were selected that are co-dominants on the exposed sand bars in Lough Neagh (Jewson & Briggs, Citation1993). Cymbellonitzschia diluviana Hustedt has small, low-growing cells that can move, but rarely do so (Jewson & Lowry, Citation1993). They are frequently found in dense aggregations in depressions or grooves that offer some protection (Jewson & Lowry, Citation1993). In contrast, Martyana martyi (Hérib.) F.E. Round (synonyms Opephora martyi Hérib. and Fragilaria martyi (Héribaud) Lange-Bertalot) has cells that are thick-walled, upright and non-motile yet are dispersed at low densities all over grains, particularly on raised surfaces (Witkowski et al., Citation1996). Although differing in their structure and behaviour, both species possessed the ability to form resting cells. This is potentially important in a habitat where burial periods are highly variable and may last from days to months. In this study, the factors controlling resting cell formation, duration and recovery were investigated in relation to the demands of living on sand grains.
Materials and methods
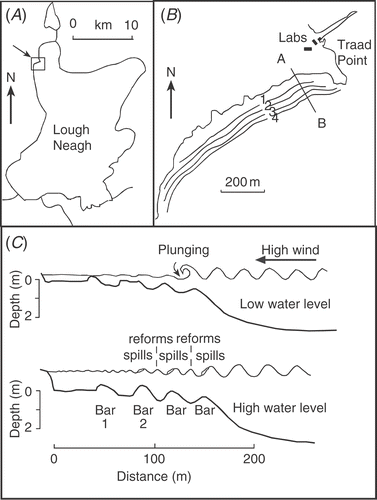
Bottom profiles were determined weekly at right angles to the shore along a fixed transect 150 m west of Traad Point () during 1987 and 1988, with less intensive sampling until 1996. The transect was marked with galvanized metal posts at 20-m intervals. Depths were recorded every metre along a measuring tape suspended between the posts. When water levels became too high for wading, readings were supplemented by bottom profiles using an echo sounder (Model X-16 Lowrance Electronics Inc., Tulsa, Oklahoma, USA). Water levels were recorded at a fixed station in a boathouse about 300 m from the site and calibrated against the continuous water level recording at Toome (Hydrometrics Unit, Department of Agriculture Northern Ireland). Cores were taken using thin-walled (3 mm) plastic tubes (7 cm diameter) pushed into the sand by hand. All cores were taken from the crest of bars unless stated otherwise. The cores were then returned to the nearby laboratory () and extruded in a darkened room. A rubber bung was placed on the surface of the sand to prevent any slumping or disturbance when the water above the core was decanted. The bottom bung was removed next and, by holding the tube at 45°, the core was slid carefully into half of a split length of tube of the same diameter. Extrusion in this way kept the core intact with little water loss. It was then sectioned at the appropriate depths and subsamples (approx. 1 cm3) taken from the centre of the main core with a modified Size 7 cork borer (11 mm diameter). For pigment analysis, sand was placed in 10 ml of 90% methanol and analysed following the method of Marker et al. (Citation1980). For analysis of phaeopigments, 10−3 M HCl was used in the acidification step. After analysis, the sand was dried and weighed. Results could then be expressed either per unit volume or per g sand. To test the frequency of disturbance, two layers of coloured sand were buried at 0.04 and 0.18 m depth below the sand surface in an area of approximately 10 m2 on the crest of Bar 2 on 28 May 1987. The original sand was replaced on top of each layer. During the following 12 months cores were taken to determine whether the layers had been disturbed.
Fig 1. Site map of (A) Lough Neagh, N. Ireland and (B) the position of the four main longshore bars at Traad Point on the NW shore (54°43′N, 6°31′W). The line A–B marks the position of the fixed transect for bottom profiles and sand sampling, (C) diagram of bars and wave characteristics along the transect A–B in low and high water levels.
Autofluorescence of cells on sand grains was measured in a Turner Filter Fluorometer (Model 111, Amsco Instrument Turner, California, USA). Sand grains were held in a modified Pasteur pipette that was fitted vertically into a specially modified holder of the high sensitivity door. Sand grains were placed in the broader section of the pipette (diameter 5 mm) and then filtered lake water was added above. Grains were evenly packed into the narrower section (diameter 1 mm) by gently tapping the glass as they fell. Excess water was then removed from above the sand. One advantage was that the sand grains were relatively uniform in size, from 150 to 350 µm, which aided good packing and avoided ‘jamming’ in the pipette. Four readings were then taken for each sample, with the pipette turned by 90° each time. Standard deviation was generally below 5% of the mean. After these initial measurements, the pipette was carefully flushed with 10−5 M 3(3,4-dichlorophenyl)-1, 1-dimethyl urea (DCMU), which left the grains in place for re-reading. Afterwards, the sand was easily removed from the pipette by turning it upside down and flushing with a jet of water. A blank of cleaned sand and Milli-Q water was used between samples. All samples were dark adapted before reading. The results were recorded on chart paper and then used to calculate the ratio Fv/Fm (Cullen, Citation1982; Falkowski & Kolber, Citation1995; Cullen & Davis, Citation2003) where Fm was the fluorescence after addition of DCMU and Fv = Fm − Fo. Fo was the initial dark-adapted fluorescence.
Resting cell recovery experiments used a thin layer (1–2 grains depth) of sand in 100-ml conical flasks with 50 ml filtered lake water added. For dark recovery, flasks were wrapped in tin foil and then placed in a dark, orbitally shaken incubator. For analysis, sand was removed with a pipette under low infrared light. For light recovery, flasks were placed in an orbitally shaken, illuminated incubator (Gallenkamp, UK) that was kept at ambient lake temperature and 30 µmol m−2 s−1 on an 8:16 h light:dark cycle. For microscopic examination of cells, a layer of sand one grain thick was placed on a slide with filtered lake water and coverslip. Live, resting or dividing cells were observed in situ in both transmitted and epifluorescent light (Leitz Dialux 20 EB epifluorescent microscope). It was possible to use the same sand from the fluorescence measurements, with the advantage that autofluorescence of cells was enhanced and stable for longer after DCMU addition. Sand grains for SEM observation were first air-dried and then attached to a brass stub with double-sided tape. They were then sputter coated with gold and observations made using a JEOL JSM 840 operating at 25 Kv.
Hydrogen sulphide concentration of interstitial water was determined using a colorimetric method with N,N-dimethyl-p-phenylenediamine (Anon, Citation1982). Redox measurements were made with platinum and calomel reference electrodes (see Hargrave, Citation1972).
For nutrient assays, a thin layer of sand was covered by 50 ml of filtered lake water (taken from above the bars) in 100-ml conical flasks, which were placed into an orbitally shaken, illuminated incubator (Gallenkamp, UK) that was kept at ambient lake temperature and 30 µmol m−2 s−1 on an 8:16 h light:dark cycle. Selected nutrients (Si, N, P) were added singly and in combination (Si + P, Si + N, N + P, Si + N + P) in the form of K2HPO4, NaNO3 and NaSiO3 to give final concentrations of 34 µM, 350 µM and 200 µM respectively. The proportion of dividing to non-dividing cells of Martyana martyi was determined on 100 individuals at the beginning of each assay and after 3 days. Dividing cells were those in which there was a clear cell wall between daughter cells, or they were still attached but ‘hinging’ before separation. Water samples for silicate, phosphate and nitrate determinations were taken weekly in the middle of the lake and every 2 weeks over the sand and in the bay offshore (1 km). Analyses were as described in Gibson (Citation1981) and Gibson & Fitzsimons (Citation1982).
Irradiance was measured with a Li-Cor Ll-190SB Quantum sensor (Li-Cor Inc., Nebraska, USA.; Jewson, Citation1993). Diatom samples were prepared for counting using standard methods (Battarbee, Citation1986). Cells were removed from the sand with 30% hydrogen peroxide in a water bath at 90°C (Renberg, Citation1990), and then washed with distilled water and a weak solution of ammonium added to the final wash. Diatom suspensions were settled and dried onto cover slips and mounted using Naphrax mounting medium; both broken and whole frustules were counted but, unless stated, only whole frustules are reported in this study.
Site
The study area () was a system of multiple sand bars in Lough Neagh, N. Ireland, which is a large (383 km2), relatively shallow, eutrophic lake (see Wood & Smith, Citation1993), with fetches up to 28.5 km (Hueston, Citation1993). The shoreline is predominantly rocky but there are long stretches that are sandy (Jewson & Briggs, Citation1993) and many of these have multiple longshore bars running parallel to the coast (Carter & Balsillie, Citation1983). The study site is 150 m west of Traad Point on the NW shore (54°43′N, 6°31′W). The beach is 1 km in length and adjacent to the University of Ulster Freshwater Laboratory (), which closed in 1999. Offshore and inshore wave and wind characteristics at the time of this study were analysed by Hueston (Citation1993) and changes in wave height and bottom shear stress have also been studied at a nearby site by Carter (Citation1993) and Carter & Carter (Citation1983). During calm weather and lower water levels, the innermost bar often broke up and re-orientated into a series of transverse shoals at right angles to the shore (Carter & Balsillie, Citation1983; see also Carter, Citation1988, fig. 60C, D). During storms, waves reaching the beach were typically 0.2–0.5 m in height but occasionally reached 1 m. The sand was largely glacial in origin and had a relatively uniform size, mostly between 0.125–0.250 mm, with 75% fine sand and 25% medium sand (Hueston, Citation1993). The only macrofauna found on the more exposed bars were larvae of a chironomid, Stictochironomus sticticus, which feeds on detritus settling on the sand surface. A more complex assemblage of chironomid larvae and oligochaetes occurred in the sheltered areas inshore of the bars and in the troughs (Carter & Carter, Citation1983).
Results
Sand habitat and sediment movements
Between 1986 and 1996, there were usually four sand bars running parallel to the shore during winter () but this increased to six during very high water levels (above 12.8 m O.D. Belfast) and reduced to three during low water in summer (below 12.5 m O.D. Belfast). These large changes resulted from a complex interaction of physical factors but, during this study, three main processes were identified that affected the epipsammic community (see summary in ).
-
Spilling waves – the shallow onshore slope off Traad Beach favoured multiple breaking, where waves ‘spill’, i.e. losing 75–95% of their energy before reforming and breaking (‘spilling’) again on the next inner bar (). The waves lost energy gradually as they passed over each bar, which raised plumes of sand into the water and caused movement of surface layers by sand rippling. The frequency of ripples on Traad Beach was usually between 12 and 16 m−1 but with a range between 8 and 20 m−1.
-
Plunging waves – these mainly occurred during lower water levels and high onshore winds. They lost all of their energy at the point of wave breaking, which caused scouring and erosion of a bar, with the undertow depositing sand offshore (see ).
-
Shifts in wave ‘break point’ occurred when there were changes in water level during onshore winds and particularly affected deeper layers of sand by changing the height and position of bars. The degree of change in the bars depended on the size and duration of the water level change and the duration and energy of the waves (see below). Water levels in the lake were regulated and mostly kept within statutory control limits of 12.46 and 12.61 m (O.D. Belfast) but, during periods of prolonged rainfall, the capacity of the outflow was sometimes exceeded and levels rose by 0.5–1 m once or twice a year (e.g. 13.2 m in January 1988). Short-term changes in water level due to seiches also had some effect, especially on the inner bars in low water. Rises in water level were occasionally 0.30 m but were usually less than 0.15 m.
The net effect of these processes was frequent surface disturbance (0–0.05 m) and longer-term shifts in bar height and position that caused exchange of sand grains between the surface and deeper layers. The bottom profiles in illustrate the extreme positions of bars, which moved inshore and offshore by up to 20 m. However, movements were usually less than 5 m during any one year. also shows how much sand was moved off the bar platform and down the outer slope when water levels were low. The sand was later returned up the slope and onto the bars again during higher levels. This effectively retained sand within the site. A good example of how the different physical processes interact occurred during a storm in autumn 1993. Bottom profiles were taken before and after strong winds that lasted for two days () when water levels were low (12.41 m O.D.). The largest waves lost most of their energy as plunging waves on the outer bar (). The effect was to lower the crest of Bar 4 by about 0.14 m, with much of its sand eroded offshore, i.e. down the slope outside the breaker zone. This happened because, when the plunging waves collapsed, they curled over and created a jet of water that hit the sand surface and carried the dislodged sand offshore in the undertow. At the same time as this was occurring, medium and smaller waves passed over the outer bar or lost only some of their energy as spilling waves, before reforming and spilling again on each of the inner bars (e.g. ). This shifted bars inshore, as sand was pushed over the crest, mainly by rippling, and bars became wedge-shaped in profile, with the gentler slope on the offshore side and the steeper slope inshore (). In two days of storms, Bar 2 moved inshore by about 3 m and Bar 1 by 2.5 m. Bar 2 also had a large net gain of sand (approx. 23%), representing over 500 tonnes on this single beach. Most of the extra sand came from Bar 3, which remained in the same position but its height dropped by about 0.09 m, losing about a third (35%) of its sand. The net loss occurred because it was not being re-supplied from further offshore.
Fig 2. Bottom profiles along the fixed transect on 19 February 1988 (broken line) and 14 June 1990 (solid line). Bars in June 1990 are in positions that were troughs in February 1988. Note that the different horizontal and vertical scales exaggerate the slope.
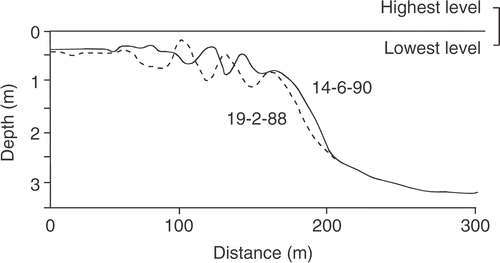
Fig 3. Bottom profiles of sand bars on (A) 26 August (solid line) and 9 September (broken line) 1993 and (B) 26 August (solid line) and 20 November (broken line) 1993.
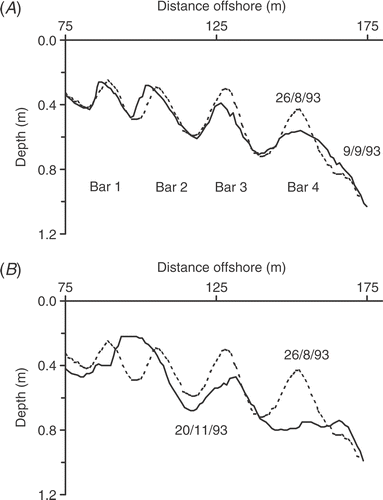
The bars then remained in largely the same positions for the next three months, because water levels remained low (12.46–12.53 m). However, there were two periods of strong, onshore winds in mid November. The first of these was a brief storm (8 November) that was followed by four days of wind (16–19 November). A profile on the day after () showed that Bar 4 had been almost completely erased by plunging waves, with sand shifted offshore and down the slope outside of the breaker zone. Again, smaller waves had passed over Bar 4 and spilt on Bar 3, causing bottom rippling and further transport of sand inshore. Bar 2 could not grow vertically because of the shallow water depth and so had begun to break up. However, conditions then changed when there was a prolonged period of wet weather and a rise in water level that continued until March the following year. During this time, the continuous bars reformed parallel to the shore. This included sand that had been eroded offshore beyond the wave-break point in the earlier storms and was now returned up the slope and back onto the bars by wave action. Such irregular periods of movement and stability were characteristic of the sand environment throughout the study. As an independent test of disturbance, two layers of coloured sand were buried on Bar 2 at 0.04 and 0.18 m on 28 May 1987. The layer at 0.04 m was dispersed in a few weeks but the deeper layer stayed intact for 12 months (10 May 1988), with viable cells beneath it that showed little reduction in chlorophyll a.
Light environment
Light was attenuated rapidly in the overlying water, with surface values reduced to 1% by 2–3 m (Jewson, Citation1993; Jewson & Briggs, Citation1993). This meant that much of the sand bar habitat was in the light zone, except during high water levels or turbidity caused by onshore winds. Also, because grains were relatively free of organic matter, some light penetrated below the sand surface. Incident light was reduced to 1% within approximately 3–5 mm of the sediment surface but this meant that some growth was possible by cells (on the crests of bars) down to a depth of about 4–5 sand grains. However, surface sand was rarely static. During spilling waves, surface layers were continually being turned over by rippling, with some grains lifted into the water in a plume and experiencing a rapidly changing light climate.
Distribution on sand grains: cell form and division
Diatom cells were found predominantly in depressions on the surface of sand grains or in areas that offered some protection (see ). In a seasonal study over 2 years (1987/1988), Cymbellonitzschia diluviana made up 45% of the total diatom population (SD ± 10, n = 53), with concentrations of 1.99 × 106 cells g−1 dry sand. The mean cell size was relatively small (110 µm3) so that, when expressed as cell volume, the species was only 24% of the total diatom biovolume.
Fig 4. Scanning electron micrographs of sand from Traad Beach. (A) Sand grain showing most cells in grooves, except Martyana martyi. Scale bar: 100 µm. (B) Cells and stalks of M. martyi. Scale bar: 10 µm. (C) Cymbellonitzschia diluviana. Scale bar: 1 µm.
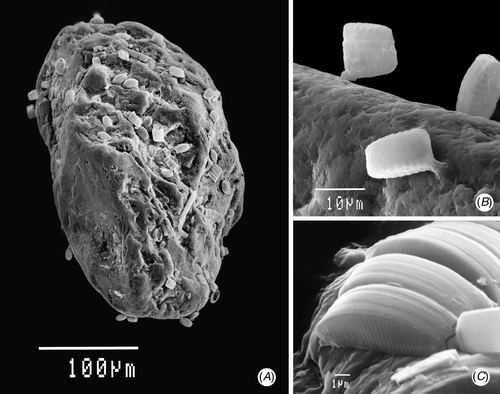
Cymbellonitzschia diluviana was low-growing (see ), with the dorsal margin of the frustule (i.e. ‘height’ or transapical dimension) projecting only 3–4 µm above the surface. The longer, ventral side of the frustule lay parallel to the grain surface. This aided attachment, because the raphe structures that secreted the mucilage for attachment ran along the outside edge of the ventral side of both valves (see , ). This arrangement also helped during division, because each of the parent valves were able to remain securely attached to the surface while the new valves of the two daughter cells were formed between them (), so minimizing losses from being swept away or dislodged by abrasion. Daughter cells of subsequent divisions usually remained alongside each other, if there was enough space (). Therefore, cell numbers built up in more favourable, ‘protected’ areas. Even so, one indication of the abrasive conditions is the proportion of broken valves. In a typical example, 28% of C. diluviana cells on 20 Jan 1987 were broken, having had the upper parts of their frustule sheared off leaving the ventral ‘bases’ still attached in their original rows.
Fig 5. Diagram (not to scale) of how the stalked species Martyana martyi divides (A), the daughter cells ‘hinge’ to bridge the gap between grains (B), and finally separate (C). This contrasts with Cymbellonitzschia diluviana: (D) side view of cell and secreted mucilage from the raphe (along the ventral side); (E) cross-section of cell before and after division, showing position of mucilage attachment and arrangement of the flattened chloroplast; (F) view from above showing shape of chloroplasts in both normal and resting cells.
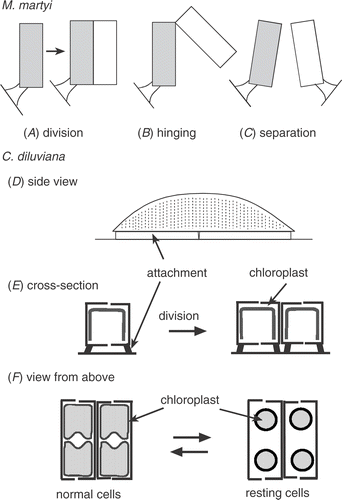
The spatial distribution of the co-dominant species, Martyana martyi, was quite different. Its cells projected out from the surface by 10–20 µm () and were rarely found aggregated, occurring at low concentrations all over grains, especially on raised areas. In this study, M. martyi made up 16% of the diatom cells present on grains over one year (SD ± 7, n = 53), with mean cell concentrations of 0.63 × 106 cells g−1 dry sand. When expressed as cell volume, its proportion of the total diatom population was 37%, because of its larger cell size (mean 495 µm3). An important reason for its spatial distribution was what happened after cell division. This was determined by directly observing live cells. One daughter cell remained attached to the grain by the original stalk while, at the opposite end, the mucilage pad joining the two daughter cells acted like a hinge (). The free end of the second cell was then able to swing out and bridge across to attach to an adjacent sand grain. The two cells then separated. However, if there was no other surface immediately within range, cells remained in the hinged position. In this way, cells can disperse without the need to be motile. It also meant that cells were more likely to attach to more raised parts of adjacent grains. In the deeper layers of sand, the mucilage stalk attachment of M. martyi became much weaker and cells were easily dislodged. This is not necessarily a disadvantage, because it may aid wider dispersal when the sand is exposed again.
Other diatom species that were characteristic of the sand flora and present all year round at exposed sites included Cocconeis disculus Schumann (9%), Navicula scutelloides Wm. Smith (5%), Amphora ovalis var. pediculus Kütz. (5%) and Achnanthes clevei Grun. (2%). Also present, but rare on the most exposed sites, was Amphora calumetica (B. W. Thomas ex Wolle) Perag. Apart from diatoms, some single-celled and colonial cyanobacteria (1–2 µm cell diameter) and green algae were present but in low concentrations at the surface of exposed sites. However, although still in relatively low concentrations, they survived better than diatoms in the deepest layers. In laboratory isolations that were left unshaken (i.e. less abrasion), they rapidly outgrew the diatoms.
Nutrients in overlying water
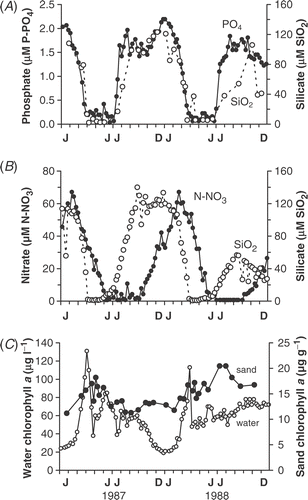
Silicate, nitrate and phosphate concentrations were measured in the water over the bars as well as in the bay immediately offshore and in the middle of the lake (). The timings of spring decline and late summer increase were broadly similar at all three sites, as indicated by silicate concentrations in the water over the bars () and in the middle of the lake (). Although there were occasional brief inputs from small streams draining into areas inshore of the bars (e.g. 9 November 1988), the water chemistry over the bars was similar to offshore for most of the year. Changes in phosphate concentration at all sites showed a similar pattern to silicate but the summer decline in nitrate occurred later and remained undetectable from July–September () due to uptake by planktonic cyanobacterial populations throughout the summer (over 50 µg chl a l−1, ).
Fig 6. Seasonal variation in concentrations of: (A) inshore silica (open circles) and open lake phosphate (filled circles); (B) open lake silica (open circles) and nitrate (filled circles); (C) chlorophyll a in the open lake (open circles) and on surface sand from Bar 3 (filled circles). The silica and nitrate data for the open lake were supplied by Aquatic Science Division, Department of Agriculture Northern Ireland.
Nutrient assays
Nutrient assays were carried out using additions of the three nutrients that declined to undetectable concentrations in the water column (i.e. silicate, phosphate and nitrate). Addition of vitamins (B1, B12 & Biotin) was also tested but did not enhance growth either in assays or cultures (data not shown). Response was monitored using the number of dividing cells of the dominant species (by cell volume) Martyana martyi (). These could be easily recognized because of the process of ‘hinging’ (see above), where the normally solitary cells did not separate immediately after division. When actively growing, between half and three quarters of cells were hinged in this way, whereas during nutrient limitation the proportion fell to between 6 and 12% of cells (see controls in ).
Table 1. Relative increase in the number of dividing cells of M. martyi after 3 days’ incubation in 8:16 h light:dark cycles with both single and combined nutrient additions. The results are expressed as multiples of the control (percentage of dividing cells in lake population at start of assay)
A preliminary assay was carried out in August 1987 using silicate alone. The proportion of cells dividing in the lake at this time was low (6%) but increased to 60% within 3 days of silica addition. This sharp increase occurred because most cells were held up in an extended, pre-division state. When silica was supplied, they formed cross walls between the daughter cells and divided. A complete series of assays was begun in September (), when silicate and phosphate concentrations were increasing but nitrate had become undetectable (, ). The addition of nitrate resulted in large increases in dividing cells () and continued to stimulate growth until late October when nitrate became detectable again in the water (). In the following winter (e.g. 26 February 1988), when all nutrient concentrations were high (, ), there was no change with added nutrients. This was still the case by early spring (11 March 1988), when 73% percent of M. martyi cells were dividing but 13 days later, on 24 March 1987, silicate in the water above the bars was down to 8 µM (due to uptake by planktonic diatoms) and addition of silicate, either singly or in combination, stimulated growth significantly ().
However, silica limitation was temporarily lifted when some of the declining plankton crop settled onto the surface of the bars. Analysis of the water over the bars showed an increase in silica concentration to 37 µM that did not occur at the two offshore sites (, ), i.e. the rise was very localized in time and space. Assays on 8 April 1988 showed a smaller but significant response to added silica. The settled material was then resuspended by onshore winds and spilling waves on 13 and 14 April. Follow-up assays with silica additions on 15 April resulted in an increase of dividing cells from 21–66%, so silica limitation had returned and this was still evident on 29 April (). By 20 May, the number of dividing cells in the lake was down to 12%, with most cells in the pre-division state described above, so that, when supplied with silica, the number of cells dividing increased rapidly to 76% (). In the lake, the number of dividing cells on the sand continued to decline, remaining at around 5% during the rest of summer.
Chlorophyll fluorescence was also monitored in the assays and was effective in detecting silica limitation but was less sensitive than the growth assay in detecting nitrogen limitation, where shorter incubation times would probably be needed.
Seasonal changes in biomass
Chlorophyll a concentrations on the surface of the sand showed relatively little seasonal variation. For example, during 1987 and 1988 the mean values on Bar 3 were 14.9 µg chl a g−1 dry sand (n = 34), with a range from 11 to 21 µg chl a g−1. This contrasted with the much larger changes taking place in the water column, from 25 to 131 µg chl a l−1 in 1987 and from 21 to 113 µg chl a l−1 in 1988 (). One major difference between the two habitats was the size of the spring peak. In 1987, chlorophyll a concentrations on the sand increased by only 54% (i.e. from 11 to 17 µg chl a g−1 between 26 January and 23 April) before silica limitation. There was a further small rise on 9 May 1987 but this was due to a brief settlement of the collapsing plankton crop (cyanobacteria and diatoms). In the spring of the following year (1988), the increase in chlorophyll was also small, from 12 to 17 µg chl a g−1 between 4 February and 23 April, when silica was again limiting (See ; ). However, later in the year, biomass changes were similar in both habitats (), with a gradual decline in summer 1987 and a gradual rise in summer 1988, controlled by nutrient supply. By contrast, in winter, biomass levels were maintained on the sand while they dropped from 60 to 21 µg chl a l−1 in the water column ().
Depth distribution of biomass
The depth distribution of chlorophyll a was measured on 33 occasions during 1987 and 1988. The results are summarized in . The greatest depth distribution of chlorophyll a was found on Bar 3, where there was little reduction from the surface down to 0.35 m and then a slow decline to about a third of surface values by 0.5 m (). Bars 2 and 4 showed greater changes, with chlorophyll concentrations gradually declining to nearly half the surface values by 0.40 m (, ). This was because the deeper layers were less frequently disturbed than Bar 3 but for different reasons. In the case of the outer one (Bar 4), it effectively became ‘fossilized’ when the water depth above it increased beyond 1.5 m (e.g. February 1988). In contrast, in low water levels, this bar could virtually disappear, because it was eroded by plunging waves () and its sand transported further offshore. Bar 2 was affected in different ways. It was the furthest inshore (i.e. least wave energy) and was the shallowest of the three bars sampled (usually between 0.22 and 0.35 m), so it had slightly higher surface concentrations of biomass (17.4 µg chl a g−1 dry sand; ). However, it was the most variable in height, being built up during high water and decapitated in low water levels (, ). Overall, Bar 3 had the highest biomass per unit area, with 6.5 g chl a m−2 (mean annual integral), compared with 5.6 g chl a m−2 on Bar 2 and 4.4 g chl a m−2 on Bar 4.
Fig 7. Depth distribution below the sand surface of mean chlorophyll a concentration (µg g−1 dry sand) during 1987 and 1988 on (A) Bar 2, (B) Bar 3 and (C) Bar 4. Phaeopigment values (open circles) are included for Bars 3 and 4. The error bars are standard errors of the mean (n = 20, 34 & 40 for Bars 2, 3 & 4, respectively).
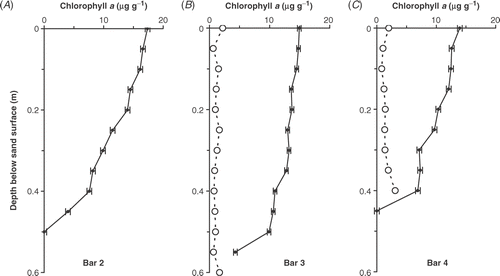
The concentrations of phaeopigment were generally low, between 0.4 and 1.7 µg g−1 but, in the deepest layers, concentrations increased (up to 3 µg g−1) when conditions became reducing and the sulphide concentration increased. Generally, sulphide concentrations were undetectable (below 0.01 mM) in the upper layers (0–0.25 m) but increased in deeper layers. For example, on 4 April 1989 concentrations were 0.1–0.6 mM between 0.30 and 0.45 m but then rose sharply to 2.1 mM at 0.5 m. Below this there was a sharp transition to coarser, anoxic gravels in which no live cells were found.
Resting cells – depth distribution
Both chloroplast shape and fluorescence characteristics were used to assess the depth distribution of resting cells. In C. diluviana the normally expanded chloroplasts reduced to a circular shape when resting (). They could be identified and counted easily using autofluorescence because they were the only species where cells remained alongside each other in rows, with a characteristic appearance of two parallel rows of red dots. The physiological condition of cells was followed using the decline in fluorescence relative to the surface and the fluorescence ratio Fv/Fm, which can be related to the maximum photochemical quantum efficiency (see Cullen, Citation1982; Falkowski & Kolber, Citation1995; Cullen & Davis, Citation2003). Typical depth distributions are shown in , where there was little change in chlorophyll a with depth but a rapid increase in cells with rounded chloroplasts (i.e. resting cells) below 0.05 m. By 0.15 m, all cells had rounded chloroplasts. Fluorescence also showed a rapid change with depth, declining to 23% of the surface value at 0.15 m, but decreasing more slowly to 6% at 0.5 m. Fv/Fm declined less rapidly but approached zero by 0.3 m.
Fig 8. Depth distribution below the sand surface of Bar 3 on 9 March 1988 of (A) extracted chlorophyll a (µg g−1 dry sand); (B) percentage of Cymbellonitzschia diluviana cells in the resting state (rounded chloroplasts); (C) fluorescence relative to the surface value; (D) Fv/Fm ratio. The area between the horizontal broken lines represents the transition zone (see text).
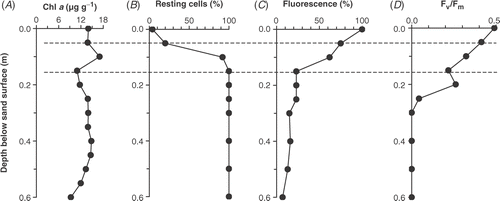
Between November 1987 and November 1988, 24 depth profiles were obtained that had a similar general pattern to the example in . Three depth zones could be distinguished. An upper zone (0–0.05 m), which was frequently disturbed and had C. diluviana cells with fully expanded chloroplasts, high fluorescence and a high Fv/Fm (above 0.4). Next was a transition zone (approximately 0.05–0.20 m), where there was an increase in the number of resting cells, a decrease in fluorescence and a decrease in Fv/Fm. Below this layer, was the broadest zone, reaching down to the base of profiles, in which all cells were resting. Fluorescence declined to below 20% of surface values and Fv/Fm approached zero. The lower limit of this bottom zone was where sulphide concentrations increased, as described above.
Preliminary measurements suggested that decline in fluorescence and rounding of chloroplasts coincided with decreases in redox potential. However, this was difficult to test in deep sand without breaking redox probes or causing disturbance. Therefore, a site was chosen in a trough between bars where the change to reducing conditions happened more rapidly (). At this site, between Bar 3 and Bar 4 on 8 March 1989, the redox potential dropped from +450 mV at the surface to +208 mV at 0.18 m (i.e. on the threshold of anoxia) and the sand appeared grey in colour. Even so, the chlorophyll a concentration was still relatively evenly distributed with depth, having values of 18.6 µg chl a g−1 dry sand at the surface, 16.7 at 0.04 m, 16.7 at 0.12 m and 16.0 at 0.18 m. In this depth profile, there was a linear relationship between redox and fluorescence () and a linear increase in the percentage of resting cells of C. diluviana as the redox fell (). So, at 0.18 m, the fluorescence decreased to 18% of that found at the surface and all cells were resting.
Fig 9. Relationship between redox potential and (A) percentage of resting cells of Cymbellonitzschia diluviana (rounded chloroplasts); (B) fluorescence (expressed as a percentage of the maximum surface fluorescence); (C) Fv/Fm ratio. The sand was taken from a trough between Bar 3 and Bar 4 on 8 March 1989.
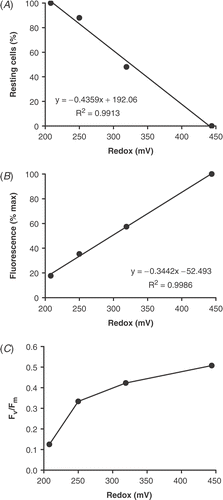
However, Fv/Fm showed a non-linear relationship with redox potential (), decreasing more rapidly near to the onset of reducing conditions. Below 0.12 m, it dropped from 0.33 to 0.13 when the redox potential fell from +250 to +208 mV. Under autofluorescence microscopy, there appeared to be a dimming of the light emitted but no further change in chloroplast shape. The results show that physiological changes continue to take place in the cell even after chloroplast rounding is complete.
Resting cell formation
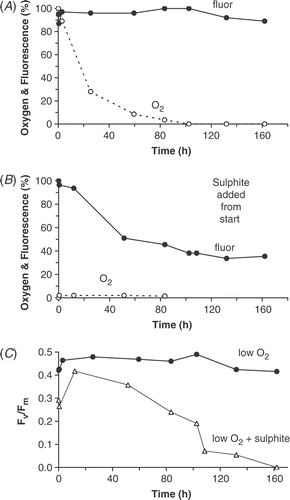
To investigate controls on formation of resting cells, a series of experiments with different oxygen concentrations and redox potentials were initiated. In a preliminary experiment (18/11/87), surface sand was placed in the dark but kept oxygenated. After 35 days, the number of C. diluviana resting cells was still relatively low (18%) but by 85 days all cells were resting. Martyana martyii was slower to begin forming resting cells but, by 72 days, 90% of cells were resting. After 43 days, however, M. martyii cells began to fall off the sand. The most likely explanation was that they were no longer able to maintain their mucilage stalk (as noted above in deeply buried cells). Chlorophyll fluorescence began to decline after 35 days, reaching 44% of the initial value after 72 days, but Fv/Fm stayed above 0.5 throughout the experiment, only dropping slightly to 0.43 after 85 days.
In a second set of experiments, the effects of lowered oxygen and onset of reducing conditions were investigated by placing sand in dark, sealed bottles (). In the first of these experiments, oxygen decreased naturally through cell respiration. Oxygen concentration fell to below 2% by 83.5 h but redox values decreased from +440 to only +300 mV, so that conditions were never reducing. By 162 h, the percentage of C. diluviana cells resting (i.e. rounded chloroplasts) was only 20% and chlorophyll fluorescence had declined by less than 10% (). Also, Fv/Fm dropped only slightly to 0.42 (). In a concurrent experiment (not shown), a similar low oxygen concentration (2%) was reached at 92 h but then 1 mM sulphite was added to create reducing conditions. The redox fell to −100 mV and the percentage of C. diluviana resting cells increased to 62% by 162 h. At the same time, the chlorophyll fluorescence fell to 46% and Fv/Fm fell sharply from 0.44 to 0.08. In a third experiment, reducing conditions were created from the beginning, with redox reduced to −200 mV (). The percentage of C. diluviana resting cells increased rapidly compared with the other two experiments, up to 91% by 102 h and 100% by 162 h. Chlorophyll fluorescence fell by half in the first 50 h but then continued to drop more slowly, reaching 38% by 162 h. Fv/Fm steadily declined to 0.19 at 102.5 h and was zero by 162 h (). Therefore, in these experiments, reducing conditions greatly increased the speed of resting cell formation.
Resting cells – recovery
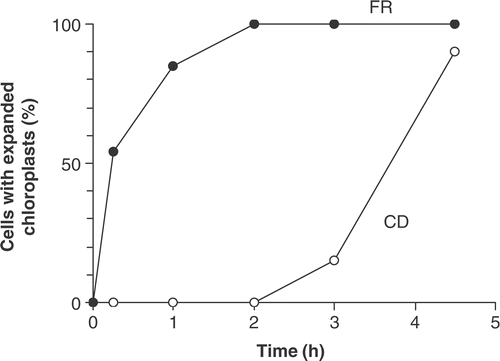
Recovery of cells from the resting state was initially investigated by following the expansion of chloroplasts (). On sand taken from 0.15 m below the sediment surface on 17 August 1987, all cells had rounded chloroplasts. They were placed in flasks in an illuminated, shaken incubator. Martyana martyi cells took only 2 h for the whole population to have fully expanded chloroplasts but Cymbellonitzschia diluviana was slower, taking 4.5 h to reach 90% recovery. An added indication of cell recovery is the time to the first division. This was relatively easily determined in M. martyii because of the characteristic ‘hinging’ of cells shortly after division (). In this experiment, M. martyii cells began to divide after 40 h.
Fig 11. Recovery of resting cells of Cymbellonitzschia diluviana (CD) and cells of Martyana martyi (FR) collected from 0.15 m below the sand surface and placed in the light (30 µmol m−2 s−1) on 17 August 1987. Results are expressed as percentage of cells with fully expanded chloroplasts (n = 100).
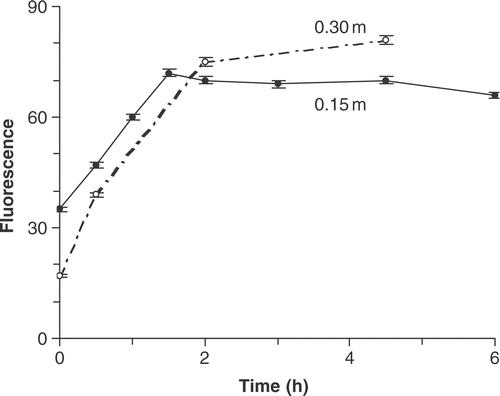
To test the effect of burial depth on recovery, sand grains from 0.15 and 0.30 m below the sediment surface (30 July 1987) were placed in an illuminated incubator and kept gently shaken. The fluorescence of cells from 0.15 m took only 1.5 h to recover to surface values () and Fv/Fm rose from 0.37 to 0.53. Although cells from 0.30 m recovered at a similar rate, they took longer because they had a lower fluorescence at the start (Fig. 12). Also, the initial Fv/Fm of 0.3 m cells was much lower (0.06) than at 0.15 m (0.37), indicating that the deeper cells were probably in, or close to, reducing conditions. Fv/Fm recovered rapidly in the deeply buried cells to reach 0.44 after 2 h and 0.48 after 4.25 h. In the cells from 0.15 m, Fv/Fm recovered to values similar to the surface material (0.53) in 2 h.
Fig 12. Community fluorescence after DCMU addition (arbitrary units) in sand collected from two depths (0.15 and 0.30 m) below the sediment surface on 7 August 1987 and placed in the light (30 µmol m−2 s−1).
To test whether recovery could occur in the dark, cells on sand grains from 0.15 and 0.30 m were put in the dark with oxygenated lake water and gently shaken. The fluorescence of cells from 0.15 m did recover but only to 75% of the fluorescence level of cells exposed in the light and over a longer time interval (4.5 h). Similarly, cells from 0.30 m reached only 62% of the fluorescence level of cells exposed to light in the same time and Fv/Fm recovered to only 0.34. Thus, there was a slow, partial recovery when oxygen was supplied in the dark.
Discussion
Although sand was moved inshore and offshore, it remained within the coastal zone (e.g. ). Therefore, by staying attached to grains, cells maintained their position and avoided being swept away. However, surviving in such a harsh environment requires specialized adaptations in both physical form and physiology. The two diatom species investigated in this study, Cymbellonitzschia diluviana and Martyana martyi, showed very different approaches.
One of the major problems for sand dwellers is abrasion. To avoid it, most species live in grooves and depressions (e.g. Meadows & Anderson, Citation1968; Stevenson & Stoermer, Citation1981; Miller et al., Citation1987). Miller et al. (Citation1987) noted that “an epipsammic diatom has little problem staying attached to its substratum, but rather a greater problem of staying sheltered”. The number of broken valves (up to 28% of C. diluviana) found in this study is a good example of this. Therefore, it was a puzzle to find one species, Martyana martyi, which actually projected from the surface (). In a study of sand communities in streams, Miller et al. (Citation1987) suggested that stalked species are better at colonizing bare surfaces and Krejci & Lowe (Citation1986) found more stalked species on quartz grains. However, this does not fully explain the distributions found here. Martyana martyi gained an advantage by colonizing exposed areas because it avoided the competition for space in more sheltered areas. However, cells must be strong enough to survive the exposure. Martyana martyi does have a thick cell wall that is strongly buttressed and a tough, flexible stalk () that can bend when sand grains grind against each other. Even so, it was still a mystery as to how this non-motile species came to be so evenly dispersed until cells were observed bridging across between sand grains after division (). This is a beautifully simple solution to dispersion that reduces the risk of being washed away. A characteristic of this species is the lack of linking spines between neighbouring cells. Instead, they have mucilage pads associated with a terminal pore field that keeps daughter cells attached to each other while allowing them to ‘hinge’ across to another grain. The cells finally separate when both are firmly attached. So, they never form long chains, which would be susceptible to abrasive damage. This is probably why closely related Fragilaria species (i.e. those with linking spines between cells) on Traad Beach were largely restricted to sites that were less disturbed.
Cymbellonitzschia diluviana had a different approach, with small cells that have a low, elongated cell shape that maximizes attachment along its raphe-bearing side (, , ). It has a form that is ideally suited to life in more protected areas of grains. Krejci and Lowe (Citation1986) suggested that occupation of grooves was not caused by loss of cells from exposed areas but was probably the result of an active migration, even happening in undisturbed laboratory experiments. C. diluviana is able to move, but the only time it has been seen to do so was when environmental conditions approached its limit for growth, e.g. below pH 7.8 (Jewson & Lowry, Citation1993). Usually cells were found in short rows on the surface of the grain (), often in dense clusters (see Jewson & Lowry, Citation1993). The reasons for this partly relate to its cell shape but also to what happens during division. In particular, having a raphe (mucilage secreting structure) on the ventral edge of the cell allowed both parent valves to remain attached to the grain surface throughout division and formation of the two daughter cells (). This greatly reduced the risk of them being sheared off and assisted the build-up of cells in a favourable area. In summary, distributions of C. diluviana and M. martyi are related to their very different strategies during division. Both solutions reduce the risk of being washed away and also allow them to coexist on the same grain.
These two species dominated in a community that showed very limited seasonal changes in algal biomass (). The main factor controlling the changes was sediment movement (, ) resulting from complex physical processes that caused frequent and abrasive mixing. So, even when 73% of M. martyi cells on the surface were dividing in March (), there was only a small net increase in biomass () because cells were either dislodged or buried. Cells were also continually being brought back to the surface from deeper layers, as bars shifted after changes in water level. This meant that population changes were ‘buffered’ by the large standing crop buried up to half a metre below the sand surface. However, as well as physical processes, low light in winter and nutrient limitation in summer (see ; ) also contributed to the low seasonal variation in algal biomass. In the case of nutrients, there was the possibility that they might be derived from the interstitial water, as has been suggested for more organic sediments (Happey-Wood & Priddle, Citation1984). The evidence from the timing of events () and nutrient assays () was that the overlying water was the main source of nutrients during this study, with silica limitation lasting from late March until August, followed by nitrogen limitation through to late October (i.e. nearly 7 months in total). Phosphate limitation was less important because periods of low concentrations were similar to periods of low silicate concentrations. However, there was some evidence from the assays that nutrients might be gained briefly from sediments in specialized circumstances, e.g. after the collapse and sedimentation of the planktonic diatom crop. Usually material deposited on the sand was quickly resuspended into deeper water but, if it did remain, then decomposition was rapid (Zlinszky, Citation1993). However, in general, the regular flushing by wave action means that there is always likely to be a lower availability of nutrients than in organic sediments.
The degree of disturbance was also important in determining the environmental conditions that developed below the sand surface. In small lakes, where wave energy is low, the transition to anoxia may happen within a few centimetres, as Moss & Round (Citation1967) described in Shear Water. The multiple sand bar systems are at the other extreme, with complete anoxia not developing for over half a metre. Sand bars are found in moderate-to-high wave-energy shores in freshwaters where there are water-level changes, e.g. the Laurentian Great Lakes (see Evans, Citation1940; Saylor & Hands, Citation1970). In such mobile sediments, survival of buried cells is aided by the ability to go into a resting or dormant state. In L. Neagh, this was not a morphological change to the frustule, which occurs in some species that form a distinct spore (see McQuoid & Hobson, Citation1996), but a change in physiological state.
The change was not a sudden switch but a gradual response, with the first sign being a rounding of chloroplasts and a decline in fluorescence (, ). This was followed by further change that was investigated here using another fluorescence indicator, Fv/Fm (Figs ). This ratio can be related to the maximum photochemical quantum efficiency (see Cullen, Citation1982; Falkowski & Kolber, Citation1995; Cullen & Davis, Citation2003), so has to be interpreted carefully because it can be influenced by a number of factors. Some of these have been discussed recently in relation to productivity of the oceans (see Cullen, Citation1982; Falkowski & Kolber, Citation1995; Cullen & Davis, Citation2003), nutrient conditions (Geider et al., Citation1993; Berges et al., Citation1996; Boyd et al., Citation1998; Young & Beardall, Citation2003) and temperature shock (Sellner et al., Citation1982). In this study, sand diatoms were silica limited from mid March until August, followed by nitrogen limitation through to late October. Surface values of Fv/Fm did vary during this time (from 0.40 to 0.58) but changes found in buried cells were much larger (, ). In laboratory experiments, Fv/Fm dropped rapidly to near zero under reducing conditions (). In contrast, the ratio decreased only slightly in cells kept in the dark but oxygenated. This suggests that the photosynthetic apparatus was not completely shut down in those cells buried in oxygenated layers just below the surface, which means that they were able to switch on relatively quickly but at the expense of higher maintenance costs.
Overall, the results show that the diatoms were in a range of physiological states. There was an upper zone, which was frequently disturbed and oxygenated, and where most cells were fully active (0–0.05 m), a transition zone, where cells were in an intermediate physiological state (Fv/Fm 0.2–0.4) but with the chloroplasts mostly contracted, and finally a bottom zone where all cells were resting (). This gradient is reflected in recovery times, with cells from intermediate depths becoming fully active sooner than those from deeper layers when exposed to light and/or oxygen (). Even cells kept in the dark but oxygenated showed some recovery, which means that, if they were exposed by storms at night, they could begin the switching on process.
One of the earliest reports of resting cells was by Nipkow (Citation1950), who described finding several Aulacoseira species in varved sediments up to 4–5 years old in Zürichsee, but the first comprehensive study of their ecological role was by Lund (Citation1954) in the planktonic Aulacoseira subarctica. He showed that formation of resting cells aided survival in surface sediments during periods when nutrients were unavailable in the water column. He also noted that, after resuspension, cells of A. subarctica took 7 days until the first division. This is much longer than M. martyi, which divided in one and a half days. The faster response of epipsammics may be because time in the light is important. Cells must switch on quickly, unless limited by nutrients, to make maximum use of time on the surface before they are buried again.
Another difference between the two habitats, is that in sand the process of resting cell formation only begins once cells are buried, so they are unable to use an environmental cue to build-up storage products beforehand and this must affect their survival time. In the water column, Lund (Citation1954) showed that A. subarctica used the decline in silica concentration below a certain threshold as a cue, and that some cells could then survive for up to 3 years in anaerobic conditions (Lund Citation1954). There have been relatively few studies of survival in anoxic conditions in freshwater benthic species. Moss (Citation1977) compared epipelic (mud) and epipsammic (sand) communities and found that the latter tolerated anaerobic conditions better. Another study of individual species, rather than community response, focused mainly on estuarine epipelic communities but included one sandy site (Admiraal & Peletier, Citation1979; Admiraal, Citation1984). They found that there was a wide range in tolerance of species exposed to anaerobic conditions, especially if there were moderate-to-high concentrations of ammonia and sulphide. Cultures of some species were able to survive in the dark for 1–2 months equally well in aerobic or anaerobic conditions. Resting stages were not investigated but Admiraal (Citation1984) noted that “it seems likely that the maintenance of diatoms under anaerobic conditions is due to an extreme reduced metabolic activity”.
During recovery of resting cells, stalked species, such as Martyana martyi (), may have an added advantage because they usually project above a covering of mucilage that can form where there are dense algal communities. The mucilage may provide a protective microclimate in unfavourable conditions but it may also reduce the rate of oxygen re-supply, as sand grains from anaerobic layers still appeared grey for some time after oxygen was restored to the surrounding water. Martyana martyi was one of the quickest species to recover, usually expanding its chloroplasts within 1–2 h () and dividing in less than 2 days. The downside is that the stalk has to be maintained and resting cells of M. martyi began falling off grains after 43 days in the dark, whereas C. diluviana were still firmly attached to grains after 18 months of burial.
Cymbellonitzschia diluviana and Martyana martyi illustrate a variety of adaptations, in both form and physiology, which aid survival and co-existence on the sand grain. In particular, they both show an adaptable resting strategy that is suited to the uncertain length of burial that may last days, weeks or even years. It is a graded response rather than an on–off switch. There is a compromise between conserving energy when buried and restoring full activity as quickly as possible, to make best use of any time on the surface. Adverse conditions during burial, such as low redox, hasten resting cell formation and deepen the resulting resting state, which then lengthens the recovery period. Since growth was only possible in the top 2–3 mm, only a small fraction (less than 1%) of the epipsammic biomass on the bars was active at any one time. The remainder represented a large ‘seed bank’ that was either resting or forming resting cells.
Acknowledgements
We thank M. Quinn for his help throughout this study, especially in collecting sand samples and bottom profiling. Hugo McGrogan helped with water analyses. Special thanks are also due to J. Zlinszky and all the staff and postgraduates of the Traad Point Field Station and also J. Quinn, R.W. Battarbee, J.A. Berges, E.B. Young, M.J. Dring and P.M.J. Rioual. The work was supported by a Natural Environment Research Council studentship to Richard Bowen. Richard Bowen died 28 May 1991 and this paper is dedicated to his memory.
References
- Admiraal , W . 1984 . “ The ecology of estuarine sediment-inhabiting diatoms ” . In Progress in Phycological Research , Edited by: Round , FE and Chapman , DJ . Vol. 3 , 269 – 322 . Bristol : Biopress .
- Admiraal , W and Pelletier , H . 1979 . Sulphide tolerance of benthic diatoms in relation to their distribution in an estuary . Br. Phycol. J. , 14 : 185 – 196 .
- Anon . 1982 . Sulphide in Waters and Effluents 1982, Tentative Methods. Methods for the Examination of Waters and Associated Materials , London : HMSO publications .
- Battarbee , RW . 1986 . “ Diatom analysis ” . In Handbook of Holocene Palaeoecology and Palaeohydrology , Edited by: Berglund , BE . 527 – 570 . Chichester : Wiley .
- Berges , JA , Charlebois , DO , Mauzerall , DC and Falkowski , P . 1996 . Differential effects of nitrogen limitation on photosynthetic efficiency of photosystems I and II in microalgae . Plant Physiol. , 110 : 689 – 696 .
- Boyd , P , Berges , JA and Harrison , PJ . 1998 . In vitro iron experiments at iron-rich and -poor sites in the NE subarctic Pacific . J. Exp. Mar. Biol. Ecol. , 227 : 131 – 151 .
- Cadee , GC and Hegeman , J . 1974 . Primary production of the benthic microflora living in the Dutch Wadden Sea . Neth. J. Sea Res. , 8 : 260 – 291 .
- Carter , RWG . 1988 . Coastal Environments , London : Academic Press .
- Carter , RWG . 1993 . “ The morphology, hydrodynamics and sedimentation in Lough Neagh ” . In Lough Neagh , Edited by: Wood , RB and Smith , RV . 35 – 93 . Kluwer, , Netherlands : Monographiae Biologicae .
- Carter , RWG and Balsillie , JH . 1983 . A note on the wave energy transmitted over near shore sand bars . Earth Surf. Proc. Landforms , 8 : 213 – 222 .
- Carter , CE and Carter , RWG . 1983 . Factors influencing the Chironomid community of a nearshore sand area . Mem. Amer. Ent. Soc. , 34 : 47 – 59 .
- Cullen , JJ . 1982 . The deep chlorophyll maximum: comparing vertical profiles of chlorophyll a . Can. J. Fish. Aquat. Sci. , 39 : 791 – 803 .
- Cullen , JJ and Davis , RF . 2003 . The blank can make a big difference in oceanographic measurements . Limnol. Oceanogr. Bull. , 12 : 29 – 35 .
- Edlund , MB and Stoermer , EF . 1999 . “ Taxonomy and morphology of Amphora calumetica (B.W. Thomas ex Wolle) Perag., an epipsammic diatom from post-pleistocene large lakes ” . In Proceeding of the XIVth Diatom Symposium 1996 , Edited by: Mayama , S , Idei , M and Koizumi , I . 65 – 76 . Koenigstein : Koeltz .
- Evans , OF . 1940 . The low and ball of the eastern shore of Lake Michigan . J. Geol. , 48 : 476 – 511 .
- Falkowski , PG and Kolber , Z . 1995 . Variations in chlorophyll fluorescence yields in phytoplankton in the world oceans . Aust. J. Plant Physiol. , 22 : 341 – 355 .
- Geider , R , La Roche , J , Greene , RM and Olaizola , M . 1993 . Response of the photosynthetic apparatus of Phaeodactylum tricornutum (Bacillariophyceae) to nitrate, phosphate, or iron starvation . J. Phycol. , 29 : 755 – 766 .
- Gibson , CE . 1981 . Silica budgets and the ecology of planktonic diatom algae in an unstratified lake (Lough Neagh, Northern Ireland) . Int. Rev. Gesamten Hydrobiol. , 66 : 641 – 664 .
- Gibson , CE and Fitzsimons , AG . 1982 . Periodicity and morphology of planktonic blue-green algae in an unstratified lake (Lough Neagh, Northern Ireland) . Int. Rev. Gesamten Hydrobiol. , 67 : 459 – 476 .
- Happey-Wood , CM and Priddle , J . 1984 . The ecology of epipelic algae of five Welsh lakes, with special reference to volvocalean green flagellates (Chlorophyceae) . J. Phycol. , 20 : 109 – 124 .
- Hargrave , BT . 1972 . Oxidation-reduction potentials, oxygen concentration and oxygen uptake of profundal sediments in a eutrophic lake . Oikos , 23 : 167 – 177 .
- Harper , MA . 1969 . Movement and migration of diatoms on sand grains . Brit. Phycol. J. , 4 : 97 – 104 .
- Harper , MA and Harper , JF . 1967 . Measurement of diatom adhesion and their relationship with movement . Brit. Phycol. Bull. , 3 : 195 – 207 .
- Hickman , M and Round , FE . 1970 . Primary production and standing crops of epipelic and epipsammic algae as measured by cell numbers and chlorophyll a . Brit. Phycol. J. , 5 : 247 – 255 .
- Hueston , AE . 1993 . Hydrodynamics of an isothermal lake, L. Neagh, with particular reference to the effect of waves D. Phil. Thesis, University of Ulster, UK
- Jewson , DH . 1993 . “ Optical properties of Lough Neagh ” . In Lough Neagh , Edited by: Wood , RB and Smith , RV . 59 – 73 . Kluwer, , Netherlands : Monographiae Biologicae .
- Jewson , DH and Briggs , M . 1993 . “ Benthic algae of Lough Neagh ” . In Lough Neagh , Edited by: Wood , RB and Smith , RV . 239 – 243 . Kluwer, , Netherlands : Monographiae Biologicae .
- Jewson , DH and Lowry , SF . 1993 . Cymbellonitzschia diluviana Hustedt (Bacillariophyceae): habitat and auxosporulation . Hydrobiologia , 269/270 : 87 – 96 .
- Kingston , JC , Lowe , RL and Stoermer , EF . 1979 . Attached winter floral assemblages from Grand Transverse Bay, Lake Michigan . Micron , 10 : 203 – 204 .
- Krejci , ME and Lowe , RL . 1986 . Importance of sand grain mineralogy and topography in determining micro-spatial distribution of epipsammic diatoms . J. N. Am. Benthol. Assoc. , 5 : 211 – 220 .
- Lund , JWG . 1954 . The seasonal cycle of the plankton diatom Melosira italica (Ehr.) Kütz. subsp. subarctica O. Müll . J. Ecol. , 42 : 151 – 179 .
- Marker , AFH , Nusch , EA , Rai , H and Riemann , B . 1980 . The measurement of photosynthetic pigments in freshwaters and standardization of methods: conclusions and recommendations . Arch. Hydrobiol. Ergebnisse Limnol. , 14 : 91 – 96 .
- McQuoid , MR and Hobson , LA . 1996 . Diatom resting stages . J. Phycol. , 32 : 889 – 902 .
- Meadows , PS and Anderson , JG . 1968 . Microorganisms attached to marine sand grains . J. Mar. Biol. Ass. UK , 48 : 161 – 175 .
- Miller , AR , Lowe , RL and Rotenberry , JT . 1987 . Succession of diatom communities on sand grains . J. Ecol. , 75 : 693 – 709 .
- Moss , B . 1977 . Adaptations of epipelic and epipsammic algae . Oecologia , 28 : 103 – 108 .
- Moss , B and Round , FE . 1967 . Observations on standing crops of epipelic and epipsammic algal communities in Shear Water, Wilts . Brit. Phycol. Bull. , 3 : 241 – 248 .
- Nipkow , F . 1950 . Ruheformen planktischer Kieselalgen im geschichteten Schlamm des Zürichsees . Schweiz. Z. Hydrol. , 12 : 263 – 270 .
- Pringle , CM and Bowers , JA . 1984 . An in situ substratum fertilisation technique. Diatom colonisation on nutrient enriched sand substrata . Can. J. Fish Aqat. Sci. , 41 : 1247 – 1251 .
- Renberg , I . 1990 . A procedure for preparing large sets of diatom slides from sediment cores . J. Palaeolimnol. , 4 : 87 – 90 .
- Round , FE . 1965 . The epipsammon; a relatively unknown freshwater algal association . Br. Phycol. Bull. , 2 : 456 – 462 .
- Saylor , JH and Hands , EB . 1970 . Properties of longshore bars in the Great Lakes . Proc. Conf. Coastal Engineering. , 12 : 839 – 853 .
- Sellner , KG , Lyons , L , Perry , ES and Heimark , DB . 1982 . Assessing physiological stress in Thalassiosira fluviatilis (Bacillariophyta) and Dunaliella tertiolecta with DCMU-enhanced chlorophyll fluorescence . J. Phycol. , 18 : 142 – 148 .
- Steele , JH and Baird , IE . 1968 . Production ecology of a sandy beach . Limnol. Oceanogr. , 13 : 14 – 25 .
- Steele , JH , Munro , ALS and Giese , GS . 1970 . Environmental factors controlling the epipsammic flora on beach and sublittoral sands . J. Mar. Biol. Ass. UK , 50 : 907 – 918 .
- Stevenson , RJ and Stoermer , EF . 1981 . Quantitative differences between benthic algal communities along a depth gradient in Lake Michigan . J. Phycol. , 17 : 29 – 36 .
- Witkowski , A , Lange-Bertalot , H and Metzeltin , D . 1996 . The diatom species Fragilaria martyi (Heribaud) Lange-Bertalot, identity and ecology . Arch. Protistenkd. , 146 : 281 – 292 .
- Wood , RB and Smith , RV . 1993 . Lough Neagh, the Ecology of a Multipurpose Water Resource , Dordrecht, , The Netherlands : Kluwer Academic Publ. .
- Young , EB and Beardall , J . 2003 . Rapid ammonium- and nitrate-induced perturbations in chl a fluorescence in nitrogen-stressed Dunaliella tertiolecta (Chlorophyta) . J. Phycol. , 32 : 332 – 342 .
- Zlinszky , J . 1993 . Bacterial activity in Lough Neagh (N. Ireland) measured by the 3H-Thymidine incorporation technique D. Phil. Thesis, University of Ulster, UK