Abstract
The formation of non-motile resting cysts within the dinoflagellate life-cycle has long been considered to be unsuitable for open oceanic environments, because a considerable part of a population might be lost due to sinking. An alternative life-cycle with the production of vegetative calcareous cells as the dominant life-cycle stage was reported for the oceanic calcareous dinoflagellate Thoracosphaera heimii, and earlier observations suggested that other oceanic calcareous dinoflagellates might have similar life-cycles. In order to test this hypothesis, we investigated the life-cycle of three oceanic calcareous dinoflagellates, Thoracosphaera heimii, Leonella granifera and Calciodinellum levantinum in culture and determined relative ploidy levels with confocal laser scanning microscopy. Whereas C. levantinum forms calcareous resting cysts within the diploid sexual life-cycle phase, T. heimii and L. granifera form vegetative calcareous cells within the haploid asexual phase. By comparison with recently published molecular phylogenies, we conclude that C. levantinum is part of a group of mainly neritic species, from which oceanic species evolved repeatedly. The life-cycle of C. levantinum is basically identical to that of its neritic relatives. A reduced dormancy period is interpreted as an adaptation to the oceanic environment. By contrast, T. heimii and L. granifera are part of a clade of dinoflagellates in which the haploid vegetative life-cycle phase has diversified and enabled their members to access new habitats. While the primary calcification during the diploid phase was lost in this group, calcification was regained secondarily in the haploid vegetative life-cycle phase in T. heimii and L. granifera. Therefore the vegetative calcareous cells are not homologous with the calcareous resting cysts formed in other calcareous dinoflagellates, which may also be expressed in different biomineralization modes.
Introduction
Dinoflagellate life-cycles are highly diverse (see Elbrächter, Citation2003) and mirror the diversity and ecological importance of this large group of aquatic protists (e.g. Taylor, Citation1987). With very few exceptions, the life-cycle of dinoflagellates is haplontic (), i.e. mitotic division only occurs in the haploid phase and the zygote is the only diploid life-cycle stage (Pfiester & Anderson, Citation1987). The principal life-cycle stage in many dinoflagellates is a motile cell with two dissimilar flagella (Taylor, Citation1987; Fensome et al ., Citation1996). These planospores are generally haploid and reproduce mitotically by binary fission.
Fig. 1. Schematic haplontic life-cycle. Spores are haploid (n) and reproduce mitotically. Syngamy produces diploid zygotes (2n) that divide meiotically to return to the spore stage.
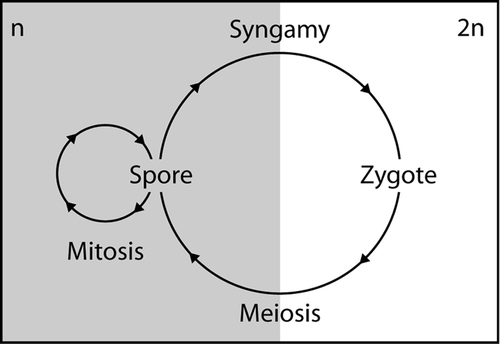
Dinoflagellates can also form coccoid non-motile stages that can occur at different stages of the life-cycle and have various functions. Within the haploid vegetative phase, these can be vegetative coccoid cells (‘vegetative cysts’) and temporary cysts (vegetative resting stages) (Fensome et al ., Citation1993; Blackburn & Parker, Citation2005). Diploid resting cysts form when two vegetative haploid cells fuse to form a planozygote, i.e. a diploid motile cell, which subsequently loses its flagella (hypnozygote) and encysts. Resting cysts are usually the only fossilizable stage within the life-cycle of dinoflagellates.
Within the large division Dinoflagellata, a distinctive sub-group is characterized by the formation of calcareous-walled, non-motile cells during part of the life-cycle (Fensome et al ., Citation1993). These are termed calcareous dinoflagellates and have an extensive fossil record (Bolli, Citation1974; Keupp, Citation1981; Kohring, Citation1993). They have recently been intensively studied by geologists in order to develop their use as indicators of past ecological conditions and palaeoceanographic change (Vink et al ., Citation2002; Hildebrand-Habel & Streng, Citation2003; Meier & Willems, Citation2003; Meier et al ., Citation2004a , Citation b ; Streng et al ., Citation2004; Vink, Citation2004). About 30 extant species of calcareous dinoflagellates are known today, of which about 10 are found predominantly in oceanic environments and 20 in neritic environments. Nonetheless neritic and oceanic associations of calcareous dinoflagellate cysts typically have very few members in common. From available evidence it has been proposed that there exists a fundamental difference between neritic and oceanic species in their strategies of producing calcareous stages (Janofske & Karwath, Citation2000).
In all neritic species studied so far, culture observations have shown that the non-motile calcifying stage is a resting cyst and that it is formed in the diploid life-cycle stage, as part of the sexual cycle (Lewis, Citation1991; Nuzzo & Montresor, Citation1999; Sgrosso et al ., Citation2001; Olli & Anderson, Citation2002). Detailed life-cycle observations are available for only one oceanic calcareous dinoflagellate, Thoracosphaera heimii, the single most common species (Tangen et al ., Citation1982; Inouye & Pienaar, Citation1983). However, only asexual reproduction was observed in these studies and the calcified cells, although non-motile, were shown to be actively photosynthesizing and capable of asexual division. Hence they were inferred to be an alternative vegetative haploid stage, rather than diploid resting cysts.
On morphological grounds, i.e. tabulation pattern of the theca and archaeopyle type, the other oceanic species have been predicted to be more closely related to the neritic species than to T. heimii (Janofske & Karwath, Citation2000; Meier et al ., Citation2002) and so were inferred to have neritic-type life-cycles, i.e. with the calcified cells being true resting cysts produced in the diploid phase rather than vegetative coccoid cells of the haploid phase. However, sediment trap studies have shown that calcareous cell production of oceanic species takes place throughout the year (Wendler et al ., Citation2002; Tanimura & Shimada, Citation2004), and not, as in neritic species, only at certain intervals during the year (Montresor et al ., Citation1998). Oceanic species often show a continuous production of calcified cells under normal culture conditions (Janofske & Karwath, Citation2000), whereas in neritic species the cells enter the sexual cycle only when forced by decreasing daylength, temperature or nutrient supply (e.g. Sgrosso et al ., Citation2001; Olli & Anderson, Citation2002). Similarly, for some oceanic species it has been observed that the calcareous stages form 50% or more of all living specimens of a culture or can even be dominant (Janofske & Karwath, Citation2000). As sexuality has so far not been observed in cultures of oceanic species, it is possible that the production of vegetative haploid calcareous stages is the rule rather than an exception in oceanic species.
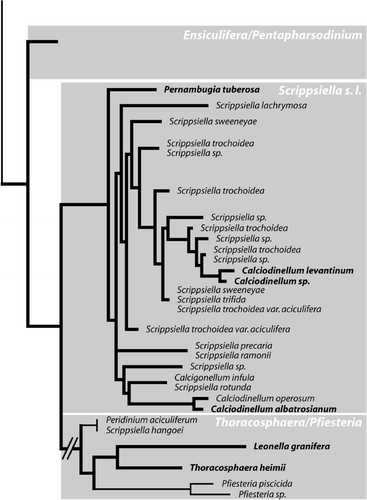
Due to its peculiar life-cycle, T. heimii was thought to be only distantly related to all other dinoflagellates (Tangen et al ., Citation1982). However, recent molecular genetic studies suggest that calcareous dinoflagellates are a monophyletic group including non-calcareous members that secondarily lost calcification (D’Onofrio et al ., Citation1999; Gottschling et al ., Citation2005a ). Therefore, T. heimii is closely related to all other calcareous dinoflagellates (), and it has been speculated that the only species that is likely to have a similar life-cycle to Thoracosphaera heimii is Leonella granifera (Gottschling et al ., Citation2005a ).
Fig. 2. Molecular phylogeny of calcareous dinoflagellates. Simplified maximum likelihood tree of all calcareous dinoflagellates based on ITS1, ITS2 and 5.8S rRNA (modified after Gottschling et al . Citation2005a ). Calcareous taxa are indicated by bold lines, and oceanic species are set in bold.
In order to test this hypothesis, we observed cultures of three typical oceanic taxa Thoracosphaera heimii, Leonella granifera and Calciodinellum levantinum (a species that, according to molecular phylogenies, is closely related to neritic species that form true resting cysts, ). We investigated ploidy levels of the different stages present in the cultures using fluorescent nuclear stains and confocal laser scanning microscopy (CLSM), and describe an evolutionary scenario for calcareous dinoflagellate life-cycles and the phylogenetic implications.
Materials and methods
Cultures
Cultures were obtained from the culture collection of the University of Bremen (Department of Geosciences, Division of Historical Geology and Palaeontology). They originate from the Atlantic Ocean and the Mediterranean Sea (), and were each established by germination of a single calcareous cell. Strains were grown in polystyrene culture flasks using culture medium K without silica (Keller et al ., Citation1987) at a constant temperature of 24°C under white fluorescent lamps at 80 µmol m−2 s−1 in a 12:12 h light–dark cycle.
Table 1. Origin of cultures used in this study. All cultures are held at the culture collection of the University of Bremen.
Sample preparation for confocal laser scanning microscopy
A 1-ml subsample of a culture was collected in a 2-ml Eppendorf tube, and the cells were centrifuged. The supernatant was discarded and the pellet was suspended in 1 ml of phosphate-buffered saline (PBS). A 1-ml suspension of 4% buffered formaldehyde was added, and the cells were left for fixation overnight. Subsequently, the cells were centrifuged and the supernatant was replaced by PBS. For staining the cell nucleus, a 3-µM solution of propidium iodide (PI) was prepared by diluting a stock solution (1.5 mM) of PI 1:500 in a staining buffer consisting of 100 mM Tris, pH. 7.4, 150 mM NaCl, 1 mM CaCl2, 0.5 mM MgCl2. The fixed cells were centrifuged to form a pellet, and the supernatant was replaced with the PI staining solution. After 15 min, the sample was centrifuged again and the staining solution was replaced by fresh buffer. A drop of the sample was embedded in citifluor antifading solution on a glass slide with cover slip and sealed with varnish. Fluorescence microscopy was carried out within a few hours after slide preparation to ensure a strong fluorescence signal.
Microscopy
For culture observations, Zeiss Axiovert 200 and Olympus CK2 inverted transmitted light microscopes were used. Images were taken with a NikonCoolpix E4500 digital camera attached to the photo tube. Fluorescence microscopy was carried out using a Leica TCS SP confocal laser scanning microscope running Leica Confocal Software version 2.61. Propidium iodide (PI) is a red fluorescent dye with an absorption maximum at 535 nm, and a fluorescence emission maximum at 617 nm. We used a 488 nm (blue–green) argon ion laser for excitation. With this setup, it was possible to detect the nucleus stained with PI as well as the chloroplasts that show a red autofluorescence. The slightly different fluorescence of the PI-stained nucleus and the autofluorescent chlorophyll were distinguished with the Leica dye-finder software.
Results
Culture observations
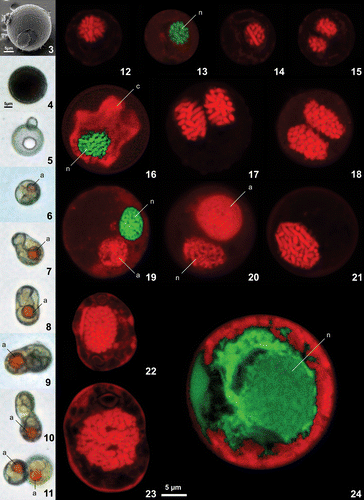
The various strains of Thoracosphaera heimii and Leonella granifera all showed very similar behaviour. The calcareous non-motile cells were the dominant life-cycle stage (), and motile cells were observed only in low numbers, in cultures with large cell densities. Under normal culture growth conditions, a cell hatched from a calcareous coccoid cell and divided immediately into two daughter cells. This could be either a flagellate motile planospore or a non-motile spherical aplanospore. In the first case, the cells settled within a few minutes to a few hours on the bottom of the culture flask and formed aplanospores. The aplanospores started to calcify subsequently and to form calcareous coccoid stages. In weakly growing cultures of L. granifera strain GeoB*192, binary fission of weakly calcified cells also occurred without excystment. This did not occur in well-calcified cells with strong walls. The weakly growing cells of L. granifera strain GeoB*192 formed an orange accumulation body that stayed with one cell during binary fission () and was newly formed by the other cell subsequently (). No evidence of sexual reproduction was observed in any of the strains, syngamy and meiosis were not seen, nor were likely planozygote stages or resting cysts.
Figs 3–24. Scanning electron, light and confocal laser scanning micrographs of Leonella granifera, Thoracosphaera heimii and Calciodinellum operosum. . Light and scanning electron micrographs of Leonella granifera. . strain GeoB*153, well calcified cells that were the dominant life-cycle stage. . strain GeoB*192, weakly calcified cells showing different stages of binary fission. An orange accumulation body (a) was frequently observed in these cells. . Confocal laser scanning microscopy images of cells from Thoracosphaera heimii, strain GeoB*101 (), Leonella granifera, strains GeoB*153 () and GeoB*192 (), and Calciodinellum levantinum, strain GeoB*165 (), stained with propidium iodide. Nuclei with permanently condensed chromosomes visible in all specimens of T. heimii and L. granifera (). Chloroplast (c) and propidium iodide fluorescence of the nucleus (n) were distinguished with Leica dye finder software (, , ). Cells with two nuclei were frequently observed (, , ). Accumulation body (a) observed in weakly growing cultures of L. granifera strain GeoB*192 was autofluorescing (, ). . Small thecate cell of C. levantinum with relatively small nucleus, interpreted to be haploid. . Large thecate cell of C. levantinum with relatively large nucleus, interpreted to be diploid. . Calcareous cyst of C. levantinum that was less well stained, so that chromosomes are not clearly visible; nucleus (n) visualized with Leica dye finder software; because of relatively large size of nucleus, cell also interpreted to be diploid. Scale bars: 5 µm.
The strain of C. levantinum showed a different behaviour. The motile flagellate stage is thecate and dominated in young cultures. Pairs of flagellate cells attached to each other were regularly observed, but we were not able to discern whether they were fusing or dividing. Calcareous stages were observed only in ageing cultures, but production was continuous once it had started. Excystment usually occurred a few days to a few weeks after encystment as indicated by the increasing number of empty calcareous cells in the cultures.
Confocal laser scanning microscopy
The nuclei of the calcareous cells of T. heimii and L. granifera were very similar (). The condensed chromosomes were clearly visible, and cells with two nuclei were frequently observed in both species. This resulted apparently from nuclear division occurring inside the calcareous cell. In L. granifera strain GeoB*153, the nucleus was surrounded by the chloroplast (). The accumulation body of the cells from L. granifera strain GeoB*192 had autofluorescence (, ). Planospores could not be analysed, as they were too rare.
In C. levantinum, two different types of thecae were present in the samples (, ), which differ in size of the cell and the nucleus. Small thecae of approximately 15 µm had a relatively small nucleus, whereas larger thecae of about 22 µm had a larger nucleus. In both, the condensed chromosomes were visible. The calcareous cells of C. levantinum were less well stained, which made it difficult to distinguish between the nucleus and other cell contents, and the chromosomes were not visible (). The calcareous cells were considerably larger than the thecae (about 30 µm), and the nucleus was larger than in the small thecae but similar in size to that of the larger thecae.
Discussion
Life-cycle
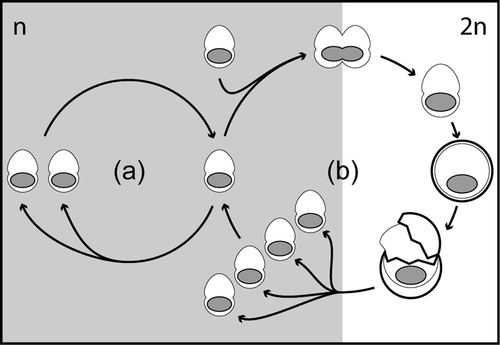
As described above, the dinoflagellate life-cycles are characteristically haplontic (), i.e. asexual reproduction (mitosis) occurs only in the haploid phase, and the zygotes are the only diploid life-cycle stage (Pfiester & Anderson, Citation1987). Although we did not directly observe sexual reproduction in the cultures of Calciodinellum levantinum, the different sizes of the nuclei indicate that the small thecae are haploid and the calcareous cells are diploid. The larger thecae might represent diploid planozygotes. From this we infer that the calcareous stages of C. levantinum are true resting cysts formed during the sexual life-cycle phase (), as observed in neritic calcareous dinoflagellates, mostly of the genus Scrippsiella (e.g. Lewis, Citation1991; Nuzzo & Montresor, Citation1999; Sgrosso et al ., Citation2001; Olli & Anderson, Citation2002). The cysts of C. levantinum are considerably larger than the thecae as in many other calcareous dinoflagellates (Janofske & Karwath, Citation2000; Gottschling et al . Citation2005b ), but also larger than the possible planozygotes. Therefore, intrathecate cyst production seems unlikely in C. levantinum, but it is unknown so far whether the thecae are shed or disintegrate before calcification takes place.
Fig. 25. Life-cycle of Calciodinellum levantinum. Within the vegetative life-cycle phase (a) the motile stages are haploid (n) and can reproduce by mitotic division. The fusion of two haploid motile cells results in the formation of a diploid (2n) planozygote that subsequently undergoes encyst-ment (b). After excystment, four haploid daughter cells are formed by meiotic division. This life-cycle is essentially similar to that of many dinoflagellates except that the resting cyst stage is calcareous rather than organic walled.
The life-cycle of Thoracosphaera heimii was described in detail by Tangen et al . (Citation1982) and by Inouye and Pienaar (Citation1983) and, so far, the life-cycle with primarily coccoid dinoflagellates that possess a calcareous cell wall in the asexual life-cycle phase has been thought to be unique. The observed behaviour of T. heimii in our cultures agrees well with their descriptions, although our strains of T. heimii did not display the division of weakly calcified cells reported by Tangen et al . (Citation1982). A nearly identical reproduction pattern is documented for Leonella granifera for the first time (), including the division of weakly calcified cells and red body formation that have been reported in T. heimii (Tangen et al ., Citation1982; Inouye & Pienaar, Citation1983). The presence of chloroplasts indicates that the vegetative calcareous coccoids represent the main photosynthetically active life-cycle stage, whereas the planospore stage is subordinate. Our analyses thus support the hypothesis of Janofske and Karwath (Citation2000), that calcification occurs in the dominant, asexually reproducing phase of the life-cycle. The coccoid and planospore stages must have the same ploidy level, since they occur within the same asexual phase of the life-cycle. Whether they are haploid or diploid could not be unequivocally determined but, as dinoflagellate life-cycles are generally haplontic, it seems more likely that the coccoid and planospore cells observed in the strains of T. heimii and L. granifera are also haploid. Sexual reproduction was not observed, but could possibly take place in an alternative sexual cycle with the formation of planozygotes. Tangen et al . (Citation1982) speculated that motile cells observed in ageing cultures might be planozygotes that are produced in response to unfavourable conditions in older cultures.
Fig. 26. Life-cycle of Leonella granifera (Thoracosphaera heimii is similar). The dominant life-cycle stages are calcareous vegetative cells (a, 1–2) that are presumably haploid (n). After a cell hatches from the calcareous shell (a, 3), it divides (a, 4) and forms aplanospores, either directly (a, 6) or via the production of planospores (a, 5). The aplanospores start to calcify (a, 7), and weakly calcified cells are capable of mitotic division (b). Sexuality was not observed but might occur in a separate sexual cycle (2n) starting from the planospore stage (c).
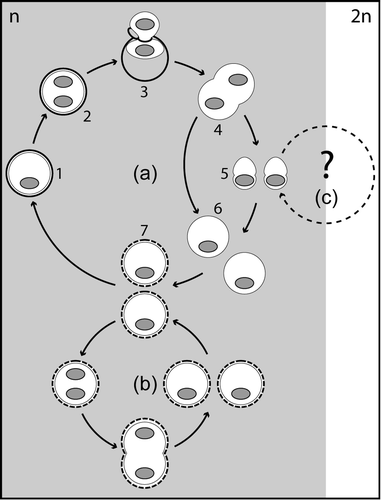
The haploid vegetative coccoid stages of Thoracosphaera heimii and Leonella granifera are, therefore, different developmental stages and cannot be regarded as being homologous to the sexual calcareous cysts that are formed in C. levantinum and other calcareous dinoflagellates investigated so far.
Phylogenetic implications
The two different life-cycles observed in our study are also reflected in molecular phylogenies. Calciodinellum levantinum is part of a clade that is formed mainly by species of the genus Scrippsiella (; Gottschling et al ., Citation2005a , Citation b ). Most taxa investigated so far within this Scrippsiella sensu lato clade are neritic species with haplontic life-cycles and formation of resting cysts. In addition to C. levantinum, there are two other oceanic species in this clade, i.e. Calciodinellum albatrosianum and Pernambugia tuberosa (). Their behaviour in cultures is similar to that of C. levantinum, i.e. young cultures are dominated by motile stages and production of calcareous cells is continuous once it starts (Janofske & Karwath, Citation2000). As Janofske & Karwath (Citation2000) observed no sexual reproduction in cultures of these species, they concluded that the calcareous stages cannot be hypnozygotes, implying that they formed during vegetative reproduction in the haploid phase. This conclusion is questionable, as the absence of sexual reproduction cannot be proven by a non-observation, as is shown by the example of our observations on C. levantinum. Therefore it seems possible – and, considering the close relationship in molecular phylogenies, even very likely – that sexual reproduction does also occur in C. albatrosianum and P. tuberosa. The three oceanic species are isolated from each other within the Scrippsiella sensu lato clade (). This indicates that the oceanic species within this clade have a different origin, and that oceanic species evolved from neritic ancestors at least three times during the evolution of calcareous dinoflagellates (Gottschling et al . Citation2005b ). The continuous cyst production and short dormancy periods that have been observed for these species in cultures and in sediment trap studies is probably an adaptation to the oceanic environment, as it prevents the resting cysts from sinking to water depths from which they cannot return to the photic zone. Sinking rates of cysts from the neritic calcareous dinoflagellate Scrippsiella trochoidea have been reported to be about 11 m d−1 (Anderson et al ., Citation1985). Oceanic calcareous dinoflagellates can be expected to have lower sinking rates, as they are generally smaller and less well calcified.
The sister clade of Scrippsiella sensu lato includes the sister taxa T. heimii and L. granifera, as well as Pfiesteria piscicida, Scrippsiella hangoei and Peridinium aciculiferum (, Gottschling et al ., Citation2005a ). The latter three are all non-calcareous dinoflagellate species that are known from either estuarine (P. piscicida), brackish (S. hangoei) or freshwater (P. aciculiferum) environments (Larsen et al ., Citation1995; Steidinger et al ., Citation1996; Rengefors & Anderson, Citation1998). A large variety of life-cycles was reported for these species. Recent research on S. hangoei has shown that up to 95% of the cysts produced in cultures are actually vegetative-resting stages (Kremp & Parrow, Citation2006) and not sexual-phase-resting cysts as previously reported (Kremp & Heiskanen, Citation1999). Only field observations on the life-cycle of P. aciculiferum are available so far, and a life-cycle with the production of resting cysts within the sexual life-cycle was reported (Rengefors & Anderson, Citation1998). However, the sequences of the ITS, SSU and LSU of S. hangoei and P. aciculiferum are nearly identical, and it is possible that the formation of cysts in the vegetative phase has been overlooked also in P. aciculiferum (Rengefors, pers. comm.). The life-cycle of P. piscicida is more complex, but also includes the production of coccoid cells in the vegetative life-cycle phase (Litaker et al ., Citation2002; Parrow et al ., Citation2002), and even the formation of amoeboid stages and chrysophyte-like cysts is discussed (Burkholder & Glasgow, Citation1997). This group also exhibits a higher physiological diversity, represented by different feeding and protection strategies, which include photoautotrophic (T. heimii, L. granifera, S. hangoei, P. aciculiferum) and heterotrophic/phagotrophic (P. piscicida) nutrition, as well as the production of toxins (P. aciculiferum, Rengefors & Legrand, Citation2001, P. piscicida, Steidinger et al ., Citation1996). This diversification of the life-cycle might in fact be the key character that enabled the members of the Pfiesteria/Thoracosphaera/Leonella clade to adapt to new ecological niches or to be highly successful in environments shared with other dinoflagellates. In T. heimii and L. granifera, the reduction of the sexual phase may have been advantageous for accessing the oceanic habitat, as the relatively fast alternation between coccoid and planospore stages within the haploid phase of the life-cycle, as well as the relatively small size of the cells, reduce the risk of sinking.
As indicated by the long branches in , the nucleotide substitution rates in the Pfiesteria/Thoracosphaera/Leonella clade are much higher than those in the Scrippsiella sensu lato clade. This evidence for faster molecular evolution appears to correlate with the high variability of life-cycles, habitats and feeding strategies and so perhaps to indicate generally more rapid evolution in this clade.
Biomineralization
Molecular genetic data suggest that the potential to form calcareous structures is an apomorphy for calcareous dinoflagellates (Gottschling et al ., Citation2005a ). Calcification during the sexual stage of the life-cycle is a synapomorphy of basal calcareous dinoflagellates (Ensiculifera/Pentapharsodinium) and the Scrippsiella clade. Calcification was secondarily lost in a subclade of the calcareous dinoflagellates, which includes taxa from different habitats (Pfiesteria, Thoracosphaera, Leonella). Within this subclade, calcification was regained in the clade formed by Thoracosphaera and Leonella, this time during the haploid phase of the life-cycle. A similar evolutionary process has been inferred for coccolithophores (Young et al ., Citation1999; Billard & Inouye, Citation2004), where calcification evolved only once, probably also in the diploid life-cycle phase with subsequent transition to the haploid phase. This transition also resulted in two very different biomineralization modes (holococcolith v. heterococcolith) of the two phases. There are some indications that different biomineralization modes are present also in calcareous dinoflagellates. For Thoracosphaera heimii, which is calcifying in the haploid phase, crystallites are formed within cell vesicles that may be Golgi derived and then assembled to cell wall elements outside the cytoplasm (Inouye & Pienaar, Citation1983), whereas in Scrippsiella minima, which produces diploid resting cysts, wall formation includes calcification of mucofibrous protrusions formed within amphiesmal vesicles inside the cell wall (Gao et al ., Citation1989). This is especially important for calcareous dinoflagellate taxonomy, which is based partly on the crystallography of the calcareous wall (Janofske, Citation1996, Citation2000; Janofske & Karwath, Citation2000). The current wall type classification has been shown to be only partly in agreement with molecular phylogenies, especially for T. heimii and L. granifera (Gottschling et al ., Citation2005a ). As T. heimii is known from the fossil record since the upper Maastrichtian, it is very likely that the split between T. heimii/L. granifera and all other calcareous dinoflagellates had already occurred in the Cretaceous. Therefore, some fossil calcareous dinoflagellate species are likely to be vegetative stages. A re-evaluation of calcareous dinoflagellate biomineralization is necessary for future taxonomic studies and may prove useful for identifying fossil vegetative coccoid cells.
Acknowledgements
This study was funded by the European Commission while the first author (KJSM) held a Marie Curie Intra-European Fellowship at the Natural History Museum (project DINO-CULT). We thank Malte Elbrächter for a stimulating discussion on life-cycles and terminology and two anonymous reviewers for their comments and corrections.
Additional information
Notes on contributors
K. J. Sebastian Meier
CEREGE, Europôle Méditerranéen de l' Arbois – BP 80 –, 13545 Aix en Provence cedex 04, FranceNotes
CEREGE, Europôle Méditerranéen de l' Arbois – BP 80 –, 13545 Aix en Provence cedex 04, France
References
- Anderson , DM , Lively , JJ , Reardon , EM and Price , CA . 1985 . Sinking characteristics of dinoflagellate cysts . Limnol. Oceanogr. , 30 : 1000 – 1009 .
- Billard , C and Inouye , I . 2004 . “ What is new in coccolithophore biology? ” . In Coccolithophores – From Molecular Processes to Global Impact , Edited by: Thierstein , H and Young , J . 1 – 30 . Berlin–Heidelberg, , Germany : Springer-Verlag .
- Blackburn , S and Parker , N . 2005 . “ Microalgal life cycles: Encystment and excystment ” . In Algal Culturing Techniques , Edited by: Andersen , RA . 399 – 417 . Amsterdam, , The Netherlands : Elsevier .
- Bolli , HM . 1974 . Jurassic and Cretaceous Calcisphaerulidae from DSDP Leg 27, Eastern Indian Ocean . Init. Rep. DSDP , 27 : 843 – 907 .
- Burkholder , JM and Glasgow , HB . 1997 . Pfiesteria piscicida and other Pfiesteria-like dinoflagellates: behavior, impacts and environmental controls . Limnol. Oceanogr , 42 : 1052 – 1075 .
- D'Onofrio , G , Marino , D , Bianco , L , Busico , E and Montresor , M . 1999 . Toward an assessment on the taxonomy of dinoflagellates that produce calcareous cysts (Calciodinelloideae, Dinophyceaea): A morphological and molecular approach . J. Phycol. , 35 : 1063 – 1078 .
- Elbrächter , M . 2003 . Dinophyte reproduction: progress and conflicts . J. Phycol. , 39 : 629 – 632 .
- Fensome , RA , Taylor , FJR , Norris , G , Sarjeant , WAS , Wharton , DI and Williams , GL . 1993 . A classification of modern and fossil dinoflagellates . Micropaleontology, Special Publication , 7 : 1 – 351 .
- Fensome , RA , Riding , JB and Taylor , FJR . 1996 . “ Chapter 6, Dinoflagellates ” . In Palynology: Principles and Applications , Edited by: Jansonius , J and McGregor , DC . 107 – 169 . Texas, , USA : American Association of Stratigraphic Palynologists Foundation, College Station .
- Gao , X , Dodge , JD and Lewis , J . 1989 . An ultrastructural study of planozygotes and encystment of a marine dinoflagellate, Scrippsiella sp . Br. Phycol. J , 24 : 153 – 165 .
- Gottschling , M , Keupp , H , Plötner , J , Knop , R , Willems , H and Kirsch , M . 2005a . Phylogeny of calcareous dinoflagellates as inferred from ITS and ribosomal sequence data . Mol. Phylog. Evol. , 36 : 444 – 455 .
- Gottschling , M , Knop , R , Plötner , J , Kirsch , M , Willems , H and Keupp , H . 2005b . A molecular phylogeny of Scrippsiella sensu lato (Calciodinellaceae, Dinophyta) with interpretations on morphology and distribution . Eur. J. Phycol. , 40 : 207 – 220 .
- Hildebrand-Habel , T and Streng , M . 2003 . Calcareous dinoflagellate associations and Maastrichtian-Tertiary climatic change in a high latitude core (ODP Hole 689B, Maud Rise, Weddell Sea) . Palaeogeogr. Palaeoclimatol. Palaeoecol. , 197 : 293 – 321 .
- Inouye , I and Pienaar , RN . 1983 . Observations on the life cycle and microanatomy of Thoracosphaera heimii (Dinophyceae) with special reference to its systematic position . S. Afr. J. Bot. , 2 : 63 – 75 .
- Janofske , D . 1996 . Ultrastructure types in Recent “calcispheres” . Bull. Inst. Oceanogr. Monaco , 14 : 295 – 303 .
- Janofske , D . 2000 . Scrippsiella trochoidea and Scrippsiella regalis nov. comb. (Peridiniales, Dinophyceae): A comparison . J. Phycol. , 36 : 178 – 189 .
- Janofske , D and Karwath , B . 2000 . “ Oceanic calcareous dinoflagellates of the equatorial Atlantic Ocean: cyst-theca relationship, taxonomy and aspects on ecology ” . In Ecological Studies on Living and Fossil Calcareous Dinoflagellates of the Equatorial and Tropical Atlantic Ocean , Edited by: Karwath , B . 93 – 136 . Bremen, , Germany : Universität Bremen .
- Keller , MD , Selvin , RC , Claus , W and Guillard , RRL . 1987 . Media for the culture of oceanic ultraphytoplankton . J. Phycol. , 23 : 633 – 638 .
- Keupp , H . 1981 . Die kalkigen Dinoflagellaten-Zysten der borealen Unterkreide (Unter-Hauterivium bis Unter-Albium) . Facies , 5 : 1 – 190 .
- Kohring , R . 1993 . Kalkdinoflagellaten aus dem Mittel- und Obereozän von Jütland (Dänemark) und im Pariser Becken (Frankreich) im Vergleich mit anderen Tertiär-Vorkommen . Berl. Geowiss. Abh. , E6 : 1 – 164 .
- Kremp , A and Heiskanen , A-S . 1999 . Sexuality and cyst formation of the spring bloom dinoflagellate Scrippsiella hangoei in the coastal northern Baltic Sea . Mar. Biol. , 134 : 771 – 777 .
- Kremp , A and Parrow , MW . 2006 . Evidence for asexual resting cysts in the life cycle of the marine peridinoid dinoflagellate . Scrippsiella hangoei. J. Phycol. , 42 : 400 – 409 .
- Larsen , J , Kuosa , H , Ikävalko , J , Kivi , K and Hällfors , S . 1995 . A redescription of Scrippsiella hangoei (Schiller) comb. nov – a ‘red tide’ forming dinoflagellate from the northern Baltic . Phycologia , 34 : 135 – 144 .
- Lewis , J . 1991 . Cyst-theca relationships in Scrippsiella (Dinophyceae) and related orthoperidinoid genera . Bot. Mar. , 34 : 91 – 106 .
- Litaker , RW , Vandersea , MW , Kibler , SR , Madden , VJ , Noga , EJ and Tester , PA . 2002 . Life cycle of the heterotrophic dinoflagellate Pfiesteria piscicida (Dinophyceae) . J. Phycol. , 38 : 442 – 463 .
- Meier , KJS and Willems , H . 2003 . Calcareous dinoflagellate cysts in surface sediments from the Mediterranean Sea: Distribution patterns and influence of main environmental gradients . Mar. Micropaleontol. , 48 : 321 – 354 .
- Meier , KJS , Janofske , D and Willems , H . 2002 . New calcareous dinoflagellates (Calciodinelloideae) from the Mediterranean Sea . J. Phycol. , 38 : 602 – 615 .
- Meier , KJS , Höll , C and Willems , H . 2004a . Effect of temperature on culture growth and cyst production in the calcareous dinoflagellates Calciodinellum albatrosianum, Leonella granifera and Pernambugia tuberosa . Micropaleontology , 50 ( suppl. 1 ) : 93 – 106 .
- Meier , KJS , Zonneveld , KAF , Kasten , S and Willems , H . 2004b . Different nutrient sources forcing increased productivity during eastern Mediterranean S1 sapropel formation as reflected by calcareous dinoflagellate cysts . Paleoceanography , 19 doi:10.1029/2003PA000895
- Montresor , M , Zingone , A and Sarno , D . 1998 . Dinoflagellate cyst production at a coastal Mediterranean site . J. Plankton Res. , 20 : 2291 – 2312 .
- Nuzzo , L and Montresor , M . 1999 . Different excystment patterns in two calcareous cyst-producing species of the dinoflagellate genus . Scrippsiella. J. Plankton Res. , 21 : 2009 – 2018 .
- Olli , K and Anderson , D . 2002 . High encystment success of the dinoflagellate Scrippsiella cf. lachrymosa in culture experiments . J. Phycol. , 38 : 145 – 156 .
- Parrow , M , Burkholder , JM , Deamer , NJ and Zhang , C . 2002 . Vegetative and sexual reproduction in Pfiesteria spp. (Dinophyceae) cultured with algal prey, and inferences for their classification . Harmful Algae , 1 : 5 – 33 .
- Pfiester , LA and Anderson , DA . 1987 . “ Dinoflagellate reproduction ” . In The Biology of Dinoflagellates , Edited by: Taylor , FJR . 611 – 648 . Oxford, UK : Blackwell Scientific Publications .
- Rengefors , K and Anderson , DM . 1998 . Environmental and endogenous regulation of cyst germination in two freshwater dinoflagellates . J. Phycol. , 34 : 568 – 577 .
- Rengefors , K and Legrand , C . 2001 . Toxicity in Peridinium aciculiferum – an adaptive strategy to outcompete other winter phytoplankton? . Limnol. Oceanogr. , 46 : 1990 – 1997 .
- Sgrosso , S , Esposito , F and Montresor , M . 2001 . Temperature and daylength regulate encystment in calcareous cyst-forming dinoflagellates . Mar. Ecol. Prog. Ser. , 211 : 77 – 87 .
- Steidinger , KA , Burkholder , JM , Glasgow , HB and Hobbs , CB . 1996 . Pfiesteria piscicida gen. et sp. nov. (Pfiesteriaceae fam. nov.), a new toxic dinoflagellate with a complex life cycle and behavior . J. Phycol. , 32 : 157 – 164 .
- Streng , M , Hildebrand-Habel , T and Willems , H . 2004 . Long-term evolution of calcareous dinoflagellate associations since the Late Cretaceous: comparison of a high- and a low-latitude core from the Indian Ocean . J. Nannoplankton Res. , 26 : 13 – 45 .
- Tangen , K , Brand , LE , Blackwelder , PL and Guillard , RRL . 1982 . Thoracosphaera heimii (Lohmann) Kamptner is a dinophyte: observations on its morphology and life cycle . Mar. Micropaleontol. , 7 : 193 – 212 .
- Tanimura , Y and Shimada , C . 2004 . Calcareous dinoflagellates from a northwestern Pacific sediment trap and their paleoceanographic implications . Micropaleontology , 50 : 344 – 356 .
- Taylor , FJR , ed. 1987 . The Biology of Dinoflagellates , Oxford, , UK : Blackwell Scientific Publications .
- Vink , A . 2004 . Calcareous dinoflagellate cysts in South and equatorial Atlantic surface sediments: diversity, distribution, ecology and potential for palaeoenvironmental reconstruction . Mar. Micropaleontol. , 50 : 43 – 88 .
- Vink , A , Brune , A , Höll , C , Zonneveld , KAF and Willems , H . 2002 . On the response of calcareous dinoflagellate cysts to oligotrophy and stratification of the upper water column in the equatorial Atlantic Ocean . Palaeogeogr. Palaeoclimatol. Palaeoecol. , 178 : 53 – 66 .
- Wendler , I , Zonneveld , KAF and Willems , H . 2002 . Production of calcareous dinoflagellate cysts in response to monsoon forcing off Somalia: a sediment-trap study . Mar. Geol. , 46 : 1 – 11 .
- Young , JR , Davis , SA , Bown , PR and Mann , S . 1999 . Coccolith ultrastructure and biomineralisation . J. Struct. Biol. , 126 : 195 – 215 .