Abstract
The formation and secretion of heterococcoliths by the non-motile life phase of the coccolithophore Coccolithus pelagicus was investigated using electron microscopy and time-lapse bright field imaging. Coccolithogenesis in C. pelagicus exhibited sequential mineralization of single coccoliths in Golgi-derived and nuclear-associated vesicles, a pattern similar to the formation of heterococcoliths in Emiliania huxleyi. Our TEM data show that only on maturation does the single coccolith vesicle migrate away from the nucleus before secretion. A reticular body, distinct from the Golgi body, was also clearly visible at the distal surface of the developing coccolith vesicle, suggesting this is a common structural feature in placolith cells that mineralize and secrete coccoliths one at a time. Time-lapse imaging revealed that the coccolith secretion process is rapid, taking 60–190 seconds, and involves considerable contractile activity to eject and position the coccolith on the surface of the cell. An intact flagellar root apparatus was discovered at the anterior pole of this non-motile cell from which polarized secretion of coccoliths occurs, which may indicate a novel role for such cytoskeletal structures. Freeze-fracture preparations revealed columnar deposits and adhesions linking the scales and coccolith baseplates to the cell, across the periplasmic space providing points of attachment for cellular movement. Rotatory movements of the cell relative to external coccoliths were exhibited by all actively calcifying cells. These movements enable the cell, while exhibiting morphologically polarized secretion, to locate and secrete a mature coccolith in a spatially well-defined manner. Finally, the time-lapse imaging approach described here provides an opportunity to quantify the regulation of coccolith production in single cells with high temporal resolution allowing responses of calcification to rapidly fluctuating environmental conditions such as light–dark transitions to be examined in detail, which has not been possible with bulk calcification studies.
Introduction
Calcifying coccolithophores are an extremely successful group of phytoplankton, many of which exhibit a heteromorphic life cycle, alternating between haploid and diploid phases (Geisen et al ., Citation2002; Houdan et al ., Citation2004). In the case of the ubiquitous Emiliania huxleyi (Lohmann) Hay et Mohler, ornate calcite plates (coccoliths) are generated by non-motile diploid cells, which interlock to form an external layer surrounding the cell (coccosphere). Coccolithophores such as E. huxleyi and Coccolithus pelagicus (Wallich) Schiller have a wide ecological distribution (McIntyre & Be, Citation1967; Brand, Citation1984) and in the case of E. huxleyi can form blooms thousands of square kilometres in area (Holligan et al ., Citation1993). Coccolithophores are the most abundant and one of the most significant producers of calcite on earth and thus play an integral role in the marine carbon cycle and upper ocean carbonate chemistry (Rost & Riebesell, Citation2004). The elemental composition of calcite coccoliths in marine sediments is also an important source of palaeo-proxy data enabling reconstruction of past climate and ocean chemistry (Rickaby et al ., Citation2002; Stoll et al ., Citation2002). In spite of their influential role in biogeochemical cycles, the cellular mechanism and environmental regulation of coccolith production are not well understood. Recent work in coccolithophore biology has led to considerable advances in our understanding of the biodiversity, ecology, life history and cell physiology of these globally important phytoplankters (Paasche, Citation2002; Thierstein & Young, Citation2004). Nevertheless, the functional roles of coccolith production and the coccosphere have not been unequivocally demonstrated (Young, Citation1994).
The sequence of heterococcolith formation has been the subject of many biochemical (van der Wal et al ., Citation1983a , b) and ultrastructural studies (Braarud & Nordli, Citation1952; Manton & Leedale, Citation1969; Klaveness, Citation1972, Citation1976) and involves the formation of an organic scale or baseplate within a Golgi-derived compartment followed by nucleation of calcite crystals, which alternate in their c-axis orientations, on the peripheral rim of the baseplate (Westbroek et al ., Citation1989; Marsh, Citation1999; Young et al ., Citation1999). The so-called protococcolith ring thus comprises alternating V- and R-crystal units, but it is the R-units that continue to extend proximally as the coccolith matures (Young et al ., Citation1992, Citation2004). Both the assembly and precise control of calcite crystal formation are highly dependent on calcium binding proteins (Corstjens et al ., Citation1998) in addition to polysaccharides, which have been identified as a result of their robust association with coccolith forming structures and calcite (van der Wal et al ., Citation1983a ; Marsh, Citation1994; Henriksen et al ., Citation2004). Nevertheless, in spite of decades of detailed studies of coccolith structure and function, little progress has been made in understanding the precise cellular mechanisms that lead to heterococcolith formation and extrusion through the plasma membrane to maintain a consistent periplasmic coccosphere. Indeed, the act of coccolith secretion remains un-described (Paasche, Citation2002).
The goal of the present study was therefore to determine the key structural features and temporal characteristics of heterococcolith formation and secretion in C. pelagicus using a combination of electron microscopy and high resolution live cell imaging. We show that the formation of a coccosphere involves light-dependent coccolithogenesis followed by light-independent rapid polar secretion. Significantly, we show that cellular contractile activity and the ability of the cell to rotate with respect to the external coccosphere is critical to the secretion and maintenance of an intact coccosphere. In this regard, a novel functional role for the layer of organic scales is proposed.
Materials and methods
Culture maintenance and growth conditions
A strain of C. pelagicus was obtained from the Plymouth Culture Collection (PLY182g). Coccolithus pelagicus has recently been divided into two sub-species on the basis of holococcolith morphology, heterococcolith size and molecular genetics (Geisen et al ., Citation2002; Saez et al ., Citation2003). Strain PLY182g was included in C. pelagicus braarudii by Saez et al . (Citation2003) on the basis of molecular genetics. Throughout this study our strain remained in the non-motile heterococcolith producing phase, and the coccoliths produced showed the typical features of C. pelagicus braarudii (). Replicate cultures were maintained in filtered seawater (FSW) medium containing, 500 µM NaNO3, 32 µM K2HPO4, 100 µM Na2SiO3, and Guillard's f/2 vitamins (Guillard & Ryther, Citation1962). A trace metal solution was supplemented into this medium containing, 2.8 µM Na2-EDTA, 20 nM ZnSO4, 460 nM MnCl2, 50 nM CoCl2, 20 nM CuSO4, 2 µM Na2MoO4 and 200 nM H2SeO3 (Davey et al ., Citation2003). The cultures were grown at 15°C, an irradiance of 100 µmol photons m−2 s−1, and a 12:12 h light–dark cycle. Semi-continuous batch cultures were maintained by sub-culturing at the mid-log growth phase, determined by regular cell counting using a Neubauer haemocytometer.
Transmission electron microscopy
Cells were fixed in 2.5% (v/v) glutaraldehyde for 1 h, centrifuged (3,000 g for 10 min) and re-suspended in 0.1 M sodium cacodylate buffer (pH 7.2). Centrifugation and resuspension was repeated twice before the cells were treated with a secondary fixative of 1% osmium tetroxide in sodium cacodylate buffer (0.1 M, pH 7.2) for 1 h. The pellet of cells was rinsed twice in buffer and dehydrated in an ethanol series before embedding in Spurr's resin. The hardened resin blocks were sectioned using an Ultracut microtome (Reichert-Jung, Leica, Milton Keynes, UK) with a diamond knife. The sections were mounted on copper grids and counterstained with a saturated solution of uranyl acetate in ethanol and Reynolds lead citrate for 15 min. Sections were examined using a Jeol 1200 EX II TEM (Jeol, Welwyn Garden City, UK) and the images captured with an SIS mega view III.
Freeze-fracture scanning electron microscopy
Aliquots of log growth phase C. pelagicus cells were spun to a pellet, mounted onto a fracture rivet and plunge frozen in liquid ethane before storage in liquid nitrogen. Rivets were mounted into the cryo-chamber of a JSM-6100 scanning electron microscope (Jeol, Welwyn Garden City, UK) under liquid nitrogen. The frozen rivet was fractured and the specimen viewed for integrity before etching by briefly (60–120 s) adjusting the stage temperature to −85°C. Following successful etching the specimen was returned to −135°C then positioned into the transfer chamber of the cryo-SEM for gold-palladium sputter coating before viewing and analysis.
Light microscopy and time-lapse analysis
Coccolithus pelagicus cells in mid-log growth phase were completely decalcified by dissolving the coccoliths in an EGTA buffered calcium–free artificial seawater medium at pH 8.2 as previously described (Taylor & Brownlee, Citation2003). We calculated a division rate at exponential growth of approximately 0.5 d−1 in our cultures. A mature fully expanded C. pelagicus cell is covered with approximately 10–18 coccoliths. The pre-existing coccoliths are shared between the daughter cells on division. Thus between 5 and 9 new coccoliths must be produced per cell after division to maintain a complete cover at these growth rates. Since most production occurs during the 12-h light period, this equates to a production rate of between 0.4–0.75 new coccoliths per hour, which is close to the maximum production rate we measured (0.4 h−1, see below) in decalcified cells. Therefore coccolith production rates in intact cells are not significantly different to those observed in the decalcified cells harvested at exponential phase.
Decalcified cells were placed into a small chamber consisting of a 35-mm petri dish with a glass cover slip cemented over a central aperture. The coverslip was treated with 0.01% poly-L-lysine (Sigma-Aldrich, Gillingham, UK) to promote adhesion of the cells. EGTA buffered seawater was removed and replaced with fresh f/2 filtered seawater medium. The chamber was mounted onto the stage of a Nikon Diaphot (Nikon, Kingston Upon Thames, UK) inverted microscope and cells viewed with oil immersion objectives under bright field illumination. The external coccoliths were completely removed, but the organic layer of scales remained after EGTA treatment (). The organic layer of the cells adhered to the coverslip anchoring the cells and enabling long-term observations of the developing intracellular coccoliths. An Orca C4742-95 CCD camera (Hamamatsu Photonics, Germany) was coupled to the microsope and coccolith secretion monitored using time-lapse image capture over 10–18 h. Image sequences were analysed with Wasabi software (Hamamatsu Photonics, Welwyn Garden City, UK) and exported as bitmaps for layout using Corel Draw (Corel, Ottawa, Canada). Time intervals for coccolith secretion and formation in individual cells were obtained by frame counting from one secretion event to another. Cells were exposed to different photosynthetically active radiation (PAR) levels by adjusting the voltage supply to the halogen bulb in the condenser. Light levels at the specimen were calibrated using a quantum light sensor (QSL-2110, Biospherical instruments, San Diego, USA). The dynamic range of the camera was such that image capture was possible in the absence of any significant PAR. Thus for experiments in the dark, images were obtained by inserting an infrared filter (100% cut off < 730 nm) above the condenser.
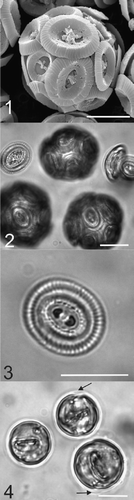
Figs. 1–4. Micrographs of Coccolithus pelagicus. . SEM image of a whole C. pelagicus cell (182g strain) showing coccolith morphology consistent with the temperate sub-species braarudii. . Light micrograph of C. pelagicus showing complete coccospheres and loose coccoliths, which were also present in the cell suspension. . Light micrograph showing detail of the distal shield and central bar of the coccoliths produced by C. pelagicus 182g. . EGTA-decalcified cells showing internal coccoliths at various stages of development and in two cells there is evidence of the loose periplasmic organic layer of scales (black arrows, see also ). Scale bars: 10 µm.
Results and discussion
General ultrastructural observations
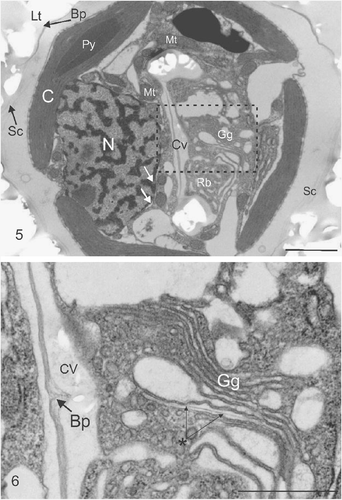
The organelles and ultrastructure seen using TEM analysis of the 182g strain of C. pelagicus () were generally consistent with those described in other coccolithophore species (Parke & Adams, Citation1960; Manton & Leedale, Citation1969; Klaveness, Citation1973; Inouye & Pienaar, Citation1984). Briefly, the exterior of the cell was covered by coccoliths overlying an organic layer consisting of coccolith baseplates and organic scales. The coccolith calcite was dissolved during preparation of ultrathin sections, leaving an impression in the embedding resin. Two large lobed chloroplasts with embedded pyrenoids were located just beneath the plasma membrane, on opposite sides of the cell (). A large nucleus surrounded by nuclear endoplasmic reticulum was located towards the posterior end of the cell. Numerous sections revealed a developing coccolith within a Golgi-derived coccolith vesicle closely associated with the nucleus. Mitochondria were clearly located around the nucleus and the developing rim of the coccolith vesicle.
Figs. 5, 6. Ultrastructure of coccolith developing within a C. pelagicus cell. . TEM showing impression left by external coccoliths (Lt), baseplate of coccolith (Bp) and layer of organic scales (Sc) on the outside of the cell. Internal organelles comprise the chloroplast (C) showing a single thylakoid traversing the pyrenoid (Py), Nucleus (N) surrounded by the nuclear envelope, indicated by white arrows, and mitochondria (Mt), closely associated Golgi-derived coccolith vesicle (Cv) in the early stages of development. The Golgi body (Gg) and mass of anastomosing vesicles resembling a reticular body (Rb) are located in the distal pocket of the developing coccolith vesicle. . Detail of (indicated by box) showing the Golgi body and the central region of the coccolith vesicle containing an unmineralized section of the coccolith organic baseplate. A newly developing organic baseplate or scale is located within a distal Golgi cisterna (marked with asterisk). Scale bars: 2 µm () and 1 µm ().
The Golgi and reticular body
A single large Golgi body () was always present on the distal side of the developing coccolith vesicle, which is consistent with the closely related placolith forming Umbilicosphaera sibogae Weber-van Bosse (Inouye & Pienaar, Citation1984). This is in marked contrast to E. huxleyi where the Golgi body occupies a location often peripheral to the margins of the coccolith vesicle (Klaveness, Citation1972; van der Wal et al ., Citation1983a ). Detailed examination of the Golgi body () revealed the presence of dilated cisternae, typical of scale and coccolith forming prymnesiophytes (Pienaar, Citation1969; Klaveness, Citation1972; Inouye & Pienaar, Citation1988; Hawkins & Lee, Citation2001) and a Golgi vesicle containing a developing amorphous organic baseplate or scale.
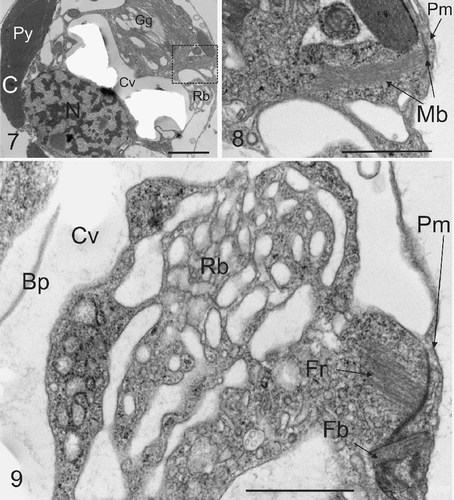
In addition to the Golgi body, a prominent mass of anastomosing tubular vesicles remarkably similar to the reticular body of E. huxleyi (Wilber & Watabe, Citation1963; van der Wal et al ., Citation1983a ; Westbroek et al ., Citation1984) was consistently co-localized with the Golgi body in the distal pocket of cytoplasm formed by the developing coccolith (, see also , ). This was not previously reported for C. pelagicus (Manton & Leedale, Citation1969) and it was subsequently proposed (Paasche, Citation2002) that the absence of a reticular body in C. pelagicus is related to the fact that coccoliths form directly within Golgi cisternal compartments. The presence of the reticular body was noted as being unique to E. huxleyi and Gephyrocapsa Kamptner, and correlated to a model whereby smaller coalescing Golgi vesicles form the coccolith compartment (Paasche, Citation2002). However the precise ontology of the coccolith vesicle in E. huxleyi remains uncertain. Many coccolithophores do not exhibit a reticular body associated with calcification, for example the cricolith forming Pleurochrysis carterae Braarud & Fagerland (Pienaar, Citation1969; Outka & Williams, Citation1971) and the tremalith-forming Ochrosphaera neapolitana Schussnig (Fresnel & Probert, Citation2005). In these species coccolith mineralization may occur simultaneously in more than one compartment (van der Wal et al ., Citation1983b) and maturation occurs in Golgi-derived vesicles that move distally towards the plasma membrane and are not associated with the nucleus. In the case of O. neapolitana however, a small tubular structure appears to be associated with the coccolith vesicle and peripheral endoplasmic reticulum during coccolith calcification (Fresnel & Probert, Citation2005). In a morphological examination of U. sibogae, a placolith forming species closely related to C. pelagicus, a reticular body was not defined, although tubules associated with the endoplasmic reticulum system were noted as extending to and connecting with the coccolith vesicle and distally located Golgi body complex (Inouye & Pienaar, Citation1984). Given the above it would seem that, regardless of mechanism or morphology, a close association between the endoplasmic reticulum and the coccolith vesicle during active calcification is a common feature among coccolithophores and is probably related to the high capacity of this endomembrane system to sequester and transport calcium to the site of mineralization (Brownlee & Taylor, Citation2004).
Figs. 7–9. Microtubule bundles and flagellar root. . TEM section showing the typical location of microtubule bundles with respect to coccolith vesicle and the reticular mass (Rb). Abbreviations are as in legend for . . Detail from box in showing bundle of microtubules (Mb) projecting from the plasma membrane (Pm) into the interior of the cell. This structure resembles a root of a flagellar apparatus as described below. . A further TEM section showing the detail of a section through a compound flagellar root, in proximity with the reticular mass. A flagellar base (Fb) can be seen associated with microtubular flagellar roots (Fr). Other abbreviations are as . Scale bars: 1 µm.
An endoplasmic tubular reticular body may be a common feature among the placolith-forming species that produce coccoliths one at a time. However, within the class Prymnesiophycae, E. huxleyi and C. pelagicus are only distantly related (Young et al ., Citation2005). Moreover, C. pelagicus is a member of the order Coccolithales within which there are classes that represent species that produce single (reticular body present) or multiple (reticular body absent) coccoliths. A possible explanation is that the reticular body is a morphological feature that appeared early in the evolution of placolith producing coccolithophores and has been subsequently lost or modified in a number of groups.
Compound flagellar root and contractile microtubules
Bundles of microtubules, resembling those commonly associated with flagellar roots of motile coccolithophores (Manton & Peterfi, Citation1969; Gayal & Fresnel, Citation1983; Inouye & Pienaar, Citation1988; Green & Hori, Citation1994; Fresnel & Probert, Citation2005), were observed in several ultrathin sections of the non-motile heterococcolith-bearing C. pelagicus (). The microtubular bundles were located in the gap between the two chloroplasts, extending some distance into the interior of the cell. We also observed well-developed compound flagellar roots, with associated microtubular bundles (). Such compound flagellar roots have previously been described in the motile stage of C. pelagicus (Klaveness, Citation1973) and were not reported in the previous ultrastructural study of non motile C. pelagicus, where flagellar bases alone were noted with no further comment (Manton & Leedale, Citation1969). Interestingly, both flagellar and haptonemal bases are present in the non motile placolith forming U. sibogae (Inouye & Pienaar, Citation1984). The retention of compound flagellar roots in the non-motile stages of placolith forming coccolithophores raises the question of their functional role (see below).
Organic un-mineralized scales and columnar deposit
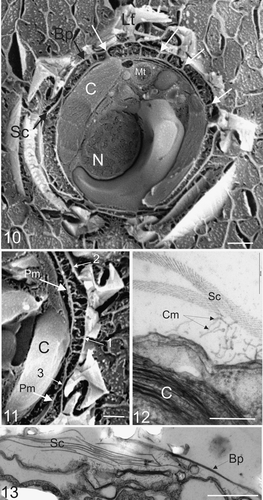
Scanning EM observations of plunge frozen and freeze fractured specimens () provided excellent resolution of all the major conspicuous organelles without the harsh chemical fixation procedures required for TEM sections (cf. ). Thus intact cells were well preserved with minimum disruption to the intracellular morphology. Coccoliths surrounding the cell were present and the arrangement of the coccosphere, underlying organic scales and organic baseplates were clearly resolved. A columnar deposit located between the plasma membrane and organic scale layer (), as originally described by Manton & Leedale (Citation1969), was clearly visible. The periplasmic space between the plasma membrane and organic layer was present in every freeze fracture specimen, and varied between 0.1 and 1 µm, which is consistent with the periplasmic space observed in TEM specimens. This shows that the protoplast is not tightly apressed to the organic layer and overlying coccosphere, which is consistent with the lack of significant turgor pressure in these cells (unpublished observations). Nevertheless, a close association between the cell and the coccosphere is maintained by the extracellular columnar deposits (). In addition, several points of direct contact between the plasma membrane, the organic scales and the organic baseplate of the coccoliths, similar to focal adhesions found in algae, plants and animals (Reuzeau & Pontlezica, Citation1995; Henry et al ., Citation1996) are present (). This provides strong evidence that the columnar strands observed in TEM sections (), which are a common feature in scale forming coccolithophores (Manton & Leedale, Citation1969; Manton & Peterfi, Citation1969; Hawkins & Lee, Citation2001), act to adhere the organic scales to the surface of the cell and prevent premature detachment of both scales and coccoliths. Moreover, this adhesive function is likely to be especially important in retention of coccoliths in circumstances where the coccosphere is incomplete. This is supported by our observations of coccolith production and secretion during re-calcification of naked cells (see , ).
Figs. 10–13. External and internal morphology of C. pelagicus using freeze fracture SEM and TEM. . Freeze fracture through the centre of a whole C. pelagicus cell showing fractured external interlocking coccoliths (Lt) with baseplate (Bp), layer of organic scales (Sc) connected to the plasma membrane by columnar strands and local adhesions spanning the periplasmic space (examples indicated with white arrows). The nucleus (N), Chloroplast (C) and mitochondria (Mt) are also easily distinguished. . Detail of a further freeze-fracture showing columnar strands forming attachments between the plasma membrane (Pm) and both the coccolith baseplate (1) and organic scales (2). Occasional close associations between the layer of organic scales and plasma membrane are observed (3). . TEM showing detail of columnar strands (Cm) connecting the plasma membrane to organic scales (Sc). The chloroplast (C) is clearly visible. . TEM section at the surface of cell showing organic scales interspersed between coccolith baseplates. Scale bars: 2 µm (), 1 µm (), 200 nm () and 1 µm ().
Coccolithogenesis
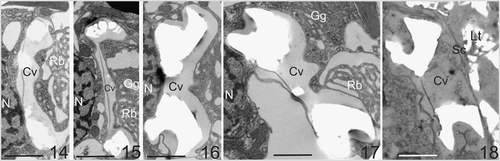
The crystal deposition of coccoliths followed a pattern previously described for E. huxleyi (de Vrind-de Jong et al ., Citation1994) and inferred for C. pelagicus from light micrographs (Manton & Leedale, Citation1969). Both TEM (Figs ) and time-lapse imaging () confirm that, as in E. huxleyi, a protococcolith ring of calcite (Westbroek et al ., Citation1989; Young et al ., Citation1999) forms first at the peripheral edge of the organic baseplate and is extended in both directions. At maturity, the calcite coccolith is structurally connected only to the peripheral margins of the baseplate (Figs ). The coccolith vesicle retains a close association with the nucleus until fully mature (). It then moves away from the nucleus prior to secretion (), which occurs between the two chloroplasts at the anterior pole of the cell. Release of the coccolith to the exterior () occurs after fusion of the coccolith vesicle membrane with the plasma membrane. The coccolith must then perforate the layer of organic scales before interlocking with neighbouring coccoliths on the surface of the cell. The organic layer consists of scales as previously described (Manton & Leedale, Citation1969) that overlap to varying degrees in layers up to eight scales thick. In some areas beneath the baseplate of coccoliths, organic scales are absent (see ). Scales were always observed in the gaps between two interlocking coccoliths. This probably arises as a result of the newly emerging coccolith being pushed through and perforating the layer of organic scales before interlocking with other coccoliths to form the external coccosphere.
Figs. 14–18. Typical ultrastructural sequence of coccolith formation in C. pelagicus. . Newly forming coccoliths with rim of calcite deposit. The coccolith vesicle (Cv) is closely associated with the nucleus (N). Reticular (Rb) and Golgi bodies (Gg) are located in the distal pocket of the coccolith vesicle. . A mature coccolith within a vesicle that remains closely associated with the nucleus. . A mature coccolith vesicle disassociating from the nucleus prior to release. Again, the Golgi body and reticular mass are clearly located in the distal pocket formed by the coccolith vesicle. . A mature coccolith at the surface of the cell shortly after vesicle fusion. Note the proximal coccolith vesicle (Cv). membrane is intact and the distal coccolith vesicle membrane is no longer present. The coccolith has not emerged through the layer of scales (Sc) below the external layer of coccoliths (Lt) in this section. Scale bars: 2 µm.
Live cell imaging of coccolith secretion
Previously no TEM data on the extrusion of the coccolith from the C. pelagicus cell has been published. This suggests that, for the majority of coccolithogenesis, the coccolith stays closely apposed to the nucleus (Figs ), only leaving this position in the final stages when it is extruded from the cell in a relatively rapid sequence of events. Thus, detailed observation of the secretory event using TEM has been remained elusive. High resolution time-lapse live cell imaging of previously decalcified cells provided an alternative approach to observe calcification and coccolith secretion in detail. shows a typical live-cell secretion sequence where a coccolith was presented roughly perpendicular to the plasma membrane. The secretory process from initiation of exocytosis to final settlement of the coccolith onto the cell surface was rapid, occasionally taking just 60 s but on average 164 ± 8 s (n = 77). The time taken for coccolith exocytosis was not significantly different between the different light treatments (data not shown). The coccolith subsequently emerged in a smooth curved trajectory accompanied by considerable contractile activity of the protoplast (see also ), which pushed the emerging coccolith out and away from the cell. A close association between the proximal surface of the coccolith and the cell remained, and the coccolith was drawn back as the cell rounded up, eventually settling on the cell surface. The columnar deposits and plasma membrane adhesions described above are likely to play an important role here.
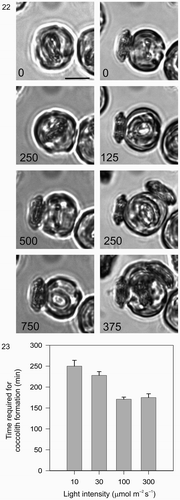
Fig. 19. Time-lapse video analysis of coccolith secretion. Bright field images of a decalcified C. pelagicus cell with mature internal coccolith taken every 30 s (time in seconds indicated on each panel) during a coccolith secretion event. The coccolith initially emerges tangentially (0–60 s) followed by a curved trajectory (90–180 s) before lying flat on the cell surface. The secretion event is accompanied by considerable contractile activity of the protoplast (e.g. 120 s), see also . Scale bars: 10 µm, time indicated is s.
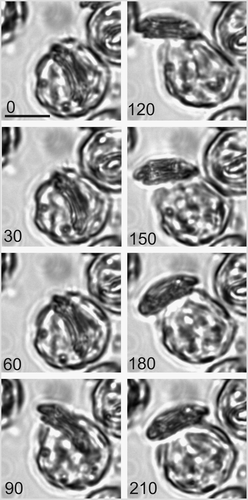
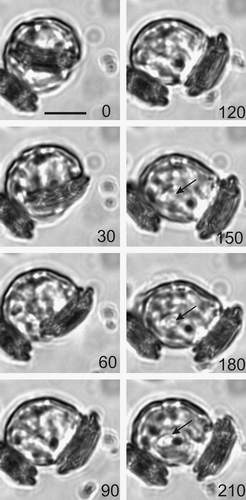
Fig. 20. Location of coccolith secretion. Time-lapse images of a second coccolith secreted by a cell after decalcification. The sequence shows a cell with one previously secreted coccolith. The cell rotated approximately 90° with respect to the first coccolith before secretion of the second coccolith. The second coccolith emerged in a 60 s period (30–90 s) followed by contractile activity that ‘pushes’ the coccolith away from the cell. Formation of the third coccolith can be detected within 60 s of coccolith secretion (indicated by arrow at 150 s). Scale bar: 10 µm.
The contractile behaviour of the cell that accompanies coccolith extrusion indicates that the co-ordinated activity of the cytoskeleton is critical in this process. The presence of a compound flagellar root with associated bands of microtubules () that extend into the cytoplasm from a location near the exit site at the anterior pole of the non-flagellated heterococcolith-bearing cell is intriguing in this respect. Flagellar root contractions that are dependent on calcium, ATP and the contractile protein centrin, have been described in the prasinophycean Tetraselmis Stein (Salisbury et al ., Citation1984) and in a range of dinoflagellates (Cachon et al ., Citation1994). In Chlamydomonas Dangeard such flagellar root contractions are responsible for rapid nuclear movement during deflagellation (Salisbury et al ., Citation1987) and in dinoflagellates they play a role in visceral contractions and excretion via contractile vesicles (Cachon et al ., Citation1983). Whether or not the microtubule-based flagellar root apparatus described here has any role in coccolithogenesis and secretion in this non-motile strain of C. pelagicus remains to be determined. While the present study does not provide evidence for centrin-containing structures, the co-ordinated contractile activity during coccolith extrusion suggests the involvement of a contractile, cytoskeletal-based mechanism. This hypothesis is supported by the observation that filamentous actin is specifically localized to the site of scale production and secretion in Pleurochrysis Pringsheim (Hawkins et al ., Citation2003). Moreover, it has been proposed that microtubular structures associated with the flagellar apparatus in Pleurochrysis pseudoroscoffensis Gayral et Fresnel may underlie protoplast contractions associated with internal cricolith migration (Gayral & Fresnel, Citation1983). Cytoskeletal dynamics in relation to coccolith formation, cellular polarity and coccolith exocytosis certainly now warrant detailed examination.
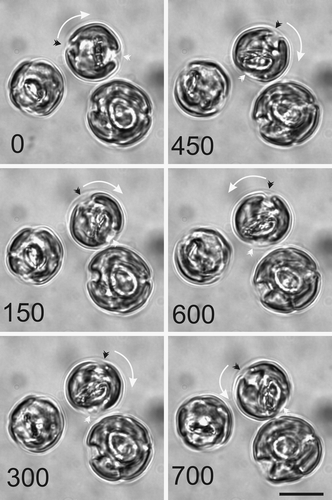
The polar nature of secretion has been postulated in many models of coccolith formation (Westbroek et al ., Citation1984). Our TEM data support the contention that coccolith secretion occurs at the anterior pole of the cell between the chloroplasts. If the site of coccolith secretion from the cell is fixed, the question arises of how the cell fills any gaps in a coccosphere, or indeed how the cell co-ordinates the precise location of coccolith secretion, such that the new coccolith emerges and interlocks between two external coccoliths. Time-lapse observations revealed that decalcified C. pelagicus cells, while remaining in a fixed location on the microscope slide, can secrete coccoliths in different locations. This is demonstrated in , where an external coccolith is present and the cell secretes the second coccolith in a different location on the cell surface. Further observations of sequences of coccolith secretion show that the position of coccolith secretion, while broadly polar in surface position, varies in precise location of release (, see also ). Significantly and unexpectedly, we show that the variable location of coccolith secretion arises as a result of the cell rotating relative to the layer of organic scales. illustrates the typical motile behaviour of actively calcifying cells. Cells exhibit continuous visceral contractions and slow rotary movements in variable directions within the layer of organic scales (by which the cell is fixed to the glass slide). The ability of the cell to rotate freely, relative to the coccosphere, not only provides evidence for a novel functional role of this layer but also explains how cells can secrete a new coccolith, away from the precise location of the previously deposited coccolith, and thus effectively maintain a complete and continuous coccosphere. The columnar deposits and focal adhesions to the organic layer and scales described above may provide temporary anchorage points during protoplast movements.
Fig. 21. Cell motion during coccolith development. A series of light micrographs acquired in sequence illustrate the rotary motility typically exhibited by actively calcifying C. pelagicus cells. The anterior-posterior axis of the uppermost cell is marked by a black and a white arrowhead. This axis has rotated first clockwise (0–450 s) followed by an anticlockwise movement (450–700 s). The direction of rotation is shown with a white arrow. The other cells in this field are also exhibiting movement with respect to their fixed position on the microscope slide. Scale bars: 10 µm.
Time-lapse analysis also confirmed that light-dependent formation of coccoliths in C. pelagicus is a sequential but uninterrupted process. Coccoliths were produced one by one, with the intracellular nucleation of a new proto-coccolith ring evident immediately on secretion of a mature coccolith (). The fact that only one developing coccolith was ever observed is consistent with sequential coccolith production in this species. The observation of rapid onset of calcite precipitation on a coccolith baseplate, observable only seconds after a secretion event, points to complex intracellular signalling that regulates coccolith calcification and co-ordinates this with secretion, such that only one coccolith is produced at a time.
The light dependency of coccolith formation in a single cell of C. pelagicus was also readily demonstrated using time-lapse imaging at different light levels (). Coccolithogenesis in low light (10 µmol m−2 s−1) took an average of 250 min (±14 SE) per coccolith. Coccolith production rate became light saturated between 30 and 100 µmol m−2 s−1, with a minimum average time of 170 min (±5 SE). These single-cell observations of coccolith formation in the light in C. pelagicus are consistent with tracer studies on populations of cells, showing co-dependence of calcium uptake on light (Linschooten et al ., Citation1991) and of whole cell calcification on light and photosynthesis in E. huxleyi (Balch et al ., Citation1992). Although calcite production was not quantified, our experiments support the hypothesis that rate of formation and secretion of individual coccoliths in C. pelagicus can account for the light-dependency of calcite production rather than a significant alteration in calcite incorporation per coccolith, which has been demonstrated in some strains of E. huxleyi (Paasche, Citation1999). Moreover, coccolith mineralization was observed to occur in the dark at a very low rate (), showing that calcification in C. pelagicus proceeds, albeit very slowly, in the absence of photosynthesis. This is consistent with observations of dark calcification in E. huxleyi where 14C incorporation into calcite was found to vary between 80 and 20% of light saturated rates in calcified cells (Balch et al ., Citation1992) and occurred at approximately 10% of light saturated rates in previously decalcified cells (Paasche, Citation1966). Moreover, in E. huxleyi experiments with respiratory inhibitors have shown that dark respiration provides the energy source for the low rates of calcification observed in the dark (Sekino & Shiraiwa, Citation1996). It is thus likely that the energetic requirements for dark calcification in C. pelagicus are similarly met by dark respiration.
Figs. 22, 23. Light dependency of coccolith formation. . A single cell was monitored after decalcification. First, in the dark (0–750 min) followed by a period in the light (0–375 min, 100 µmol m−2 s−1). During the dark period, a secretion event of an existing mature coccolith occurred followed by the initiation of a new coccolith that developed only very slowly and which was not secreted during the first 750 min recording period. During the subsequent 375 min light period, the cell rapidly completed the formation and secretion of three coccoliths. Note the location of the second coccolith (250 min), which reflects the fact that the cell rotated approximately 90° with respect to the first coccolith before secreting. The third coccolith was secreted between the first and second. Cell size increased noticeably in the light compared to the dark. Scale bar: 10 µm. . Graphical representation of the effect of light on coccolith formation in C. pelagicus. Each bar represents the average time (±SE) of coccolithogenesis from a minimum of 20 cells at each light level, the experiment was repeated at least three times for each light treatment.
Conclusion
We show here that the pattern of coccolith formation in C. pelagicus is broadly consistent with that of other placolith-bearing species, such as E. huxleyi. We report the novel finding that rapid secretion of individual coccoliths is associated with both contractile and rotatory movements of the protoplast, which position the site of polarized coccolith secretion. This is supported by freeze-fracture and TEM studies showing ultrastructural connections between the protoplast surface and the overlying external organic components. We also show that the rapid process of coccolith secretion is light-independent while coccolithogenesis is strongly light dependent. Time-lapse imaging provides a tool for detailed temporal analysis of coccolith formation in individual cells under varying environmental treatments.
Acknowledgements
We thank Roy Moate (University of Plymouth Electron Microscopy Unit) for helpful technical discussions and two anonymous referees for their helpful comments. This work was supported by BBSRC 226/P15068 to ART and CB and NERC grant-in-aid to the Marine Biological Association.
References
- Balch , WM , Holligan , PM and Kilpatrick , KA . 1992 . Calcification, photosynthesis and growth of the bloom-forming coccolithophore, Emiliania huxleyi . Cont. Shelf Res. , 12 : 1353 – 1374 .
- Braarud , T and Nordli , E . 1952 . Coccoliths of Coccolithus huxleyi seen in an electron microscope . Nature , 170 : 361 – 362 .
- Brand , LE . 1984 . “ Physiologial ecology of marine coccolithophores ” . In Coccolithophores , Edited by: Winter , A and Siesser , WG . 39 – 49 . Cambridge , , UK : Cambridge University Press .
- Brownlee , C and Taylor , AR . 2004 . “ Calcification in coccolithophores: a cellular perspective ” . In Coccolithophores: From Molecular Processes to Global Impact , Edited by: Thierstein , HR and Young , JR . 31 – 49 . Berlin , , Germany : Springer .
- Cachon , J , Caboche , M and Boillot , A . 1983 . Flagellar rootlets as myonemal elements for pusule contractility in dinoflagellates . Cell Motil. Cytoskel. , 3 : 61 – 77 .
- Cachon , J , Cachon , M , Greuet , C and Huitorel , P . 1994 . Nanofilament dependent motility in dinoflagellates . Biol. Cell , 81 : 1 – 10 .
- Corstjens , P , Van Der Kooij , A , Linschooten , C , Brouwers , GJ , Westbroek , P and De Vrind-De Jong , EW . 1998 . GPA, a calcium-binding protein in the coccolithophorid Emiliania huxleyi (Prymnesiophyceae) . J. Phycol. , 34 : 622 – 630 .
- Davey , MS , Suggett , DJ , Geider , RJ and Taylor , AR . 2003 . Phytoplankton plasma membrane redox activity: effect of iron limitation and interaction with photosynthesis . J. Phycol. , 39 : 1132 – 1144 .
- De Vrind-De Jong , EW , Van Emburg , PR and De Vrind , JPM . 1994 . “ Mechanisms of calcification: Emiliania huxleyi as a model system ” . In The Haptophyte Algae , Edited by: Green , JC and Leadbeater , BSC . 149 – 166 . Oxford , , UK : The Systematics Association, Clarendon Press .
- Fresnel , J and Probert , I . 2005 . The ultrastructure and life cycle of the coastal coccolithophorid Ochrosphaera neapolitana (Prymnesiophyceae) . Eur. J. Phycol. , 40 : 105 – 122 .
- Gayral , P and Fresnel , J . 1983 . Description, sexualité et cycle de développement d'une nouvelle coccolithophoracée (Prymnesiophyceae): Pleurochrysis pseudoroscoffensis sp. nov . Protistologica , 19 : 245 – 261 .
- Geisen , M , Billard , C , Broerse , ATC , Cros , L , Probert , I and Young , JR . 2002 . Life-cycle associations involving pairs of holococcolithophorid species: intraspecific variation or cryptic speciation? . Eur. J. Phycol. , 37 : 531 – 550 .
- Green , JC and Hori , T . 1994 . “ Flagella and flagella roots ” . In The Haptophtye Algae , Edited by: Green , JC and Leadbeater , BSC . 47 – 71 . Oxford , , UK : Clarendon Press .
- Guillard , RRL and Ryther , JH . 1962 . Studies on marine planktonic diatoms. 1. Cyclotella nana Hustedt and Detonula confervavae (Cleve) Gran . Can. J. Bot. , 8 : 229 – 239 .
- Hawkins , EK and Lee , JJ . 2001 . Architecture of the Golgi apparatus of a scale-forming alga: biogenesis and transport of scales . Protoplasma , 216 : 227 – 238 .
- Hawkins , EK , Lee , JJ and Correia , M . 2003 . Polar localization of filamentous actin in cells of the scale-forming alga Pleurochrysis sp . Protoplasm a , 220 : 233 – 236 .
- Henriksen , K , Stipp , SLS , Young , JR and Marsh , ME . 2004 . Biological control on calcite crystallization: AFM investigation of coccolith polysaccharide function . Am. Mineral. , 89 : 1709 – 1716 .
- Henry , CA , Jordan , JR and Kropf , DL . 1996 . Localized membrane-wall adhesions in Pelvetia zygotes . Protoplasma , 190 : 39 – 52 .
- Holligan , PM , Fernandez , E , Aiken , J , Balch , WM , Boyd , P , Burkill , PH , Finch , M , Groom , SB , Malin , G , Muller , K , Purdie , DA , Robinson , C , Trees , CC , Turner , SM and Van Der Wal , P . 1993 . A Biogeochemical study of the coccolithophore, Emiliania-huxleyi, in the North-Atlantic . Glob. Biogeochem. Cy. , 7 : 879 – 900 .
- Houdan , A , Billard , C , Marie , D , Not , F , Saez , AG , Young , JR and Probert , I . 2004 . Holococcolithophore-heterococcolithophore (Haptophyta) life cycles: flow cytometric analysis of relative ploidy levels . Systematics and Biodiversity , 1 : 453 – 465 .
- Inouye , I and Pienaar , RN . 1984 . New observations on the coccolithophorid Umblilicosphaera sibogae var. foliosa (Prymnesiophyceae) with reference to cell covering, cell structure and flagellar apparatus . Br. Phycol J. , 19 : 357 – 369 .
- Inouye , I and Pienaar , RN . 1988 . Light and electron microscope observations of the type species of Syracosphaera, S. pulchra (Prymnesiophyceae) . Br. Phycol. J. , 23 : 205 – 217 .
- Klaveness , D . 1972 . Coccolithus huxleyi (Lohmann) Kamptner I -- Morphological investigations on the vegetative cell and process of coccolith formation . Protistologica , 8 : 335 – 346 .
- Klaveness , D . 1973 . The microanatomy of Calyptrosphaera sphaeroidea, with some supplementary observations on the motile stage of Coccolithus pelagicus . Norw. J. Bot. , 20 : 151 – 162 .
- Klaveness , D . 1976 . Emiliania huxleyi (Lohmann) Hay & Mohler. III Mineral deposition and the origin of the matrix duirng coccolith formation . Protistologica , 12 : 217 – 224 .
- Linschooten , C , Van Bleijswijk , JDL , Van Emburg , PR , De Vrind , JPM , Kempers , ES , Westbroek , P and De Vrind-De Jong , EW . 1991 . Role of the Light-dark cycle and medium composition on the production of coccoliths by Emiliania-huxleyi (Haptophyceae) . J. Phycol. , 27 : 82 – 86 .
- Manton , I and Leedale , GF . 1969 . Observations on the microanatomy of Coccolithus pelagicus and Cricosphaera carterae, with special reference to the origin and nature of coccoliths and scales . J. Mar. Biol. Assoc. UK , 49 : 1 – 16 .
- Manton , I and Peterfi , LS . 1969 . Observations on the fine structure of coccoliths, scales and the protoplast of a freshwater coccolithophorid. Hymenomas roseola Stein, with supplementary observations on the protoplast of Cricosphaera carterae . Proc. R. Soc. Lond. Ser. B , 172 : 1 – 15 .
- Marsh , ME . 1994 . Polyanion-mediated mineralization -- assembly and reorganization of acidic polysaccharides in the Golgi system of a coccolithophorid alga during mineral deposition . Protoplasma , 177 : 108 – 122 .
- Marsh , ME . 1999 . Coccolith crystals of Pleurochrysis carterae: crystallographic faces, organization, and development . Protoplasma , 207 : 54 – 66 .
- McIntyre , A and Be , AWH . 1967 . Modern coccolithophoridae of the Atlantic ocean. Placoliths and cyrtoliths . Deep Sea Res. , 14 : 561 – 597 .
- Outka , DE and Williams , DC . 1971 . Sequential coccolith morphogenesis in Hymenomonas-carterae . J. Protozool. , 18 : 285 – 297 .
- Paasche , E . 1966 . Adjustment to light and dark rates of coccolith formation . Physiol Plant , 19 : 271 – 278 .
- Paasche , E . 1999 . Reduced coccolith calcite production under light- limited growth: a comparative study of three clones of Emiliania huxleyi (Prymnesiophyceae) . Phycologia , 38 : 508 – 516 .
- Paasche , E . 2002 . A review of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae), with particular reference to growth, coccolith formation, and calcification-photosynthesis interactions . Phycologia , 40 : 503 – 529 .
- Parke , M and Adams , I . 1960 . The motile (Crystallolithus hyalinus Gaarder & Markali) and non-motile phases in the life history of Coccolithus pelagicus (Wallich) Schiller . J. Mar. Biol. Assoc. UK , 39 : 263 – 274 .
- Pienaar , RN . 1969 . The fine structure of Hymenomonas (Cricosphaera) carterae. II. Observations on scale and coccolith production . J. Phycol. , 5 : 321 – 331 .
- Reuzeau , C and Pontlezica , RF . 1995 . Comparing plant and animal extracellular matrix-cytoskeleton connections – are they alike? . Protoplasma , 186 : 113 – 121 .
- Rickaby , REM , Schrag , DP , Zondervan , I and Riebesell , U . 2002 . Growth rate dependence of Sr incorporation during calcification of Emiliania huxleyi . Glob. Biogeochem. Cycle , 16 doi: 10.1029/2001GB001408
- Rost , B and Riebesell , U . 2004 . “ Coccolithophores and the biological pump: responses to environmental changes ” . In Coccolithophores, from Molecular Processes to Global Impact , Edited by: Thierstein , HR and Young , JR . 99 – 125 . Heidelberg , , Germany : Springer .
- Saez , AG , Probert , I , Geisen , M , Quinn , P , Young , JR and Medlin , LK . 2003 . Pseudo-cryptic speciation in coccolithophores . Proc. Natl. Acad. Sci. USA. , 100 : 7163 – 7168 .
- Salisbury , J , Baron , A , Surek , B and Melkonian , M . 1984 . Striated flagellar roots: isolation and partial characterization of a calcium- modulated contractile organelle . J. Cell Biol. , 99 : 962 – 970 .
- Salisbury , J , Sanders , M and Harpst , L . 1987 . Flagellar root contraction and nuclear movement during flagellar regeneration in Chlamydomonas reinhardtii . J. Cell Biol. , 105 : 1799 – 1805 .
- Sekino , K and Shiraiwa , Y . 1996 . Evidence for the involvement of mitochondrial respiration in calcification in a marine coccolithophorid, Emiliania huxleyi . Plant Cell Physiol. , 37 : 1030 – 1033 .
- Stoll , HM , Rosenthal , Y and Falkowski , P . 2002 . Climate proxies from Sr/Ca of coccolith calcite: calibrations from continuous culture of Emiliania huxleyi . Geochim. Cosmochim. Acta , 66 : 927 – 936 .
- Taylor , AR and Brownlee , C . 2003 . A novel Cl inward-rectifying current in the plasma membrane of the calcifying marine phytoplankton Coccolithus pelagius . Plant Physiol. , 131 : 1391 – 1400 .
- Thierstein , HR and Young , JR . 2004 . Coccolithophores, from Molecular Processes to Global Impact Springer, Berlin , , Germany
- Van Der wal , P , Dejong , EW , Westbroek , P , Debruijn , WC and Mulderstapel , AA . 1983a . Ultrastructural polysaccharide localization in calcifying and naked cells of the coccolithophorid Emiliania-huxleyi . Protoplasma , 118 : 157 – 168 .
- Van Der Wal , P , Dejong , EW , Westbroek , P , Debruijn , WC and Mulderstapel , AA . 1983b . Polysaccharide localization, coccolith formation, and Golgi dynamics in the coccolithophorid Hymenomonas-carterae . J. Ultrastruct. Res. , 85 : 139 – 158 .
- Westbroek , P , Dejong , EW , Van Der Wal , P , Borman , AH , De Vrind , JPM , Kok , D , De Bruijn , WC and Parker , SB . 1984 . Mechanism of calcification in the marine alga Emiliania-huxleyi . Philos. Trans. R. Soc. Lond. Ser. B-Biol. Sci. , 304 : 435 – 444 .
- Westbroek , P , Young , JR and Linschooten , K . 1989 . Coccolith production (biomineralization) in the marine alga Emiliania-huxleyi . J. Protozool. , 36 : 368 – 373 .
- Wilber , KM and Watabe , N . 1963 . Experimental studies on calcification in molluscs and the alga Coccolithus huxleyi . Ann. NY Acad. Sci. , 109 : 82 – 112 .
- Young , JR . 1994 . “ Functions of coccoliths ” . In Coccolithophores , Edited by: Winter , A and Siesser , WG . 63 – 82 . Cambridge , , UK : Cambridge University Press .
- Young , JR , Didymus , JM , Bown , PR , Prins , B and Mann , S . 1992 . Crystal assembly and phylogenetic evolution in heterococcoliths . Nature , 356 : 516 – 518 .
- Young , JR , Davis , SA , Bown , PR and Mann , S . 1999 . Coccolith ultrastructure and biomineralisation . J. Struct. Biol. , 126 : 195 – 215 .
- Young , JR , Henriksen , K and Probert , I . 2004 . “ Structure and morphogenesis of the coccoliths of the CODENET species ” . In Coccolithophores: From Cellular Processes to Global Impact , Edited by: Thierstein , HR and Young , JR . 191 – 216 . Heidelberg , , Germany : Springer .
- Young , JR , Geisen , M and Probert , I . 2005 . Review of selected aspects of coccolithophore biology with implications for paleobiodiversity estimation . Micropaleontology , 51 : 267 – 288 .