Abstract
Adhesion of raphid diatoms to natural surfaces, which is mediated by the secretion of extracellular polymeric substances (EPS), is an important strategy for growth and survival and contributes to the economically important process of biofouling. An understanding of adhesion processes requires that the genes involved in the EPS biosynthetic pathways and their regulation be characterized. Phaeodactylum tricornutum provides a model system in which to do this but the quantitative adhesion characteristics of the various morphotypes and isolates of this species are currently unknown. The present paper reports on the use of a calibrated fully turbulent flow cell to characterize the whole cell adhesion properties of morphotypes and strains of this species. It has been shown that only the oval cell morphotype adheres to a surface. There are strain/isolate differences in adhesion strength: some strains including strain Pt 1.8.6, the genome of which has been sequenced, show adhesion strengths comparable to other raphid diatoms. In common with some other raphid diatom species, adhesion strength of oval cells of some isolates of P. tricornutum was greater on a hydrophobic surface (Silastic T2 silicone elastomer), than on hydrophilic acid-washed glass. These studies provide a baseline for future molecular genetic and gene expression studies.
Introduction
Adhesion to a substratum through secretion of sticky extracellular polymeric substances (EPS), is an important strategy for growth and survival of motile benthic diatoms (recently reviewed by Underwood & Paterson, Citation2003; Chiovitti et al ., Citation2006). Adhesion provides traction for the ‘gliding’ form of motility used by motile pennate diatoms to adjust their position in relation to fluctuating environmental circumstances. The biochemical composition of EPS not only varies between different diatom species, but can also vary depending on the environmental conditions the diatom is being exposed to (Abdullahi et al ., Citation2006). EPS molecules of pennate diatoms are secreted through pores in the frustules and through the slit-like raphe, and recent studies using Atomic Force Microscopy (reviewed in Chiovitti et al ., Citation2006) clearly demonstrate differences in mechanical properties of EPS materials secreted from these two locations. EPS molecules secreted through the raphe link the cell cytoplasm to the substratum providing a method for ‘gliding’ motility via the adhesion complex (AC) involving an actin–myosin system located adjacent to each raphe (Edgar & Pickett-Heaps, Citation1984; Poulsen et al ., Citation1999). The cells once attached can rapidly divide giving rise to colonies that eventually coalesce to form a compact biofilm, which may achieve 500 µm in thickness (Callow, Citation1996). This can be beneficial where the diatoms help to stabilize benthic aquatic habitats and also represent an important food source for bacteria and grazers (Decho, Citation1990; Paterson, Citation1989; Underwood & Paterson, Citation2003).
Recent studies have also shown that some raphid diatom species exhibit differential adhesiveness in relation to the wettability of the substratum with cells. Finlay et al . (Citation2002) showed that Amphora coffeaeformis adheres more strongly to hydrophobic model surfaces in the form of self-assembled monolayers of methyl-terminated alkanethiols, than to OH-terminated alkanethiols, and Holland et al . (Citation2004) showed that Navicula perminuta, A. coffeaeformis and Craspedostauros australis adhered more strongly to hydrophobic surfaces in the form of silicone elastomers than to hydrophilic surfaces such as acid-washed glass. Clearly diatoms have the ability to distinguish the properties of a surface and modulate their adhesiveness accordingly. The mechanism of surface perception and response is unknown, although it presumably lies in quantity and/or quality of EPS produced. A fuller understanding of aspects of biosynthesis, structure and function of adhesive EPS at molecular, biochemical and physical levels (Chiovitti et al ., Citation2006) requires the use of model systems with known genome sequences, which are tractable to the tools of advanced molecular genetics, such as RNAi. In the case of motile pennate diatoms, the emerging candidate for such a model is Phaeodactylum tricornutum Bohlin. There have been a number of studies on the physiology, biochemistry and molecular biology of this species (Lewin et al ., Citation1958; Ford & Percival, Citation1965a , Citation b ; Scala et al ., Citation2002; Montsant et al ., Citation2005). Unlike other diatoms, it does not have an obligate requirement for silicon and can undergo morphological transitions between three possible morphotypes (Borowitzka & Volcani, Citation1978). The complete genome sequence of P. tricornutum will become available soon, along with the first microarrays (Scala et al ., Citation2002; Montsant et al ., Citation2005). Recent studies of the EPS of P. tricornutum have focused on the changes in the biochemical composition under changing environmental conditions (Abdullahi et al ., Citation2006) and nanomechanical properties of EPS adhesive fibres (Dugdale et al ., Citation2006). However, as yet there has been no systematic, quantitative study of whole cell adhesion properties of different isolates or morphotypes of P. tricornutum, nor whether they show differential adhesiveness in relation to surface wettability. This paper describes the first characterization of the whole cell adhesive strengths of different isolates of P. tricornutum as a baseline study for future attempts to manipulate adhesion through, for example, RNAi approaches, and to explore adhesion-related gene expression events through microarray analysis.
Materials and methods
Diatom culture
Nine isolates of P. tricornutum were provided by Dr C. Bowler (Stazione Zoologica, Naples, Italy). Isolate designations and the corresponding culture collection numbers are shown in . The isolates were cultured in artificial sea water supplemented with nutrients (Guillard's F/2 medium: Guillard & Ryther, Citation1962). All isolates grew with predominantly a fusiform morphology when cultured under static conditions in 25-ml vented tissue culture flasks (Falcon product number 3109) containing 10 ml medium inside a growth cabinet at 19°C with a 12:12h light–dark cycle (photon irradiance 330 µmol m−2 s−1 PAR). To obtain cultures predominantly oval in morphology, the isolates were grown under the same growth conditions as described above, but in 250-ml Pyrex conical flasks containing 100 ml media. After 3–5 days, depending on the isolate, the bases of the flasks were examined for the presence of oval cells. If present, the culture medium was removed, the flasks were rinsed with medium 2–3 times to remove any remaining fusiform cells, then 100 ml of fresh medium were added to each flask. Every 2 days the washing process was repeated until the cultures contained only oval cells. The exception to this was Pt 9, which required a cold shock to produce cells with an oval morphology; the culturing temperature for this isolate was 15°C.
Table 1. List of isolates/strains of Phaeodactylum tricornutum (Pt) provided by Dr C. Bowler (Stazioni Zoologica, Naples, Italy)
To prepare the fusiform cells for whole cell adhesion assays, the cells were taken from a log-phase culture and diluted to produce a chlorophyll a content of 0.3–0.4 µg ml−1, the precise value being verified after the experiment was set up. This corresponds to a cell concentration of 5 × 105 cells ml−1. Chlorophyll was extracted in DMSO (Shoaf & Lium, Citation1976) and quantified using the equations of Jeffrey & Humphrey (Citation1975). To obtain oval cells for adhesion assays, log-phase cultures were again used. Culture medium was replaced with fresh medium and the cells were removed from the bottom of the flask by dislodging them by gently shaking with 2-mm glass beads. The cells usually clumped together and, to obtain a suspension of mostly single cells, they were placed in a bead mill (Retsch MM200) at a frequency of 30 s−1 for 5 min. This procedure had no effect on the subsequent vitality and growth of the cells. The suspension was filtered through 35-µm nylon mesh and diluted to a chlorophyll a content of 0.3µg ml−1, again the precise value being confirmed after the experiment was set up.
Surfaces
The standard substratum used for adhesion assays was hydrophilic acid-washed glass (Holland et al ., Citation2004). Glass microscope slides were degreased in Decon detergent before soaking in 1M HCl for 24 h. The acid was removed by rinsing the slides in distilled water. The slides were rinsed in seawater before adding the diatom culture. The water contact angle of all the slides used in the assays was <15°.
The hydrophobic substratum used was glass microscope slides coated with the proprietary polydimethylsiloxane elastomer (PDMSE) in the form of Silastic® T2 (Dow-Corning Corporation), prepared at the University of Florida, Gainesville, as described in Hoipkemeier-Wilson et al . (Citation2004). The Silastic T2 mixture was applied to the treated glass slides, which were then placed in contact with a sheet of plate glass. The thickness of the PDMSE layer, typically 700 µm, was closely controlled by spacers within the mould. The elastomer was cured at 50°C for 5 h. No mould-release agents were used in the fabrication process. The modulus of the T2 PDMSE is 1.4 MPa, the water contact angle is typically 109 ± 3.5° and the calculated surface energy 23 ± 0.4 mN m−2 (for details of surface characterization, see Feinberg et al ., Citation2003).
Whole cell adhesion strength assays
Settlement assay and cell enumeration
Slides were placed in individual compartments of ‘quadriperm’ polystyrene culture dishes (Fisher) and 10 ml of either fusiform or oval cells at the correct dilution were added. Six replicates were used for each treatment. The cells were allowed to settle for 4 h at room temperature after which the slides were removed from the dishes and the gently rinsed to remove unattached cells by dipping each slide into a beaker of ASW. Three replicate slides were then fixed in 2.5% glutaraldehyde in sea water, desalted by washing first in 50 : 50 sea water: distilled water, followed by distilled water and dried before counting. Cells were counted in 30 fields of view from each of three replicate slides to provide initial cell adhesion data (i.e. control treatment, not exposed to flow). The remaining three replicates were used to evaluate the strength of diatom attachment in the flow channel, as detailed below. The cells were enumerated as above and the mean number of cells remaining attached to the surface after exposure to turbulent flow was compared with the mean number adhered to the control slides that were not exposed to turbulent flow. Data are expressed as percentage removal with 95% confidence limits calculated from arcsine-transformed data.
Cell detachment: water channel
The fully turbulent water channel exposes cells to calibrated wall shear stresses comparable to those that would be experienced around the hull of a ship under normal operational conditions (Schultz et al ., Citation2000; Finlay et al ., Citation2002; Schultz et al ., Citation2003). Slides were exposed to the fully-developed turbulent flow for 5 min at a range of wall shear stresses up to the maximum of 32 Pa, as previously described (Holland et al ., Citation2004).
Results
Fusiform v. oval cells
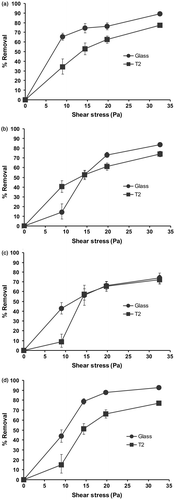
The adhesive capabilities of fusiform v. oval morphotypes were initially explored for isolate Pt 1.8.6. Results showed that oval cells adhered to both acid-washed glass and PDMSE but that fusiform cells did not adhere to either surface, or at least the adhesion was so weak that the brief, gentle rinsing step removed them (). Oval cells adhered more strongly to PDMSE compared with glass since a single shear stress (15 Pa) detached a smaller proportion of attached cells (approximately 21.5%) compared with 84% from glass. Experiments on fusiform morphotypes of the other strains also revealed very low initial levels of adhesion.
Fig. 1. (a) Cell density of Phaeodactylum tricornutum adhered to acid-washed glass or Silastic T2, before and after exposure to a shear stress of 15 Pa generated in a flow channel. (b) Mean percentage removal of fusiform or oval cells from the two surfaces after exposure to flow. Error bars are 95% confidence limits from arcsine-transformed data.
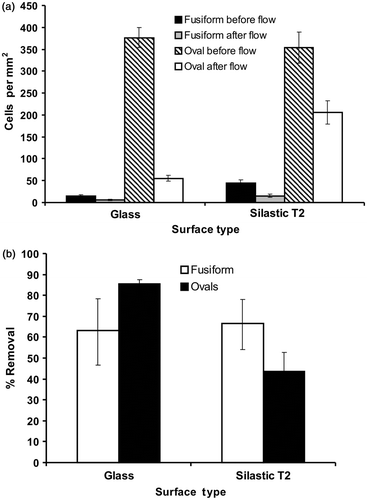
Adhesion assays for oval cells of all strains
Having established that only cells of the oval morphotype adhere, subsequent more detailed detachment assays were performed on the oval morphotypes of other P. tricornutum strains, using a full range of wall shear stresses (9, 14, 20 and 32 Pa). The strain Pt 4 which grows at a slower rate than the other strains and prefers brackish water, did not produce enough cells for a reliable assay. Comparison of the detachment curves () showed considerable variation between strains in adhesion strength. This is most easily seen by calculating the shear stress for 50% removal (). Pt 5 was the most strongly adhered (16.4 Pa for 50% removal). Three strains showed 50% removal at approximately 12–14 Pa (Pt 1.8.6, 13.60 Pa; Pt 7, 12.09 Pa; and Pt 8, 11.76 Pa). The remaining strains showed only low levels of adhesion with 65–90% removal at 10 Pa, the lowest shear stress tested.
Fig. 2. Mean percentage removal of Phaeodactylum tricornutum isolates with oval morphology from acid-washed glass at a range of wall shear stress values generated in a flow channel. For clarity the eight strains are depicted on two graphs. Error bars are 95% confidence limits from arcsine-transformed data.
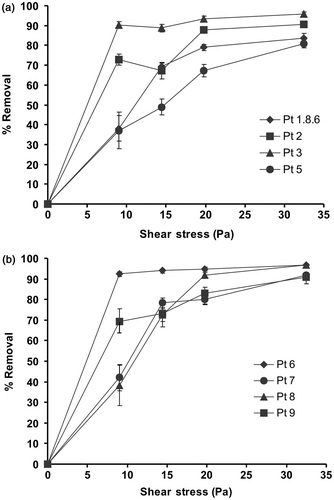
Table 2. Wall shear stress required to remove 50% of Phaeodactylum tricornutum cells with the oval morphology from acid-washed glass.
Hydrophobic v. hydrophilic surfaces
Adhesion strengths of oval cells on hydrophilic and hydrophobic surfaces was compared on those strains that demonstrated high to moderate adhesion strength on acid-washed glass (Pt 1.8.6, Pt 5, Pt 7 and Pt 8). The detachment curves () reveal that 2 of the 4 strains (Pt 1.8.6 and Pt 8) showed a clear trend of stronger adhesion to the hydrophobic silicone compared with the hydrophilic glass. Two strains (Pt 5 and Pt 7) showed a somewhat anomalous performance with a clear difference between the two surfaces only observed at low shear stresses.
Discussion
Settlement, the initial act of diatom adhesion to a surface, is not an active process. Unlike, for example, motile spores of algae, diatoms arrive at a surface through passive mechanisms, settling out usually under gravity. The initial weak attachment is probably through the EPS covering the cells (Callow, Citation2000; Finlay et al ., Citation2002) but then a more active, surface-sensing mechanism has been suggested to occur, (Cooksey & Wigglesworth-Cooksey, Citation1992; Wigglesworth-Cooksey & Cooksey, Citation1992; Chiovitti et al ., Citation2006), although direct evidence to support this is still lacking. The diatom will then become either motile or sessile with adhesion to the surface mediated by the secretion of EPS from the raphe (Edgar & Pickett-Heaps, Citation1984; Hoagland et al ., Citation1993). Becker (Citation1996) showed that the quantity of EPS produced by Amphora coffeaeformis differs depending on the surface tension of the substratum and that this species expresses hydrophobic polysaccharides in its EPS that are better suited to adhere to hydrophobic substrata.
The present study is the first in which the adhesion characteristics of Pt isolates and morphotypes have been studied through quantitative hydrodynamic methods. Lewin et al . (Citation1958) first observed that Phaeodactylum tricornutum can switch between fusiform and oval cell morphotypes depending on environmental conditions. Typically (Lewin et al ., Citation1958; Iwasa & Shimizu, Citation1972; Borowitzka & Volcani, Citation1978; Gutenbrunner et al ., Citation1994; Abdullahi et al ., Citation2006) the oval morphotype is enriched in cultures grown on solid media such as F/2 agar. The fusiform morphotype is dominant when cultures are subjected to phosphate and salinity stresses. In the present study we show that the oval cell morphotype is highly enriched (85–90%) if the cells are allowed to adhere to a glass surface of a culture flask and the non-adherent fusiform cells are removed by washing cycles. The non-adherent nature of fusiform cells was confirmed by the standard quantitative adhesion assay. The likely basis of these morphotype differences in adhesion lies in the dynamics and qualitative composition of the various types of EPS produced. In an exhaustive study of different EPS fractions of Pt morphotypes under different environmental conditions, Abdullahi et al . (Citation2006) showed significant differences in the proportions of various monosaccharides, chain terminal saccharides and the degree of sulphation. They speculated that such changes may influence EPS adhesive properties.
Diatoms responsible for biofouling not only differ in adhesive strength depending on the surface they are exposed to but also in terms of the difference in adhesive strength between species (Holland et al ., Citation2004). The isolate Pt 1.8.6 has been used in nearly all of the P. tricornutum studies described in the literature (Borowitzka & Volcani, Citation1978; Scala et al ., Citation2002; Montsant et al ., Citation2005; Dugdale et al ., Citation2006). It is from this isolate that the genome has been sequenced and the EST libraries created (Montsant et al ., Citation2005). There is clearly some variation in the adhesive characteristics of the various P. tricornutum isolates. Pt 5 in the present experiments was the most strongly adhering; its adhesion strength under shear was comparable to that shown by A. coffeaeformis and C. australis, raphid diatoms that effectively colonize surfaces. De Martino (pers. comm.) has identified several distinct genotypes amongst the nine isolates used in the present study, based on ITS 2 sequences and AFLP analysis. The groupings would seem to follow along the lines of the isolates' geographical locations. Those isolated from the UK (Pt 1.8.6, Pt 2 and Pt 3) grouped together with the tropical form Pt 9. Isolates Pt 6, Pt 7 and Pt 8 from the USA form their own clade, with Pt 4 (Finland) and Pt 5 (USA) appearing to be the most genetically distinct. However, the original geographical location of the isolates seems to have little bearing on their adhesive capabilities since isolates from within each clade demonstrated a range of adhesive strengths.
A further adhesion characteristic shared by at least some P. tricornutum isolates and other motile diatoms, is the stronger adhesion to the hydrophobic surface presented by the Silastic T-2 silicone elastomer (Holland et al ., Citation2004). This characteristic of strong adhesion to silicones has a practical consequence in the context of biofouling, it being commonly observed that ‘foul-release’ silicone elastomer coatings commonly become colonized by a slimy, brown diatom-rich biofilm (e.g. Terlizzi et al ., Citation2000). The basis of this preference is not known but it provides an interesting contrast with the adhesion characteristics of other fouling organisms such as barnacles and seaweeds that adhere more strongly to hydrophilic surfaces and weakly to hydrophobic silicones (e.g. Swain, Citation1999; Kavanagh et al ., Citation2001; Stein et al ., Citation2003; Finlay et al ., Citation2005; Sun et al ., Citation2005). The availability of a good model system in P. tricornutum, with publication of its complete genome sequence, and the development of microarrays (Scala et al ., Citation2002; Montsant et al ., Citation2005), now facilitates the analysis of surface preferences for adhesion through molecular genetic and gene expression studies. Microarrays can be used in ‘expression analysis’ or expression profiling. The array is typically hybridized with cDNA from two samples to be compared (e.g. non-adherent fusiform cells v. adherent ovals, cells from hydrophilic v. hydrophobic surfaces). These are labelled with two different fluorophores, the samples mixed and hybridized to the array, which is then scanned. This allows not only the visualization of up-regulated and down-regulated genes, but also an indication of the differential expression between the two samples being compared. The P. tricornutum adhesion assays reported in this paper provide clearly defined conditions for microarray analysis. This should provide information on the genes and pathways involved in the adhesive process, how the expression of these genes varies between the two different morphologies at an intra-species level (strong v. poor-adhering isolates) and what the influence of surface chemical and physical properties has on gene expression patterns.
Acknowledgements
This study was supported by the European Commission Sixth Framework Programme ‘Diatomics’, Contract number: 512035. We thank Chris Bowler and Alessandra de Martino (Stazione Zoologica, Naples) for supplying the cultures of P. tricornutum and Professor A. B. Brennan (University of Florida, Gainesville) for the supply of microscope slides coated with Silastic T2 silicone elastomer.
References
- Abdullahi , AS , Underwood , GJC and Gretz , MR . 2006 . Extracellular matrix assembly in diatoms (Bacillariophyceae). V. Environmental effects on polysaccharide synthesis in the model diatom, Phaeodactylum tricornutum . J. Phycol. , 42 : 363 – 378 .
- Becker , K . 1996 . Exopolysaccharide production and attachment strength of bacteria and diatoms on substrates with different surface tensions . Microb. Ecol. , 32 : 23 – 33 .
- Borowitzka , MA and Volcani , BE . 1978 . The polymorphic diatom Phaeodactylum tricornutum: Ultrastructure of its morphotypes . J. Phycol. , 14 : 10 – 21 .
- Callow , ME . 1996 . Ship-fouling: the problem and method of control . Biodeter. Abstr. , 10 : 411 – 421 .
- Callow , ME . 2000 . “ Algal Biofilms ” . In Biofilms: Recent Advances in their Study and Control , Edited by: Evans , LV . 189 – 209 . Amsterdam , , The Netherlands : Harwood Academic Publishers .
- Chiovitti , T , Dugdale , TM and Wetherbee , R . 2006 . “ Diatom adhesives: molecular and mechanical properties ” . In Biological Adhesives , Edited by: Smith , AM and Callow , JA . 79 – 103 . Berlin/Heidelberg , , Germany : Springer-Verlag .
- Cooksey , KE and Wigglesworth-Cooksey , B . 1992 . “ The design of antifouling surfaces: background and some approaches ” . In Biofilms – Science and Technology , Edited by: Melo , LF , Bott , TR , Fletcher , M and Capdeville , B . Dordrecht , , Germany : Kluwer Academic Publishers .
- Decho , AW . 1990 . Microbial exopolymer secretions in ocean environments: their role(s) in food webs and marine processes . Oceanogr. Mar. Biol. Annu. Rev. , 28 : 73 – 153 .
- Dugdale , TM , Willis , A and Wetherbee , R . 2006 . Adhesive modular proteins occur in the extracellular mucilage of the motile . pennate diatom Phaeodactylum tricornutum. Biophys. J. , 90 : L58 – L60 .
- Dugdale , TM , Dagastine , R , Chiovitti , A , Mulvaney , P and Wetherbee , R . 2005 . Single adhesive nanofibers from a live diatom have the signature fingerprint of modular proteins . Biophys. J. , 89 : 4252 – 4260 .
- Edgar , LA and Pickett-Heaps , JD . 1984 . Diatom locomotion . Prog. Phycol. Res. , 3 : 47 – 88 .
- Feinberg , AW , Gibson , AL , Wilkerson , WR , Seegert , CA , Wilson , LH , Zhao , LC , Baney , RH , Callow , JA , Callow , ME and Brennan , AB . 2003 . “ Investigating the energetics of bioadhesion on micro-engineered siloxane elastomers ” . In Synthesis and Properties of Silicones and Silcone-modified Materials , Edited by: Clarson , SJ , Fitzgerald , JJ , Owen , MJ , Smith , SD and van Dyke , ME . 196 – 211 . Washington , , USA : American Chemical Society (ACS) Symposium Series No. 838 .
- Finlay , JA , Callow , ME , Ista , LK , Lopez , GP and Callow , JA . 2002 . The influence of surface wettability on the adhesion strength of settled spores of the green alga Enteromorpha and the diatom . Amphora. Integrat. Compar. Biol. , 42 : 1116 – 1122 .
- Finlay , JA , Chung , JY , Chaudhury , MK , Callow , ME and Callow , JA . 2005 . The influence of elastic modulus and thickness on the release of the soft-fouling green alga Ulva linza (syn. Enteromorpha linza) from poly(dimethylsiloxane) (PDMS) ideal networks . Biofouling , 21 : 41 – 48 .
- Ford , CW and Percival , E . 1965a . The carbohydrates of Phaeodactylum tricornutum. Part I. preliminary examination of the organism, and characterization of low molecular weight material and of a glucan . J. Chem. Soc. , 1965 : 7035 – 7041 .
- Ford , CW and Percival , E . 1965b . The carbohydrates of Phaeodactylum tricornutum. Part II. A sulphated glucuronomannan . J. Chem. Soc. , 1965 : 7042 – 7046 .
- Guillard , RRL and Ryther , JH . 1962 . Studies on marine planktonic diatoms. 1. Cyclotella nana Hustedt and Detonula confervacea Cleve . Can. J. Microbiol. , 8 : 229 – 239 .
- Gutenbrunner , S , Thalhamer , J and Schmid , AM . 1994 . Proteinaceous and immunochemical distinctions between the oval and the fusiform morphotypes of Phaeodactylum tricornutum (Bacillariophyceae) . J.Phycol. , 30 : 129 – 136 .
- Hoagland , KD , Rosowski , JR , Gretz , MR and Roener , SC . 1993 . Diatom extracellular polymeric substances: function, fine structure, chemistry, and physiology . J. Phycol. , 29 : 537 – 566 .
- Hoipkemeier-Wilson , L , Schacher , JF , Carman , ML , Gibson , AL , Feinberg , AW , Callow , ME , Finlay , JA , Callow , JA and Brennan , AB . 2004 . Antifouling potential of lubricious, micro-engineered, PDMS elastomers against zoospores of the green fouling alga Ulva (Enteromorpha) . Biofouling , 20 : 53 – 63 .
- Holland , R , Dugdale , TM , Wetherbee , R , Brennan , AB , Finlay , JA , Callow , JA and Callow , ME . 2004 . Adhesion and motility of fouling diatoms on a silicone elastomer . Biofouling , 20 : 323 – 329 .
- Iwasa , K and Shimizu , A . 1972 . Motility of the diatom . Phaeodactylum tricornutum. Exp. Cell Res. , 74 : 552 – 558 .
- Jeffrey , SW and Humphrey , GF . 1975 . New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton . Biochem. Physiol. Pflanze , 167 : 191 – 194 .
- Kavanagh , CJ , Schultz , MP , Swain , GW , Stein , J , Truby , K and Darkangelo-Wood , C . 2001 . Variation in adhesion strength of Balanus eburneus, Crassostrea virginica and Hydroides dianthus to fouling-release coatings . Biofouling , 17 : 155 – 167 .
- Lewin , JC , Lewin , RA and Philipott , DE . 1958 . Observations on Phaeodactylum tricornutum . J. Gen. Microbiol. , 18 : 418 – 426 .
- Monstant , A , Jabbari , K , Matheswari , U and Bowler , C . 2005 . Comparative genomics of the pennate diatom Phaeodactylum tricornutum . Plant Physiol. , 137 : 500 – 513 .
- Paterson , DM . 1989 . Short term changes in the erodibility of intertidal cohesive sediments related to the migratory behaviour of epipelic diatoms . Limnol. Ocenogr. , 34 : 223 – 234 .
- Poulsen , NC , Spector , I , Spurck , TP , Schultz , TF and Wetherbee , R . 1999 . Diatom gliding is the result of an actin–myosin motility system . Cell Motility Cytoskeleton , 44 : 23 – 33 .
- Round , FE , Crawford , RM and Mann , DG . 1990 . The Diatoms , Cambridge , UK : Cambridge University Press .
- Scala , S , Carels , N , Falciatore , A , Chiusano , ML and Bowler , C . 2002 . Genome properties of the diatom . Phaeodactylum tricornutum. Plant Physiol. , 129 : 993 – 1002 .
- Schultz , MP , Finlay , JA , Callow , ME and Callow , JA . 2000 . A turbulent channel flow apparatus for the determination of the adhesion strength of microfouling organisms . Biofouling , 15 : 243 – 251 .
- Schultz , MP , Finlay , JA , Callow , ME and Callow , JA . 2003 . Three models to relate detachment of low form fouling at laboratory and ship scale . Biofouling , 19 ( Suppl ) : 17 – 26 .
- Shoaf , TW and Lium , BS . 1976 . Improved extraction of chlorophyll a and b from algae using dimethyl sulfoxide . Limnol. Ocenogr. , 21 : 926 – 928 .
- Stein , J , Truby , K , Darkangelo-wood , C , Takemori , M , Vallance , M , Swain , G , Kavanagh , C , Kovach , B , Schultz , M , Wiebe , D , Holm , E , Montemarano , J , Wendt , D , Smith , C and Meyer , A . 2003 . Structure-property relationships of silicone biofouling-release coatings: effect of silicone network architecture on pseudobarnacle attachment strengths . Biofouling , 19 : 87 – 94 .
- Sun , Y , Guo , S , Walker , GC , Kavanagh , CJ and Swain , GW . 2005 . Surface elastic modulus of barnacle adhesive and release characteristics from silicone surfaces . Biofouling , 20 : 279 – 289 .
- Swain , GW . 1999 . Redefining antifouling coatings . Paint Coat Europe , : 18 – 25 . July 1999
- Terlizzi , A , Conte , E , Zupo , V and Mazzella , L . 2000 . Biological succession on silicone fouling-release surfaces, long-term exposure tests in the harbour of Ischia, Italy . Biofouling , 15 : 327 – 342 .
- Underwood , GJC and Paterson , DM . 2003 . The importance of extracellular carbohydrate production by epipelic diatoms . Adv. Bot. Res. , 40 : 183 – 240 .
- Wigglesworth-Cooksey , B and Cooksey , KE . 1992 . Can diatoms sense surfaces? State of our knowledge . Biofouling , 5 : 227 – 238 .