Abstract
A morphological and molecular study of Fauchea repens, the type species of Fauchea, (Faucheaceae, Rhodymeniales) and its comparison with Gloiocladia furcata, the type species of Gloiocladia, establishes that the two are closely related and belong in the same genus. Accordingly, we propose a new combination, Gloiocladia repens (C. Agardh) Sánchez et Rodríguez-Prieto, comb. nov., for the plant presently known as Fauchea repens (C. Agardh) Montagne et Bory in Montagne and designate a lectotype. We provide an emended description of the genus Gloiocladia, and transfer all the species presently placed in Fauchea to Gloiocladia.
Introduction
The red algal family Faucheaceae Strachan, Saunders & Kraft is currently placed in the Rhodymeniales (Rhodophyta) (Saunders et al ., Citation1999). The genus Fauchea Montagne et Bory (in Montagne, Citation1846: 64) includes 13 species, widely distributed in tropical, subtropical and temperate seas (Bornet, Citation1890; Weber-van Bosse, Citation1928; Baardseth, Citation1941; Segawa, Citation1941; Yamada, Citation1941; Børgesen, Citation1944; Taylor, Citation1945, Citation1960; Sparling, Citation1957; Norris & Aken, Citation1985; Xia & Zhang, Citation1999), of which F. repens (C. Agardh) Montagne et Bory (in Montagne, Citation1846) is the type species. Generic delineation of the genus Fauchea has been based primarily on vegetative characters, presence of a tela arachnoidea (network of erect persistent filaments surrounding the procarp and the gonimoblast and developing from a basal nutritive tissue) in the cystocarp, cystocarp position and morphology, and by the arrangement and division pattern of the tetrasporangia (Montagne, Citation1846; Kylin, Citation1931; Irvine & Guiry, Citation1980; Norris, Citation1991). The absence of detailed information on the reproductive structures in the type species has prevented the realization of a clear concept of Fauchea and its relationship with Gloiocladia J. Agardh (Irvine & Guiry, Citation1980; Norris, Citation1991). Based on sequence analyses, Saunders et al . (Citation1999) included both genera in the family Faucheaceae, together with Gloioderma fructiculosa (Harvey) J. Agardh reported as Gloiocladia fruticulosa (Harvey) R.E. Norris.
This paper presents a detailed description of F. repens (as Gloiocladia repens (C. Agardh) Sánchez et Rodríguez-Prieto comb. nov.) based on fresh material collected during a study of the Faucheaceae of the Iberian Peninsula (Sánchez, Citation2005), along with a reinvestigation of original material housed in the Agardh Herbarium at Lund (LD) and material from other herbarium collections. We report new morphological data relating to vegetative and reproductive characters of G. repens, and have selected a lectotype for this species and reinvestigated the generic boundaries between Fauchea and Gloiocladia. We also generated 18S rRNA gene sequences from specimens of G. repens and G. furcata (C. Agardh) J. Agardh, and compared them with sequences from other Faucheaceae available in GenBank. Taxonomic changes include: a re-assessment of the genus Gloiocladia and transfer of Fauchea species to Gloiocladia, and further support for the reinstatement of the genus Gloioderma.
Materials and methods
Morphological analyses
Herbarium acronyms follow Holmgren et al . (Citation1990). Specimens of G. repens were collected by SCUBA from the Mediterranean coasts of Spain. Preserved specimens from the original collection of C. Agardh housed at LD, and others from HGI, MA, MGC, SANT, VAL and the personal herbarium of J.J. Rodríguez y Femenías, located in the Ateneu of Maó, Minorca (Spain), were also examined. Sections were made with a razor blade or a freezing microtome, stained with acidified aqueous 1% aniline-blue and mounted in 50% Karo(r) corn syrup (Bestfoods, Englewood Cliffs, NJ, USA). Habit photographs were taken with a Pentax Program camera (Pentax, Golden, CO, USA). Photomicrographs were taken with a Spot Insight digital camera (Diagnostic Instruments, Sterling Heights, MI, USA) attached to an Axioskop 2 plus microscope (Zeiss, Berlin, Germany). Voucher specimens and slides were deposited in the Herbarium of the University of Girona, Spain (HGI).
Molecular analyses
Total genomic DNA was extracted from specimens of G. repens from Columbretes Islands, Spain and of G. furcata from Formigues Islands, Spain (desiccated in silica gel or in 95% ethanol) following Hughey et al . (Citation2001), with a final cleaning step using the Gene Clean II kit (Bio 101, Vista, CA, USA). The nuclear-encoded 18S ribosomal RNA gene was amplified and sequenced as described in Freshwater et al . (Citation2005) using the oligonucleotide primers published by Saunders & Kraft (Citation1994). Sequencing reactions were run on an ABI 3100 Genetic Analyzer (DNA Analysis Core Facility, Centre for Marine Science, UNCW) and the reaction results edited and sequence contigs created using Sequencher (Gene Codes Corp., Ann Arbor, MI, USA). The resulting sequences have been deposited in GenBank under the accession numbers DQ790749 and DQ790750.
An 18S rDNA alignment including the two newly generated sequences, seven Faucheaceae sequences available in GenBank and a Lomentaria baileyana (Harvey) Farlow sequence as the outgroup, was assembled using MacClade (v. 4, Maddison & Maddison, Citation2000). Lomentaria baileyana was chosen as the outgroup for a number of reasons: (i) The Lomentariaceae and Faucheaceae are sibling groups in all published SSU analyses (Saunders et al ., Citation1999; Freshwater et al ., Citation2005); (ii) the topological arrangement of species within the Faucheaceae is basically the same in all published SSU trees and our unpublished analyses including 49 non-Faucheaceae Rhodymeniales whether or not L. baileyana is the only outgroup; (iii) various permutations of non-Faucheaceae species as outgroups did not change the basic Faucheaceae topology; and (iv) uncertain homology among sites was small when L. baileyana was the only outgroup included in the alignment. GenBank accession numbers for previously published sequences are shown under the relevant species names in . Alignment of these sequences was straightforward and performed by eye. Sites from the 5′ and 3′ ends of the alignment were removed due to missing data, so that the final alignment included 1,676 sites. Two internal regions of the alignment totalling 10 sites were excluded from analyses because the homology of these sites was uncertain. Data set characteristics and rate models were determined using MacClade, Modeltest (Posada & Crandall, Citation1998) and PAUP (Swofford, Citation2002). The aligned sequence data set and model parameters are available from the second author (DWF). Phylogenetic analyses using maximum likelihood (ML), maximum parsimony, and distance algorithms were performed using PAUP. ML searches were performed using the Branch-and-Bound algorithm and evolutionary model derived with Modeltest. ML bootstrap analyses consisted of 463 replications of Branch-and-Bound searches with the Modeltest model. Distance analyses consisted of neighbor-joining (NJ) tree building, using distances corrected with the same model used in the ML analyses. NJ bootstrap analyses were based on 5,000 replications of NJ tree building with the same distance correction. Parsimony searches were performed using the Branch-and-Bound algorithm and parsimony bootstrap analyses consisted of 2,000 replications of Branch-and-Bound searches.
Observations
Gloiocladia repens (C. Agardh) Sánchez et Rodríguez-Prieto comb. nov. (Figs )
BASIONYM: Sphaerococcus repens C. Agardh, Citation1823: 244.
NOMENCLATURAL SYNONYMS:
Gracilaria repens (C. Agardh) J. Agardh, Citation1842: 152. | |||||
Dichophycus repens (C. Agardh) Zanardini, Citation1847: 16, pl. 6, figs a–i. | |||||
Fauchea repens (C. Agardh) Montagne et Bory, nom illeg. in Montagne, Citation1846: 64. | |||||
LECTOTYPE: Agardh herbarium, LD 25705. There are four specimens in the Agardh herbarium (LD 25705, ; LD 25704, ; LD 25702, ; LD 25703, ), all of which agree with our recently collected material. Specimens LD 25704 and LD 25705 were identified as Sphaerococcus repens by C. Agardh. We chose LD 25705 as the lectotype because it shows the diagnostic features of the species most clearly.
Figs 1–7. Gloiocladia repens. Habit. . Habit of the lectotype (LD 25705). . Habit of a fertile male and female gametophyte in the original collection (LD 25704). . Habit of a sterile specimen in the original collection (LD 25702). . Habit of a fertile female gametophyte in the original collection (LD 25703). . Habit of a tetrasporophyte (HGI-A 6320). . Habit of a sterile stipitate specimen (HGI-A 6746). . Detail of the thallus showing marginal haptera and thalli anastomoses (HGI-A 5463). Abbreviations: a: anastomosis; dh: discoid holdfast; h: hapterum; st: stipe. Scale bars: 1 cm (Figs 1–6); 0.5 cm (Fig. 7).
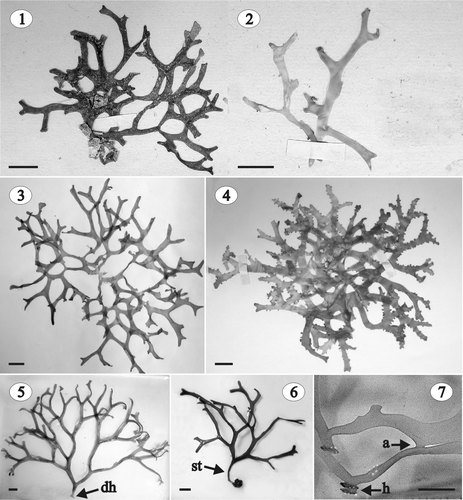
TYPE LOCALITY: Atlantic coast near Cadiz, Spain.
DISTRIBUTION: Mediterranean Sea and East Atlantic (Iberian Peninsula, Morocco and Canary Islands). In our opinion, records from the Maldives (Hackett, Citation1977) need to be verified.
HABITAT: In the Mediterranean Sea, G. repens grows in the sublittoral on rocky bottoms, on crustose corallines or on maërl, usually between 25 and 50 m deep (Feldmann, Citation1941; Gautier & Picard, Citation1957; Ballesteros i Sagarra, Citation1984). In 1889, J.J. Rodríguez y Femenías reported a specimen from 200 m, but recent studies with a Johnson-Sea-Link submersible have shown that, at present, photosynthetic macroalgae are only found down to 108 m in the Mediterranean Sea (E. Ballesteros, personal communication). In Mediterranean waters under Atlantic influence (e.g. Alboran Sea, Algeria or Sicily), G. repens has been found at 10 m depth, usually in communities of Laminariales or Fucales Feldmann, Citation1943; Giaccone, Citation1969; Rindi & Cinelli, Citation1995. On the Atlantic coast of the Iberian Peninsula, the species has been found between 6 and 14 m deep on rocky bottoms or with Laminaria ochroleuca De La Pylaie (López Varela et al ., Citation2002). Finally, in the Canary Islands, it has been found epilithic on small rhodoliths or on organic sandy bottoms at 60 m depth (Sansón et al ., Citation2002).
SEASONALITY: Spermatangia were observed in September; cystocarps occur throughout the year, and tetrasporangia were found from September to May (C. Agardh, Citation1823; Berthold, Citation1882; Hauck, Citation1885; Rodríguez y Femenías, Citation1889; Feldmann, Citation1937; Cormaci et al ., Citation1985; López Varela et al ., Citation2002; Sansón et al ., Citation2002; this work).
SPECIMENS EXAMINED: see .
Table 1. Gloiocladia repens. Examined specimens. Locality, legit, date and depth of collection, herbarium identification number and phenology are indicated. All the specimens come from the coasts of Spain
Habit and vegetative structure
Thalli decumbent, to 25 cm high and 18 cm wide (), primarily attached to the substratum by a discoid holdfast (). Plants sessile or occasionally stipitate (). The stipe, when present, cartilaginous, cylindrical, up to 13.0 mm long and 3.0 mm in diameter (). Fronds much branched, complanate and somewhat dichotomous, with dorsiventrally compressed branches, 2–6 mm wide at the base and 1–6 mm wide near the apex. Branch tips rounded and furcate with smooth margins (). Thallus 560–1,500 µm thick at the base of the plant and 500–1,000 µm at the apex. Texture cartilaginous and slippery to the touch, and the appearance shiny. Colour bright rose or reddish, but some old individuals nearly white. Fronds usually secondarily fixed to the substratum by marginal haptera and axes often anastomosing ().
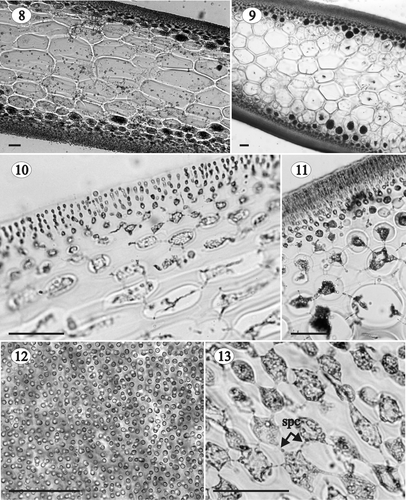
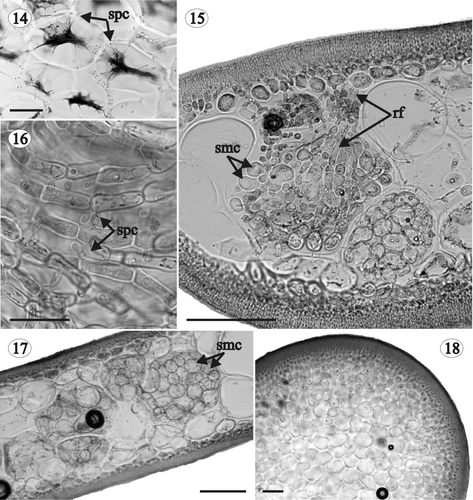
Axes multiaxial and solid (, , , , ), the outer cortex composed of compact anticlinal, simple or pseudodichotomously branched filaments arising from the subcortex (, ). Cortical filaments 5–7 cells long at the base of the plant and 3–5 cells long at the apex, with ovoid to rounded cells diminishing in size outwardly (inner cells 6–10 × 2–8 µm; outer cells 2–6 × 2–4 µm), and without lateral secondary pit-connections (). Subcortex consisting of ovoid to angular cells, 6–30 µm long, 6–20 µm wide and 6–28 µm thick, interconnected by secondary pit-connections, thus forming a network parallel to the thallus surface (). Medulla cellular and compact, composed of large hyaline cells diminishing in size in an outward direction. Medullary cells elongated in longitudinal section, 20–500 µm long, 14–240 µm wide and 16–192 µm thick (), rounded or ovoid in transversal section (), and developing numerous secondary pit-connections (up to 36 from a single cell, ). Multicellular rhizoidal filaments usually present in the basal parts of adult plants, developing from inner cortical cells or outer medullary cells, and growing between the medullary cells throughout the thallus (). Cells of rhizoidal filaments rectangular to ovoid, up to 32 × 20 µm, connected to each other and to the nearby medullary cells by secondary pit-connections (). Secondary medullary cells occasionally present in the basal parts of adult specimens, sometimes mixed with rhizoidal filaments (). Secondary medullary cells rounded, 15–40 µm in diameter, and interspersed among the large medullary cells (, ). Within the stipe, medullary cells rounded or slightly angular, not elongated in longitudinal section, and smaller than in the rest of the frond (up to 100 µm) ().
Figs 8–13. Gloiocladia repens. Vegetative structure. Aniline blue staining. . Longitudinal (8) and transverse (9) sections of a sterile individual in the middle part of the plant (HGI-A 5631, 5637). . Cortex and subcortex in longitudinal (10) and transverse (11) section (HGI-A 5631, 5637). . Outer cortical cells in a surface view (HGI-A 5629). . Network of subcortical cells showing frequent secondary pit-connections between cells (HGI-A 5637). Abbreviation: spc: secondary pit-connection. Scale bars: 50 µm.
Figs 14–18. Gloiocladia repens. Vegetative structure. Aniline blue staining. . Medullary cells in transverse section showing several secondary pit-connections between them (HGI-A 5463). . Transverse section of the basal part of the thallus showing the development of rhizoidal filaments and secondary medullary cells (HGI-A 5632). . Detail of the rhizoidal filaments connected by secondary pit-connections (HGI-A 5632). . Transverse section of the basal part of the thallus with many secondary medullary cells (HGI-A 5632). . Transverse section of the stipe (HGI-A 1793). Abbreviations: rf: rhizoidal filament; smc: secondary medullary cell; spc: secondary pit-connection. Scale bars: 50 µm (Fig. 14); 100 µm (Figs 15, 17, 18); 25 µm (Fig. 16).
Reproductive structures
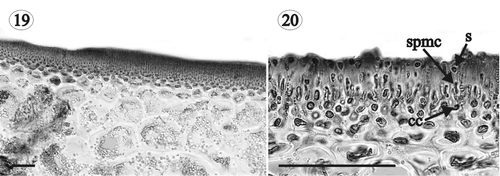
Plants monoecious. Spermatangia 1–2 µm in diameter, grouped in surface sori () and cut off singly or in pairs from ovoid spermatangial mother cells that develop from the outer cortical cells (). Female gametophytes procarpic. The carpogonial branch curved and outwardly directed, arising from a mid cortical cell (= supporting cell), and composed of three cells plus a lateral sterile cell that arises from the basal cell of the carpogonial branch (). Auxiliary cell branch situated on the supporting cell and only one cell long before fertilization (). Mature auxiliary cell branch two-celled, composed of an auxiliary mother cell and a terminal auxiliary cell containing a conspicuous globular proteinaceous inclusion (). Carpogonial branch cells fusing after fertilization and connecting to the auxiliary cell, which divides transversely to cut off a primary gonimoblast cell (= gonimoblast initial) (). Area adjacent to the supporting cell developing a subspherical mass of highly pigmented nutritive cells () connected to each other and to the supporting cell by secondary pit-connections (). These cells somewhat stellate, with the main cell body up to 23 µm in diameter and lobes up to 8 µm long (). Basal nutritive tissue developing a network of persistent filaments (= tela arachnoidea) that surrounds the fertilized procarp (, ), and is composed of stellate cells up to 23 µm in diameter with lobes up to 37 µm long (). A fusion cell is formed through enlargement of the pit-connection between the auxiliary cell and the auxiliary mother cell, which later coalesces with the fused cells of the carpogonial branch and the surrounding cells, ultimately incorporating the supporting cell. Components of the fusion cell, except the supporting cell, becoming indiscernible when the structure is mature (). The gonimoblast (120–750 µm in diameter) arising from the distal end of the primary gonimoblast cell and composed of several elongated lobes of ovoid carposporangia surrounded by the cells of the tela arachnoidea (, ). Carposporangia 20–40 µm in diameter. Cystocarps (400–1,500 µm in diameter) situated on the branch margins (), substipitate, globose, and ostiolate (, ).
Figs 19, 20. Gloiocladia repens. Light photomicrographs of male reproductive structures. Aniline blue staining. . Transverse section of a fertile area of the thallus showing a spermatangial sorus (HGI-A 6303). . Transverse section of a fertile area of the thallus showing spermatia borne on spermatangial mother cells (LD 25704). Abbreviations: cc: cortical cell, s: spermatium; spmc: spermatangial mother cell. Scale bars: 50 µm.
Figs 21–25. Gloiocladia repens. Light photomicrographs of female reproductive structures and postfertilization stages. Aniline blue staining. . Carpogonial branch (HGI-A 6303). . Procarp at a stage in which the first cell of the auxiliary branch can just be distinguished (HGI-A 6303). . Auxiliary cell branch composed of an auxiliary mother cell and an auxiliary cell with a proteinaceous inclusion (HGI-A 6334). . Young gonimoblast arising from the primary gonimoblast cell. The fusion cell between the carpogonial branch cells can also been seen (LD 25703). . Detail of the fusion cell (HGI-A 6303). Abbreviations: ac: auxiliary cell; amc: auxiliary mother cell; c: carposporangia; cbc: cells of the carpogonial branch; cc: cortical cell; cp: carpogonium; fc: fusion cell; fcab: first cell of the auxiliary branch; fcbc: fused cells of the carpogonial branch; g: gonimoblast; nc: nutritive cell; pgc: primary gonimoblast cell; pi: proteinaceous inclusion; sc: supporting cell; tr: trichogyne; stc: sterile cell; tac: cell of the tela arachnoidea. Scale bars: 25 µm (Figs 21, 22); 50 µm (Figs 23–25).
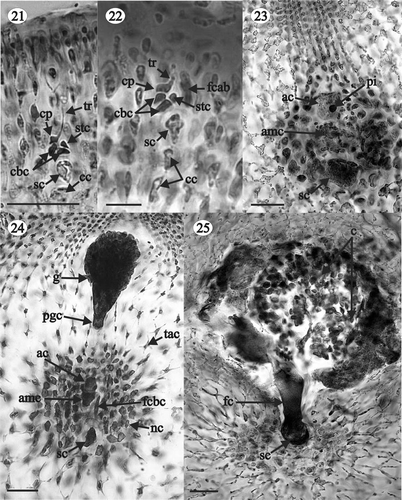
Figs 26–30. Gloiocladia repens. Light photomicrographs of the female reproductive structures with aniline blue staining. . Detail of stellate nutritive cells (HGI-A 5542). . Details of stellate cells of the tela arachnoidea (HGI-A 5542). . Cystocarps (LD 25703). . Detail of the carposporangia going out through the ostiole (HGI-A 5542). Scale bars: 50 µm (Figs 26–28, 30); 5 mm (Fig. 29).
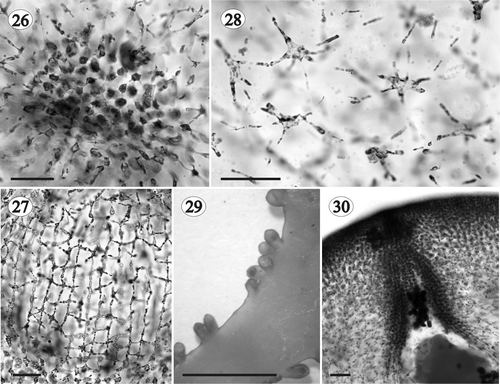
Tetrasporangia developing in extensive (<6 mm long × 2 mm wide), ovoid to elongate and prominent nemathecia situated in the middle part of the branch tips (), not produced simultaneously but with different developmental phases adjacent to one another (). Nemathecial cortical filaments longer (10–15 cells long) and their basal cells larger (10–23 × 2–6 µm) than in sterile parts of the plant (). Tetrasporangia fusiform or ovoid, up to 30–136 µm × 10–48 µm, and cruciate (), decussately cruciate () or irregularly divided (). Tetrasporangia subapical, arising from one of the first dichotomies of an outer cortical filament with the pit-connection between the tetrasporangium and the filament situated proximally, at the basal part of the tetrasporangium ().
Figs 31–35. Gloiocladia repens. Tetrasporangial development. . Nemathecia in branch surface (HGI-A 6320). . Transverse section of a well-developed nemathecium (HGI-A 5463). . Transverse section of nemathecium with cruciately dividing tetrasporangia (HGI-A 6298). . Transverse section of nemathecium with decussately cruciate dividing tetrasporangia (HGI-A 6302) (aniline blue staining). . Transverse section of nemathecium with irregularly dividing tetrasporangia (HGI-A 6302) (aniline blue staining). Scale bars: 5 mm (Fig. 31); 100 µm (Fig. 32); 25 µm (Figs 33–35).
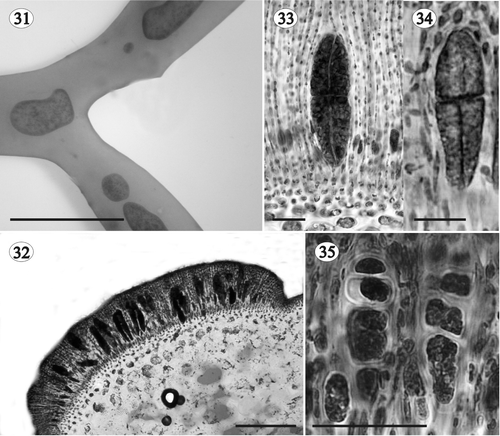
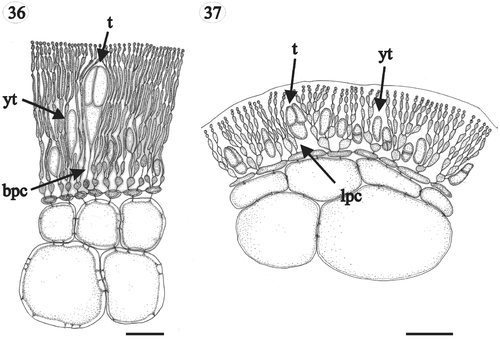
Figs 36, 37. Gloiocladia repens and Gloiocladia furcata. Tetrasporangial pit-connections with aniline blue staining. . Tetrasporangia of G. repens showing basal pit-connection with cortical filaments (HGI-A 5631). . Tetrasporangia of G. furcata showing lateral pit-connection with cortical filaments (HGI-A 5769). Abbreviations: bpc: basal pit-connection; lpc: lateral pit-connection; t: tetrasporangium; yt: young tetrasporangium. Scale bars: 50 µm.
Notes
The white colour of some adult specimens of G. repens was previously reported by López Varela et al . (Citation2001, Citation2002). It could be due to the accumulation of floridean starch, as seen in other Rhodophyta such as Kallymenia feldmannii Codomier and K. requienii (J. Agardh) J. Agardh (Rodríguez-Prieto & Vergés, Citation2001). Rhizoidal filaments and secondary medullary cells are correlated with injury to the thallus (Sparling, Citation1957; Irvine & Guiry, Citation1980; Sánchez, Citation2005; Sánchez & Rodríguez-Prieto, Citation2005) and could be involved in increasing the thallus toughness (Sánchez, Citation2005; Sánchez & Rodríguez-Prieto, Citation2005). Although this feature was not recorded for the type species, F. repens, the presence of rhizoidal filaments within the medulla was considered to be a key feature of Group 1 Rhodymeniales (Irvine & Guiry, Citation1980; Guiry & Irvine, Citation1981), which included Fauchea. Finally, López Varela et al . (Citation2001, Citation2002) described the tetrasporangia as either zonate or cruciate, and aligned in groups of fours. However, we observed only cruciate, decussately cruciate or irregularly divided tetrasporangia, even in the specimen examined by these authors (SANT-A 11760).
Molecular analyses
The 18S data set included 139 variable (8.3%) and 36 parsimony informative (2.2%) sites. A Tamura-Nei correction with invariant sites and gamma distribution was determined to be the best-fit model of sequence evolution for this data set. Maximum likelihood, distance and parsimony analyses of these data did not result in identical trees, but the major relationships were congruent () All analyses resolved a clade containing Fauchea spp. and Gloiocladia furcata, and three additional lineages leading to Faucheopsis coronata (Harvey) Kylin, Gloioderma fruticulosum and Webervanbossea splachnoides (Harvey) De Toni fil. The Fauchea/Gloiocladia clade received strong to moderate bootstrap support (maximum likelihood [M] = 78%, distance [D] = 79%, parsimony [P] = 97%), but the relationships among the four lineages vary in topology and bootstrap support among the three types of analyses. A sister relationship between the Fauchea/Gloiocladia clade and Faucheopsis was resolved with good support in parsimony and distance analyses (P = 84%, D = 85%) but was only weakly supported in the maximum likelihood analysis (M = 57%). Gloioderma and Webervanbossea De Toni were resolved as sister taxa with weak support in the maximum likelihood analysis (M = 63%), but Gloioderma was resolved as sister to the Faucheopsis/Fauchea/Gloiocladia clade with weak support in the parsimony and distance analyses (P = 62%, D = 59%).
Fig. 38. Phylogenetic trees resulting from analyses of 18S rRNA gene sequences of Faucheacean species. Accession numbers for previously published sequences are given below species names. Sequences generated in this study are shown in larger font. (A) One of four most parsimonious trees (length = 176; CI = 0.687; RI = 0.580). Inferred branch lengths are shown above branches, parsimony (P) and distance (D) bootstrap proportions are shown below branches. (B) Maximum-likelihood tree (lnL = −3340.58416) with likelihood bootstrap proportions shown above branches.
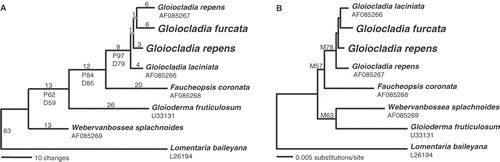
Relationships among the four specimens in the Fauchea/Gloiocladia clade are not resolved and the three specimens identified as Fauchea species do not form a separate clade. Pairwise sequence divergences for the four sequences vary from eight (0.48%) between the Spanish G. repens and California G. laciniata (J. Agardh) Sánchez et Rodríguez-Prieto comb. nov. (= Fauchea laciniata J. Agardh) specimens, to twelve (0.72%) between two specimen pairs, the Italian G. repens and Californian G. lacinata, and the Italian G. repens and Spanish G. furcata. The G. repens specimen from the Spanish Mediterranean islands of Columbretes is different from the Italian specimen identified as G. repens at 10 sites (0.60%).
Discussion
Studies of the generitype of Gloiocladia, G. furcata by Sánchez (Citation2005) and Sánchez & Rodríguez-Prieto (Citation2005), and our present observations on F. repens, the generitype of Fauchea (= G. repens), show that the main qualitative differences () between the two species are: (i) the shape of the outer cortical filaments, (ii) the location of the supporting cell, (iii) the presence or absence of a small lateral cell on the basal cell of the carpogonial branch, (iv) the inclusion of the supporting cell in the fusion cell, (v) the morphology of the fusion cell, (vi) the existence of substipitate or sessile cystocarps, (vii) the arrangement of tetrasporangia, and (viii) the location of the pit-connection between the tetrasporangium and the cortical cell (, ). The presence of coronate cystocarps, highlighted by Kylin (Citation1931) and Sparling (Citation1957), cannot be included because such coronate cystocarps are only occasionally present in G. furcata (Sánchez, Citation2005, Sánchez & Rodríguez-Prieto, Citation2005).
Table 2. Comparison of the main features of species currently placed in the genus Fauchea and transferred here to Gloiocladia
Traditionally, the genera Fauchea and Gloiocladia have been separated morphologically using some of the differences between G. furcata and G. repens which we have highlighted here, although the lack of detailed information on the reproductive structures of the type species has prevented a clear concept of Fauchea and its relationship with Gloiocladia (Irvine & Guiry, Citation1980; Norris, Citation1991). The shape of the outer cortical filaments (compact or lax) was used by Sparling (Citation1957) and the arrangement of tetrasporangia (conspicuously raised nemathecia in Fauchea and in slightly raised nemathecia or scattered in Gloiocladia), was used by De Toni (Citation1900) and Irvine & Guiry (Citation1980). Moreover, Norris (Citation1991) transferred all species of Fauchea, Gloiocladia or Gloioderma with strongly raised nemathecia to Fauchea, and those with tetrasporangia scattered or in only slightly raised nemathecia to Gloiocladia.
Information about the taxonomic characters that differentiate G. furcata and G. repens is lacking for most other species of Fauchea () and Gloiocladia (Sánchez & Rodríguez Prieto, Citation2005). Gloiocladia laciniata (as F. laciniata) is by far the best known species (Sparling, Citation1957; Dawson, Citation1963) and is closer to G. furcata than to G. repens (), having a lax outer cortex, a simple three-celled carpogonial branch and a supporting cell that does not become part of the fusion cell. It is also characterized by coronate cystocarps and cruciately divided tetrasporangia.
The utility of 18S sequence analyses in taxonomic studies of the Rhodymeniales has been demonstrated previously (Millar et al ., Citation1996; Saunders et al ., Citation1999; Freshwater et al ., Citation2005). Representative specimens of G. repens and G. furcata, the type species of Fauchea and Gloiocladia, respectively, were sequenced and analysed in this study together with other available Faucheaceae 18S sequences. Results of these sequence analyses support a close relationship between these species and suggest that a generic distinction is unwarranted. In contrast, G. furcata and Gloioderma fruticulosum are not more closely related in the 18S trees than either is to species from Faucheopsis Kylin or Webervanbossea, indicating that the transfer of at least some Gloioderma species into Gloiocladia by Norris (Citation1991) was unwarranted.
Pairwise 18S sequence divergences between specimens representing different species within the same Rhodymeniales genera range from 0.17% to 0.97% (Saunders et al ., Citation1999; Freshwater et al ., Citation2005). The pairwise sequence divergence of 0.60% between specimens identified as G. repens from the Mediterranean coastal area of Spain and G. repens from Italy is within this range, and greater than the divergence between the Spanish G. repens and a Californian specimen of G. lacinata. The type locality for G. repens is the Atlantic coast of Spain near the mouth of the Mediterranean and characters of the sequenced Spanish specimen match typical G. repens, suggesting that the Italian specimen could represent a different, cryptic species.
In the light of these observations, we reassess the genus Gloiocladia and formally propose transfer of the species of Fauchea to the earlier described genus Gloiocladia (). Although Gloioderma fruticulosum is not the generitype of Gloioderma, the distance between this and the Gloiocladia species in sequence analyses (), suggest that Gloioderma should be reinstated, at least for some species, especially those recorded from southern Australia and New Zealand.
Table 3. Formal transfers to the genus Gloiocladia
Emended description of Gloiocladia J. Agardh
Fronds much branched, complanate and somewhat dichotomous. Axes multiaxial and solid, the outer cortex composed of compact anticlinal, simple or pseudodichotomously branched filaments arising from the subcortex. Cortical filaments diminishing in size outwardly, without lateral secondary pit-connections. Subcortex consisting of ovoid to angular cells interconnected by secondary pit-connections, thus forming a network parallel to the thallus surface. Medulla cellular and compact, composed of large hyaline cells elongated in longitudinal section. Spermatangia grouped in surface sori. Plant procarpic. Carpogonial branch three-celled, with or without a lateral sterile cell arising from the first cell of the carpogonial branch. Auxiliary cell branch two-celled, composed of an auxiliary mother cell and an auxiliary cell, and situated on the supporting cell. Carpogonial branch cells fusing after fertilization and connecting to the auxiliary cell, which divides transversely to form a primary gonimoblast cell. Nutritive cells and tela arachnoidea present. Fusion cell formed through coalescence of the auxiliary and auxiliary mother cells, the fused cells of the carpogonial branch and the surrounding cells, with the supporting cell sometimes participating in the fusion process. Constituents of the fusion cell (except the supporting cell) are indiscernible when the structure is mature. Gonimoblast arising from the distal end of the primary gonimoblast cell consisting of several elongated lobes of ovoid carposporangia, and surrounded by the cells of the tela arachnoidea. Cystocarps situated on the branch margins or on the thallus surface, sessile or substipitate, prominent, globose, and ostiolated. Tetrasporangia developed in nemathecia or scattered, subapical, cruciately, decussately cruciate or irregularly divided.
Acknowledgements
We would like to thank M. Hommersand and G. Furnari for their help in improving this paper, and P. Silva for his taxonomic comments. We would also like to show our appreciation to the following people for the loan of valuable herbarium specimens: S. Riebe for the specimens from the Herbarium C. Agardh of the Botanical Museum of Sweden (LD), M. Dueñas, for the specimens from the Herbarium of the Real Jardín Botánico de Madrid (MA), F.D. Navas Fernández for the specimens from the Herbarium of the Malaga University (MGC), R. Iglesias Louzao for the specimens from the Herbarium of the Santiago University (SANT), F. Boisset for the specimens from the Herbarium of the Valencia University (VAL), and E. Ballesteros, N. Sant, F. Boisset and S. Mallol for recent collections from the Balearic Island and the Columbretes Islands. Thanks to Jordi Ros, Pere Tur and Alba Vergés, for accompanying us on dives. This work was supported by a grant from the Spanish Ministry of Science and Technology [Flora Phycologica Iberica. Bonnemaisoniales, Gracilariales, Palmariales y Rhodymeniales (Rhodophyceae), REN 2001-1473-C03-02], the US National Science Foundation [NSF-PEET no. 0328491] and the Friends of CMS DNA Algal Trust.
References
- Abbott , IA and Hollenberg , GJ . 1976 . Marine Algae of California , Stanford , , USA : Stanford University Press .
- Agardh , CA . 1823 . Species Algarum rite cognitae , Vol. 1, part 2 , 1969 Amsterdam , , The Netherlands : Reprint A. Asher & Co. .
- Agardh , JG . 1842 . Algae maris Mediterranei et Adriatici , Paris , , France : Fortin, Masson et Cie. .
- Agardh , JG . 1885 . Till algernes systematik. Nya bidrag (Fjerde afdelningen) . Acta Univ. Lund , 21 : 1 – 117 .
- Baardseth , E . 1941 . Results of the Norwegian Scientific Expedition to Tristan da Cunha 1937–1938. Report 9: The marine algae of Tristan da Cunha
- Ballesteros i Sagarra , E . 1984 . Els Vegetals i la Zonació Litoral: Espècies, Comunitats i Factors Que Influeixen en la Seva Distribució , Barcelona , , Spain : Institut d’Estudis Catalans. Tesi Doctoral, University of Barcelona .
- Berthold , G . 1882 . Über die Vertheilung der Algen im Golf von Neapel nebst einem Verzeichnis der bisher daselbst beobachteten Arten . Mitt. Zool. Stat. Neapel , 3 : 393 – 536 .
- Børgesen , F . 1944 . Some marine algae from Mauritius. III. Rhodophyceae. Part 3. Rhodymeniales . Det Kgl. Danske Vid. Selsk. Biol. Medd. , 19 : 1 – 32 .
- Bornet , E . 1890 . Note sur deux algues of the Méditerranée: Fauchea et Zosterocarpus . Bull. Soc. Bot. Fr. , 37 : 139 – 148 .
- Cormaci , M , Furnari , G and Scammacca , B . 1985 . Osservazioni sulle fitocenosi bentoniche del golfo di Augusta (Siracusa) . Boll. Sedute Accad. Gioenia Sci. Nat. Catania , 18 : 851 – 872 .
- Dawson , EY . 1963 . Marine red algae of Pacific Mexico. Part 6. Rhodymeniales . Nova Hedwigia , 5 : 437 – 476 .
- De Toni , GB . 1900 . Sylloge algarum omnium hucusque cognitarum. Vol. IV. Florideae. Sectio II , Padua , , Italy : G. B. De Toni .
- Feldmann , J . 1937 . Les algues marines de la côte des Albères. I–III. Cyanophycées, chlorophycées, phaéophycées . Rev. Algol. , 9 : 141 – 335 .
- Feldmann , J . 1941 . Les algues marines de la côte des Albères IV. Rhodophycées (suite) . Rev. Algol. , 12 : 77 – 100 .
- Feldmann , J . 1943 . Contribution à l’étude de la flore marine de profondeur sur les côtes d’Algérie . Bull. Soc. Hist. Nat. Afr. Nord , 34 : 150 – 167 .
- Freshwater , DW , Braly , SK , Stuercke , B , Hamner , RM and York , RA . 2005 . Phylogenetic analyses of North Carolina Rhodymeniales. I. The genus Asteromenia . J. N. Carolina Acad. Sci. , 121 : 49 – 55 .
- Gautier , Y and Picard , J . 1957 . Bionomie du Banc du Magaud (Est des îles d’Hyères) . Recl. Trav. Stn. Mar. Endoume , 21 : 28 – 40 .
- Giaccone , G . 1969 . Note sistematiche ed osservazioni fitosociologiche sulle laminariales del Mediterraneo occidentale . G. Bot. Ital. , 103 : 457 – 474 .
- Guiry , MD and Irvine , DEG . 1981 . A critical reassessment of infraordinal classification in the Rhodymeniales. . Proc. Int. Seaweed Symp , 8 : 106 – 111 .
- Hackett , HE . 1977 . Marine algae known from the Maldive Islands. . Atoll Res. Bull. , 210 : 1 – 30 .
- Hauck , F . 1885 . “ Die Meeresalgen Deutschlands und Oesterreichs ” . In Dr L. Rabenhorst's Kryptogamen-Flora von Deutschland, Österreich und der Schweiz , Edited by: Grunow , A , Hauck , F , Limpricht , G , Luerssen , Ch , Richter , P and Winter , G . Vol. 2 , 513 – 575 . Leipzig , , Germany : + [i]-xxiii [xxiv]. Eduard Kummer .
- Holmgren , PK , Holmgren , NH and Barnett , IC . 1990 . Index herbariorum. Part I: The herbaria of the world , Vol. 120 , New York , , USA : New York Botanical Garden .
- Howe , MA and Taylor , WR . 1931 . Notes on new or little known marine algae from Brazil . Brittonia , 1 : 7 – 33 .
- Hughey , JR , Silva , PC and Hommersand , MH . 2001 . Solving taxonomic and nomenclatural problems in Pacific Gigartinaceae (Rhodophyta) using DNA from type material . J. Phycol. , 37 : 1091 – 1109 .
- Irvine , DEG and Guiry , MD . 1980 . “ Taxonomy of the Rhodymeniales ” . In Taxonomy of Algae. Papers Presented at the International Symposium on Taxonomy of Algae held at the Centre of Advanced Study in Botany , Madras , , India : University of Madras . December 9–16, 1974 (Desikachary, T.V.Raja Rao, V.N., editors), 287–303. University of Madras
- Kylin , H . 1931 . Die Florideenordung Rhodymeniales . Acta Univ. Lund. , 27 : 1 – 48 .
- Kylin , H . 1941 . Californische Rhodophyceen . Lunds Universitets Årsskrift, Ny Foljd, Andra Afdelningen , 37 : 1 – 51 .
- López Varela , C , Bárbara , I , Veiga , AJ and Cremades , J . 2001 . Fauchea repens (C. Agardh) Montagne et Bory (Rhodymeniales, Faucheaceae) en el Noroeste de la Península Ibérica. . Algas , 26 : 4
- López Varela , C , Bárbara , I , Veiga , AJ , Cremades , J and Peteiro , C . 2002 . Fauchea repens (C. Agardh) Montagne et Bory (Rhodymeniales, Rhodophyta) en el Noroeste de la Península Ibérica . Nov. Act. Cient. Compostelana (Bioloxía) , 12 : 219 – 222 .
- Maddison , DR and Maddison , WP . 2000 . MacClade 4: Analysis of phylogeny and character evolution. Version 4.0, Sinauer Associates Sunderland , , USA
- Millar , AJK , Saunders , GW , Strachan , IM and Kraft , GT . 1996 . The morphology, reproduction and small-subunit rRNA gene sequence of Caphalocystis (Rhodymeniaceae, Rhodophyta), and new genus based on Cordylecladia furcellata J. Agardh from Australia . Phycologia , 35 : 48 – 60 .
- Montagne , C . 1846 . “ Ordo I. Phyceae Fries ” . In Exploration scientifique de l’Algérie pendant 1840–42, Sciences naturelles, Botanique, 1, Cryptogamie , Edited by: Durieu de Maisonneuve , MC . 1 – 197 . Paris , , France : Imprimerie Impériale .
- Norris , RE . 1991 . Some unusual marine red algae (Rhodophyta) from South Africa . Phycologia , 30 : 582 – 596 .
- Norris , RE and Aken , ME . 1985 . Marine benthic algae new to South Africa . S. Afr. J. Bot. , 51 : 55 – 65 .
- Posada , D and Crandall , KA . 1998 . Modeltest: testing the model of DNA substitution . Bioinformatics , 14 : 817 – 818 .
- Rindi , F and Cinelli , F . 1995 . Contribution to the knowledge of the benthic algal flora of the Isle of Alboran, with notes on some little-known species in the Mediterranean . Cryptogam. Algol. , 16 : 103 – 114 .
- Rodríguez y Femenías , JJ . 1889 . Algas de las Baleares . An. Soc. Esp. Hist. Nat. , 18 : 199 – 274 .
- Rodríguez-Prieto , C and Vergés , A . 2001 . Geographical distribution, habitat and reproductive phenology of the genus Kallymenia (Gigartinales, Rhodophyta) from Catalonia, Spain . Bot. Mar. , 44 : 479 – 492 .
- Sánchez , N . 2005 . “ Estudi taxonòmic i ecofisiològic dels membres de la família Faucheaceae (Rhodymeniales, Rhodophyta) de la península Ibèrica i de les illes Balears ” . In PhD Thesis , Girona , , Spain : Universitat de Girona .
- Sánchez , N and Rodríguez-Prieto , C . 2005 . Vegetative and reproductive morphology of the type species of Gloiocladia, G. furcata (Faucheaceae, Rhodophyta) . Phycologia , 44 : 222 – 223 .
- Sansón , M , Reyes , J , Afonso-Carrillo , J and Muñoz , E . 2002 . Sublittoral and deep-water red and brown algae new from the Canary islands . Bot. Mar. , 45 : 35 – 49 .
- Saunders , GW and Kraft , GT . 1994 . Small-subunit rRNA gene sequences form representatives of selected families of the Gigartinales and Rhodymeniales (Rhodophyta). 1. Evidence for the Plocamiales ord. nov . Can. J. Bot. , 72 : 1250 – 1263 .
- Saunders , GW , Strachan , IM and Kraft , GT . 1999 . The families of the order Rhodymeniales (Rhodophyta): a molecular-systematic investigation with a description of Faucheaceae fam. nov . Phycologia , 38 : 23 – 40 .
- Segawa , S . 1935 . On the marine algae of Susaki, Prov. Idzu and its vicinity . Sci. Pap. Inst. Algol. Res., Fac. Sci., Hokkaido Univ. , 1 : 59 – 90 .
- Segawa , S . 1941 . New or noteworthy algae from Izu . Sci. Pap. Inst. Algol. Res., Fac. Sci. Hokkaido. Univ. , 2 : 251 – 271 .
- Setchell , WA . 1912 . Algae novae et minus cognitae, 1 . Univ. Calif. Publ. Bot. , 4 : 229 – 268 .
- Sjöstedt , LG . 1926 . Floridean studies . Acta Univ. Lund. , 22 : 1 – 95 .
- Sparling , SR . 1957 . The structure and reproduction of some members of the Rhodymeniaceae . Univ. Calif. Publ. Bot. , 29 : i – iv . + 319–396
- Swofford , DL . 2002 . PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4 , Sunderland , , USA : Sinauer Associates .
- Taylor , WR . 1945 . Pacific marine algae of the Allan Hancock expeditions to the Galapagos Islands . Allan Hancock Pacif. Exped. , 12 : iv + 528
- Taylor , WR . 1960 . Marine Algae of the Eastern Tropical and Subtropical Coasts of the Americas , Ann Arbor , , USA : The University of Michigan Press .
- Weber-Van Bosse , A . 1928 . Liste des algues du Siboga. IV. Rhodophyceae. Troisième partie. Gigartinales et Rhodymeniales et tableau de la distribution des Chlorophycées, Phaeophycées et Rhodophycées de l’Archipel Malaisien . Siboga Exped. Monogr. , 59 : 393 – 533 .
- Xia , B-M and Wang , Y-Q . 2000 . Studies on some new red algae from China (I) . Acta Phytotaxon. Sin. , 38 : 77 – 85 .
- Xia , B and Zhang , J . 1999 . Flora Algarum Marinarum Sinicatum Tomus II Rhodophyta No. V Ahnfeltiales Gigartinales Rhodymeniales , Beijing , , China : Science Press .
- Yamada , Y . 1941 . Notes on some Japanese algae IX . Sci. Pap. Inst. Algol. Res. Fac. Sci. Hokkaido. Univ. , 2 : 195 – 215 .
- Zanardini , G . 1847 . Notizie intorno alle cellulari marine delle lagune e de’litorali di Venezia . Atti R. Ist. veneto Sci. , 6 : 185 – 262 .