Abstract
The cytology and life cycle of Pinnularia cf. gibba was examined in nine clones from three Scottish localities. This freshwater epipelic diatom is heterothallic and produces two isogametes per gametangium in type IC auxosporulation (Geitler's classification [1973]). The zygote undergoes a highly unusual metamorphosis before beginning expansion, becoming shortly linear–lanceolate; this is accompanied by formation of a complete covering of thin, oxidation-resistant strips and scale-like structures (at the poles), which are quite separate from the perizonium formed during auxospore expansion. Observations of similar incunabular structures in P. acidojaponica show that these elements are siliceous. The P. cf. gibba perizonium also has unusual features, including a remarkably wide primary band. Trikaryotic and haploid auxospores are sometimes formed and haploid ‘zygotes’ mature and expand like diploids, but do not develop into mature initial cells. Several phases of mucilage secretion take place, from the gametangia, zygotes and auxospores. Triplets of gametangia and polyspermy occurred with high frequency; this and the systematic significance of variation in auxospore, incunabula and perizonium structure, are discussed. Aspects of the taxonomy of the P. gibba group are treated in supplementary material provided on the European Journal of Phycology website.
Introduction
Conjugation between raphid diatoms and the formation of auxospores were first described by Thwaites in 1847. During the next 30 years, auxosporulation was reported in a wide variety of genera, including Pinnularia Ehrenberg (Carter, Citation1865; Schumann, Citation1869; Pfitzer, Citation1871; Barker, Citation1875; Schmidt, Citation1876), which is one of the largest diatom genera, with over 450 ‘accepted’ species described before 1980 (VanLandingham, Citation1978; Mann, Citation1986) and many more described since then. All the reports of Pinnularia auxospores are brief and most do little more than record that a sexual stage had been observed. Not much has been added to our knowledge of Pinnularia life cycles since 1876: Geitler (Citation1932, Citation1937, Citation1973) listed just one new record for the period up to 1973 and there are short recent reports by Hashizume (Citation1978, Citation1985) and Suzuki & Mayama (Citation1995). Overall, the literature shows only that (i) some species have type I auxosporulation sensu Geitler (Citation1973), i.e. two paired gametangia produce two auxospores (e.g. Pfitzer, Citation1871, pl. 4, ; Hashizume, Citation1978); and (ii) the auxospore possesses a robust silicified perizonium (e.g. Schmidt, Citation1876, pl. 44, ; pl. 45, ; Hashizume, Citation1978). Such characteristics are shared with many other raphid diatoms (e.g. Geitler, Citation1973; Mann, Citation1982, 1984). There are also three reports of auxosporulation in the closely related genus Caloneis Cleve (Geitler, Citation1958, 1973; Mann, Citation1989a). Like Pinnularia, Caloneis exhibits type I auxosporulation and Mann (Citation1989a) has given data on gametogenesis, plasmogamy and auxospore structure in Caloneis silicula (Ehrenberg) Cleve sensu Krammer & Lange-Bertalot (Citation1986), based on observations of incubated natural populations. There is no information on the mating system in any member of the Pinnulariaceae.
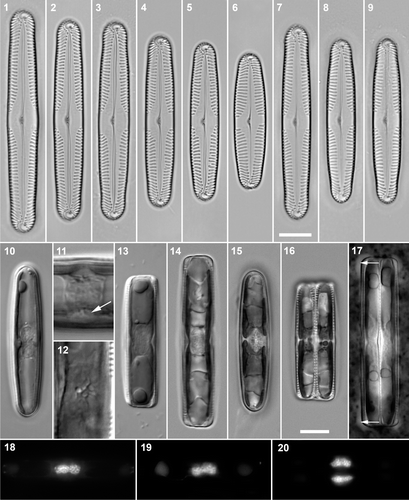
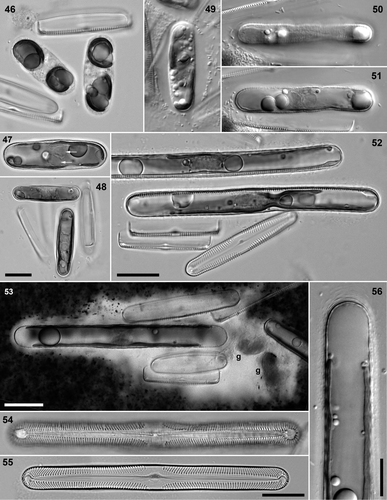
Figs 1–20. Pinnularia cf. gibba. . Cleaned valves, BF. . Craiglush clone 1. . Craiglush clone 3. . Craiglush clone 2. . Craiglush clone 4. . Craiglush clone 5. . Craiglush clone 6. . Achray, clone 9. . Menteith, clone 7. . Menteith, clone 8. . Interphase cells of clone 6 in valve view, DIC: note the cushion-like central pyrenoid in each girdle-appressed chloroplast. . Interphase cells (clone 6), DIC: centre detail, showing the invaginated pyrenoids in valve (e.g. arrow) and girdle view. . Mid-focus of interphase cell (clone 5) in girdle view, DIC, showing the central cytoplasmic bridge containing the nucleus, flanked by two large polar vacuoles. . Pre-mitotic cell (clone 3) in girdle view, BF, with undivided valve-appressed chloroplasts.. Post-cytokinetic cells (clones 4 and 6) in valve and girdle views, BF: the chloroplasts have divided transversely in each daughter cell. . Indian ink preparation: Recently divided vegetative cell (clone 3) in girdle view, with small accumulations of mucilage along the polar sections of the raphes (e.g. arrows).. DAPI-stained cells. . Interphase cells in valve () and girdle view (); note the slight central constriction of the nucleus in , caused by the pyrenoids (cf. ). . Post-cytokinetic cell in girdle view. Scale bars: 10 µm (except , which are double the magnification of the other micrographs of living cells).
Round et al. (Citation1990) and Mann (Citation2001) have suggested that, given their current circumscriptions, Caloneis may not be separable from Pinnularia without making one or other genus para- or polyphyletic. The best way to test this is through use of molecular phylogenetics, but complementary data on morphological, cytological and reproductive characteristics are also important, to reveal the evolutionary trends and developmental changes that have accompanied cladogenesis: Molecular phylogenies per se are not very interesting. We recently induced sexual reproduction in members of the species complex previously referred to as Pinnularia gibba Ehrenberg (e.g. by Krammer & Lange-Bertalot, Citation1986) and in some other freshwater Pinnularia species, and have studied stages in gametogenesis and auxospore development. Scanning electron microscopical (SEM) observations of the expanding auxospores revealed silicified structures external to the perizonium, which appear to correspond to a new class of auxospore wall elements recently reported in the unrelated genus Nitzschia Hassall by Trobajo et al. (Citation2006). Experiments with clonal cultures have allowed us to determine the mating system of several Pinnularia species and here we describe an example of heterothallism. Other types of behaviour (homothallism, automixis) will be dealt with elsewhere.
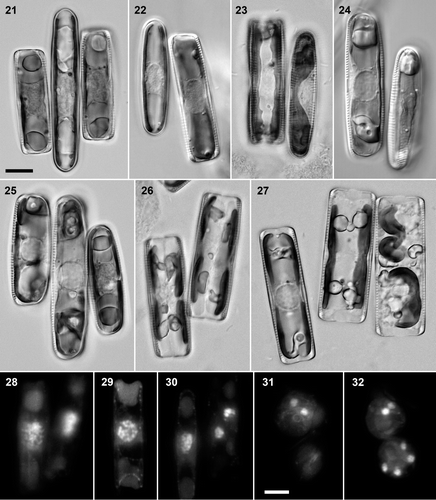
Figs 21–32. Pinnularia cf. gibba. . Clone 3 (larger, central) × clone 6, BF: triplet of recently paired cells. . Clone 6 (smaller) × clone 5, DIC: early meiotic prophase, with expanded nuclei (compare ). . Clone 4 × clone 5 (left, in girdle view), BF: mid–late meiotic prophase, with valve-appressed chloroplasts. . Clone 4 (left) × clone 5, DIC: diakinesis. . Clone 3 (larger, central) × clone 6, BF: triplet, in which the left two cells are in diakinesis, whereas the right-hand clone 6 cell is at an earlier stage of meiosis. . Clone 4 × clone 5, BF: pair of cells containing daughter protoplasts after meiosis I. . Clone 4 × clone 5, BF: triplet of cells in meiotic prophase (left), during the meiosis I division (centre) and following gametogenesis and rearrangement (right). . DAPI-stained preparations, from a clone 4 × clone 5 cross. . Pair of cells in early meiotic prophase; left cell with the chromatin concentrated towards one end of the nucleus in a synizetic knot. . Diakinesis (girdle view). . Late meiotic prophase (left) and daughter nuclei from meiosis I (right). . Gametes (cf. right-hand cell in ): the top cell contains two ± equal nuclei from meiosis II. . Gamete with two nuclei (above) and young zygote with four nuclei (below). Scale bars: 10 µm.
Materials and methods
Pinnularia cf. gibba
Pinnularia cf. gibba was collected from the Loch of Craiglush (NO 041444; 29.5 ha) on 8 December 2004, and from Loch Achray (NN 506068; 77.1 ha) and the Lake of Menteith (NN 567009; 212.4 ha) on 29 September 2005; all of these lie in Scotland, at altitudes of 100 m or less. All three lochs are classified as ‘oligotrophic’ in the Scottish Natural Heritage lochs database (data reproduced by Palmer & Roy, Citation2001) and are relatively unimpacted natural lakes, originating after the last ice age in glacial troughs or depressions in drift deposits. The pH varies from c. 6.4 in Achray to a little over 7 in Menteith to 7.5 in Craiglush, and there are corresponding gradients in alkalinity, and in the conductivity of the lake water, which ranges from c. 38 µS cm–1 (average, 1993–2004) in Achray, to 75 µS cm−1 (average, 1990–2004) in Menteith, to 127 µS cm–1 (one measurement) in Craiglush (long-term data on Achray and Menteith provided by Dr Laurence Carvalho, personal communication; other data from Wingfield et al., Citation2004).
Surface sediments containing P. cf. gibba and overlying water were collected from lakes using a glass tube, as described by Round (Citation1953), transported to the laboratory in polyethylene bottles, poured out into plastic boxes, and allowed to stand in the dark for at least 5 h. Then the supernatant was removed by suction and the mud covered with lens tissue. Under continuous low-level illumination (c. 5 µmol photons m–2 s–1), epipelic algae moved up through the lens tissue and became attached to cover-slips placed on top. These were removed at intervals and either examined immediately, or used for isolating clones, or incubated in WC medium (Guillard & Lorenzen, Citation1972). Clones were isolated by streaking harvested epipelon onto 2% agar-solidified WC medium and subculturing from discrete colonies after c. 2 weeks. They were then transferred to liquid WC medium in 25-well Repli dishes and finally to 50-mm Petri dishes. Incubated cover-slips, stock cultures and experimental crosses were kept at 15–20°C at 5–20 µmol photons m–2 s–1 and usually under a 12:12-h light–dark cycle.
For observations of cultured material, cover-slips (usually 24 × 50 mm for LM; 13-mm diameter for SEM) were placed at the bottom of Petri dishes containing culture medium, before the medium was inoculated with a clone. Cover-slips became colonized by cells and could be removed and examined after careful cleaning of the lower side. For LM examination of live cells, colonized cover-slips were mounted on drops of WC medium and ringed with white vaseline to prevent evaporation. These preparations remained healthy for some hours, but the later stages in gametogenesis and young zygotes were very sensitive to disturbance. To test for the presence of mucilage around gametangia and auxospores, cover-slips were mounted in suitably diluted Indian ink and excess ink drawn off with tissue.
Cleaned valves were prepared either with hydrogen peroxide (for preliminary inspection of frustule morphology), or by boiling in a mixture of concentrated sulphuric and nitric acids. After washing with deionized water, valves were mounted in Naphrax (currently available from Brunel Microscopes: http://www.brunelmicroscopes.co.uk/). Measurements of stria density were made alongside the raphe, near the centre.
Bright field (BF) and differential interference contrast (DIC) light microscopy (LM: planapochromat lenses, nominal numerical aperture 1.32 or 1.4) were carried out using either (i) a Reichert Polyvar 2 photomicroscope fitted with a Polaroid DMC2 digital camera capable of 1600 × 1200 pixel resolution (images were captured via Optimas image analysis software version 6.2: MediaCybernetics, Silver Spring, MD 20910, USA); or (ii) a Zeiss Axioskop with a Zeiss Axiocam MRc5 digital camera (images captured and managed via Imaging Associates/Zeiss Axiovision Version 4 imaging software). In some cases, background noise and specks were removed digitally by image division (Bayer et al., Citation2001; http://rbg-web2.rbge.org.uk/algae/microscopy_digital_microscopy.html#dust).
Samples for fluorescence microscopy were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.0. Staining was performed with 10 µl per slide of freshly prepared DAPI (4,6-diamino-2-phenylindole.2HCl; Sigma, St. Louis, MO, USA) at a concentration of 1 µg ml–1, in phosphate buffer. Preparations were sealed with nail varnish and observed under a Zeiss Axiophot microscope with ×40 objective. Digital images were captured with a monochrome, medium-resolution Axiocam system, using ISIS software (Imaging Associates, Thame, UK). Colour photographs of DAPI-stained material were acquired via a conventional lens camera, using Fuji T64 colour reversal film, and subsequently digitized and converted to grey-scale images.
For scanning electron microscopy, small round cover-slips were placed in auxosporulating cultures for several days to become colonized. The cover-slips were then removed and placed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7). The cover-slips, with fixed cells still attached, were rinsed in deionized water and dehydrated through an ethanol series (50%, 15 min; 70%, 15 min; 95%, 10 min; 100% 5 min), followed by two changes of 100% dry acetone for 5 min. Cells were then dried using an Emitech K850 critical point dryer (EM Technologies, South Stour Avenue, Ashford, Kent TN23 7RS, UK) and the cover-slips attached to aluminium stubs by carbon tabs, with silver dag painted around the slip edges to promote electrical conduction. Stubs were coated with platinum for 2 min in an Emitech K575X sputter coater and examined using a LEO Supra 55VP Field Emission SEM operated at 5kV (6 mm working distance; aperture 20 µm). Images were captured as 3 Mb TIFF files. In addition, some cover-slips bearing sexual stages were placed on a hot plate and small amounts of fuming nitric acid (>95% HNO3; below boiling point) added to remove organic matter. After the acid had evaporated, the cover-slips were washed three or four times with deionized water, dried, and coated for SEM as described above.
Voucher material and slides of clones and source populations are kept in the diatom herbarium of the Royal Botanic Garden Edinburgh.
Pinnularia acidojaponica
Auxosporulation of Pinnularia acidojaponica M. Idei & H. Kobayasi was observed in a unialgal culture, which was established from a population in acid water (pH 2.4) from Doroyu hot spring in Yuzawa-shi, Akita, Japan (see also Suzuki & Mayama, Citation1995, as Pinnularia sp.; Idei & Mayama, Citation2001, give detailed observations of vegetative valves). The natural diatom population of this habitat was composed solely of P. acidojaponica and so an aliquot of collected material was incubated in filtered water from the sampling site. Auxosporulation began within a week and auxospores and initial cells were pipetted onto cover-slips, either directly or after treatment with 1% NaClO for 1 min followed by rinsing with distilled water, dried, and coated with gold–palladium for SEM (JEOL F-15 operated at 15kV).
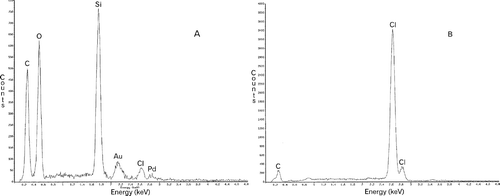
Energy Dispersive Spectrometry (EDS) analysis was carried out on the strips of material in the incunabula of P. acidojaponica using a JEOL 5800 SEM equipped with Voyager (Noran Instruments, Thermo Electron Corporation, Waltham, MA, USA), operated at 10kV, with point scanning. For EDS, auxospores were mounted on a sheet of vinyl chloride and coated by gold–palladium.
Terminology
Terminology follows the conventions of Ross et al. (Citation1979) and Round et al. (Citation1990), with the addition of ‘incunabula’ (from the plural Latin for ‘swaddling clothes’), a collective term introduced by Trobajo et al. (Citation2006) to refer to the organic and inorganic components (including silicified elements) of the auxospore wall that surround, cover or invest the auxospore and its perizonium or properizonium as the auxospore expands. As Trobajo et al. note, some evolution in the use of this term is to be expected, but there was previously no convenient way to refer to those parts of a pennate or multipolar centric auxospore wall that are formed before or after expansion begins and that are not parts of the (pro-) perizonium.
Results
Morphology of vegetative cells of P. cf. gibba
The morphology of the six Craiglush clones (, ) was highly constant, once allowances had been made for the effects of size reduction during the life cycle. Longer valves were linear with subcapitate ends (clones 1–4), whereas smaller valves (clones 5, 6) had broadly rostrate ends and became almost linear–lanceolate as a consequence (). All clones had a wide axial area and a large transverse, unthickened area without striae (fascia) at the centre; the striae were radial near the centre and convergent at the poles. There was no sign of longitudinal lines crossing the striae in any of the clones, which reflects the ultrastructure of the valve interior in that the striae lack all traces of an internal covering (i.e. the striae are simply multiseriate, rather than truly alveolate: ). Dimensions and stria densities are given in .
The Achray clones and the Menteith clone (, ) had the same central area, stria pattern and stria density as the Craiglush clones, but their valves appeared to be slightly narrower and in valves of the same length, the poles were slightly more pointed and rostrate than those from Craiglush (compare with , with ). To check the width differences, we measured 30 further valves of each of clones 3, 4, 7–9. These clones were selected because they all had similar valve lengths (). Student's t-tests and (where distributions were not normal) nonparametric statistical tests on the new data showed no significant differences in valve width either among the Achray and Menteith clones (clones 7–9) or between the Craiglush clones 3 and 4. By contrast, the small differences in width between the Menteith–Achray clones 7–9 (mean widths 8.632 ± 0.300, 8.768 ± 0.275 and 8.640 ± 0.304, respectively: mean ± s.d.) and Craiglush clones 3 and 4 (mean widths 9.208 ± 0.307 and 9.296 ± 0.468) were highly significant (p > 0.99 in all six pair-wise comparisons). The Craiglush, Achray and Menteith clones agree with the descriptions of P. gibba given by Hustedt (Citation1930) and Krammer & Lange-Bertalot (Citation1986). However, our clones and the natural populations from which they were isolated exhibited far less variation than Krammer & Lange-Bertalot or Hustedt allow and their concepts of P. gibba certainly contain several species, as recognized by Krammer (Citation1992, Citation2000). In the finer-grained taxonomy of Krammer (Citation2000), the nearest equivalent to the Craiglush clones is apparently P. parvulissima Krammer. However, because of continuing uncertainties in the taxonomy of the P. gibba complex (see Supplementary material), we will refer to all of the Scottish clones here as ‘P. cf. gibba’.
Table 1. Valve dimensions and stria densities of clones
Interphase cells had two chloroplasts, one appressed to each side of the girdle (); there was no connection between them at the centre. Each chloroplast contained a small cushion-like pyrenoid, which was penetrated by a branching system of channels (). The nucleus was apically elongate and lay in a bridge of cytoplasm between two large vacuoles, which occupied most of the cell lumen (). The nucleus was slightly constricted by the pyrenoids () and by the inward thickening of the valves around the central raphe endings (). During preparation for cell division, the chloroplasts moved beneath the valves (). As in other diatoms, the mitotic nucleus was positioned close to one side of the girdle, with the chloroplasts displaced slightly towards the opposite side (not illustrated). After mitosis (post-division nuclei are shown in ) and cytokinesis and the formation of new valves, and before the nuclei regained their central positions, the chloroplast of each new cell divided transversely (). The two daughter chloroplasts then moved around in the cell to re-establish the interphase configuration (not illustrated).
Vegetative cells moved actively and produced extracellular polymeric substances (cf. Underwood & Paterson, Citation2003), often producing a viscous mass surrounding the cells in late stationary-phase cultures. The cells were not surrounded by capsules of mucilage, either during interphase or during cell division (contrast e.g. Lyrella and Petroneis: Mann & Stickle, Citation1993; Jones et al., Citation2005), but small accumulations of mucilage were sometimes present at the poles (), apparently secreted via the raphe.
Mating system
Sexual reproduction was observed in incubated semi-natural populations and rough cultures of Pinnularia sp. from the Loch of Craiglush, Loch Achray or the Lake of Menteith, but no pairing or other evidence of sexual activity was ever seen in any of the monoclonal cultures. We therefore inoculated pairs of clones together under our standard culture conditions. In every case, we also included monoclonal controls, to check the significance of negative results in interclonal crosses. Most pair-wise combinations of the Craiglush clones (clones 1–6) were made, except two involving the largest-celled clone (clone 1). Sexual reproduction was observed in some pair-wise combinations () and there was a clear gradation in sexual response. The two clones with the largest cells, clones 1 and 2 (), did not reproduce sexually. Clone 3, which had only marginally smaller cells () did reproduce when mixed with clone 4 or clone 6 but the proportion of cells involved was low. For example, nine days after a mating experiment begun on 28 September 2005, sexualized cells comprised <0.5% of the 1,000 and 500 cells counted in clone 3 × clone 4 and clone 3 × clone 6, respectively. The largest cells found to be capable of sexual reproduction were 60 µm long. The other three Craiglush clones, with valves less than 55 µm long, reproduced vigorously when mixed together in the compatible combinations 4 × 5 and 4 × 6: e.g., c. 20% cells were sexualized in the 28 September cross of clone 4 × clone 6. The size differences between cells of the smaller-celled clones (clones 4–6) were large enough (, ) for it to be possible to determine whether individual pairs, triplets and larger aggregations contained both clones or only one. In every case, mating was interclonal.
Table 2. Results of mating experiments between Pinnularia clones in autumn 2005. Two Craiglush clones (1 and 2) remained vegetative. Other clones belong to one of two mating types.
We repeated the crosses several times between February 2005 and July 2006, with identical results in terms of compatibility but slight variation in the vigour of the sexual response (which showed no obvious pattern, apart from an increase in the sexualization of clones 3 and 9). The Craiglush population was therefore heterothallic. In accordance with the recommendations of Chepurnov et al. (Citation2005), we designate the mating type of clones 3 and 5 as PINs-1 and that of clones 4 and 6 as PINs-2 (). These designations consist of a short acronym derived from the genus and species (here unknown), and a number (1 or 2) for each mating type, the number is assigned arbitrarily, reflecting the fact that the gametangia are not obviously differentiated (see below).
Crosses carried out between PINs-1 and PINs-2 clones from the Loch of Craiglush and the three clones from the Lake of Menteith and Loch Achray showed that all three Menteith–Achray clones had the PINs-2 mating type (). There was vigorous mating between the Craiglush clone 5 and the two Menteith clones, but auxosporulation was initially much less common in the cross with the Achray clone 9, whose cells were of approximately the same length as the sexually immature Craiglush clone 2 and the sexually ‘reluctant’ clone 3. In our first experiments, although the clones from different provenances were sexually compatible, all of the auxospores aborted before or during expansion in the Craiglush × Achray cross and, although initial cells were formed in the Craiglush × Menteith crosses, they did not divide during our observations. By contrast, initial cells formed at the same time in crosses among Craiglush clones did yield vegetative progeny. After six months’ further growth in culture, new ‘allopatric’ crosses were made among the Craiglush, Achray and Menteith clones, with Craiglush 4 × Craiglush 5 as a positive control. This time, all crosses between clones of opposite mating type involved vigorous pairing and all produced viable initial cells that escaped from their perizonia. The only difference noted between the Achray × Craiglush or Menteith ×Craiglush crosses and the positive control was a slightly slower sexual response, but this could have been caused by differing inoculum densities or other extrinsic factors. Attempts to cross Menteith and Achray clones with each other were always unsuccessful, as expected given that they all had the PINs-2 mating type.
Sexual reproduction
Sexual reproduction in the various compatible combinations of PINs-1 and PINs-2 clones exhibited the same characteristics. Pairing occurred within two days of mixing. Initially, cells were in physical contact and remained so during the transition to meiosis and in the early stages of meiotic prophase (). There seemed therefore to be surface recognition between cells, but there was no constancy in the orientations of copulating cells, valve–valve (), valve–girdle () and girdle–girdle (almost achieved in ) configurations all being found. Triplets were frequent, especially where sexualization was vigorous. Thus, among 108 configurations counted in one clone 5 × clone 6 cross, there were 62 pairs, 38 triplets, and 7 clusters of four or more gametangia. In triplets, the two cells of one mating type usually flanked the single cell of the other mating type ().
When first paired, cells had similar chloroplast and nucleus configurations to vegetative cells (; compare ). On entry to meiosis, the nucleus expanded greatly and became ellipsoidal () and its boundary became much more distinct. During zygotene, the chromatin formed a synizetic knot (), which then dispersed as the chromosomes condensed towards diakinesis (). During mid- to late meiotic prophase (before diakinesis), the chloroplasts moved from the girdle to beneath the valve (). Meiosis I (, right-hand cell) was accompanied by cytokinesis in the median valvar plane (), but without the formation of new valves. The daughter protoplasts then became rearranged within the gametangia (), sliding over each other to move from their initial positions beneath the valves () to lie one towards each pole (). Almost simultaneously, the gametangial frustules dehisced and the naked gametes rounded up (, , ). Each gamete contained two almost equal nuclei from meiosis II () and one chloroplast (), which could sometimes be seen to be constricted centrally (cf. ; see also the optically sectioned chloroplasts in ).
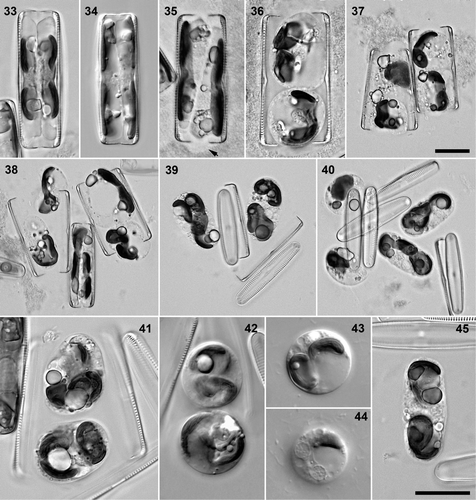
Figs 33–45. Pinnularia cf. gibba, clone 4 × clone 5 (except , clone 5 × clone 6). . Gametogenesis. . Formation of two protoplasts lying either side of the median valvar plane, BF and DIC; note that the chloroplasts are lobed but undivided. , . Dehiscence of the gametangium and rearrangement of the gametes, BF; mucilage has been secreted by the protoplasts (visible by displacement of precipitate surrounding the gametangia, e.g. at arrow). . Rearranged gametes within a pair of gametangia. . Triplet of gametangia, BF: release and movement of gametes (each with one chloroplast) from two of the gametangia; the remaining interposed gametangium is at an earlier stage of development. . Young zygotes, each with two chloroplasts, BF. . Triplet of dehisced gametangial frustules with two unfused, released gametes (left) and two zygotes (right), BF. . Young ellipsoidal zygotes, BF. . Young spherical zygotes. . Young spherical zygote, containing two chloroplasts (); two nuclei are visible in and one or two others were also present (cf. ). . Shortly cylindrical maturing zygote, before perizonium formation and expansion. Scale bars: 20 µm (bar for is in ; bar for is in ).
During meiotic prophase, cells began to produce some mucilaginous material, but this did not initially extend far from the cells. By the time gametangia dehisced, however, they were surrounded by a wide envelope with ill-defined and irregular boundaries, which persisted throughout the remainder of auxosporulation () and in particularly favourable circumstances could be visualized in SEM (). This envelope was nevertheless very watery, incorporated precipitates from the medium, and resisted the diffusion of Indian ink particles for only c. 30 min. Secretion of stiffer mucilage from the gametes occurred during and after gametangium dehiscence (: the limits of the gametic mucilage are revealed by their contact with surrounding precipitates and bacteria). Indeed, the contraction of the gametes away from the gametangial frustules before gametangium dehiscence (, , ) may have been caused by an initial phase of secretion.
Figs 46–56. Pinnularia cf. gibba, diploid auxospore development, BF (except , : DIC). . Clone 4 × clone 5. . Mature zygotes with polar chloroplasts. . Young auxospore with paired but separate gametic nuclei (arrows) at centre; chloroplasts diagonally opposed. . Young auxospores, at right angles to each other. . Clone 5 × clone 6: very young auxospore with ellipsoidal envelope of mucilage. . Clone 3 × clone 4: a half-expanded auxospore, showing the incunabular strips (peripheral focus, ) and the unfused gametic nuclei (mid-focus, ). . Clone 3 × clone 6: fully expanded auxospores containing initial epivalves. . Clone 3 × clone 6: Indian ink preparation of pair with expanded auxospore (g = remnants of unfused gametes). Note the wide mucilage envelope around the gametangia and the narrower and apparently separate envelope around the auxospore (cf. ). . Clone 3 × clone 6: initial epivalve, still contained within perizonium (causing the wide repeating pattern). . Clone 4 × clone 5: post-auxospore valve. . Seminatural Craiglush material: auxospore pole with incunabular scales and elements seen in section. Scale bars: 20 µm (bar for is in ; bar for is in ) or 10 µm ().
Plasmogamy was achieved through amoeboid movement and some swelling of the gametes (). As a result of mucilage production and the original configuration of the gametangia, gametes often had to move several tens of micrometres. During plasmogamy, the gametangial thecae were often displaced within the diffuse mucilage envelope (see below) and became arranged more or less haphazardly with respect to each other (). Fusion of the irregularly rounded gametes produced large, irregularly rounded or ellipsoidal zygotes (), which then contracted, became almost spherical and acquired a more definite outline (), suggesting the formation of a thin organic wall, which was visible as a felty material in SEM (). At this stage, the chloroplasts were closely associated and often folded around each other, but in some cases it was possible to see that two were present, as expected (, lower cell; ). DAPI staining showed the presence of four almost equal nuclei (, lower cell) in spherical zygotes and some of these nuclei could sometimes be seen with DIC optics ().
Figs 57–69. Pinnularia cf. gibba. . Clone 5 × clone 6: unfused gamete and ‘triploid’ zygote, DIC. . Clone 4 × clone 5: elongating diploid zygote and unfused haploid cell, BF. . Clone 4 × clone 5: almost fully expanded auxospore with paired, unfused gametic nuclei at centre, DAPI. . Clone 4 × clone 5: pair of mature auxospores, DAPI. The left-hand auxospore has a large, fused diploid nucleus; the right-hand auxospore has just undergone the first acytokinetic mitosis and has a functional G1-phase nucleus and a smaller, degenerating nucleus. . Clone 3 × clone 6, BF. Diploid auxospore (containing initial epivalve, visible at top). . Clone 3 × clone 6, BF. ‘Triploid’ auxospore. Note the greater width of this cell, compared with . . Clone 5 × clone 6, DIC. Detail of auxospore centre, showing three unfused nuclei. . Clone 5 × clone 6, DIC. Haploid auxospore with mucilage envelope (cf. ); undifferentiated gamete present at top. . Clone 4 × clone 5, BF. Haploid auxospore. . Clone 3 × clone 6, BF. Haploid auxospores: late stages in expansion. . Clone 5 × clone 6, DIC. Escape of the initial cell from the perizonium (empty at top); arrowheads indicate extent of primary transverse perizonial band. . Clone 4 × clone 5, DAPI. Divided initial cell still partly enclosed in the perizonium (outlined by fluorescing bacteria). Scale bars: 20 µm () or 10 µm (Fig. 63).
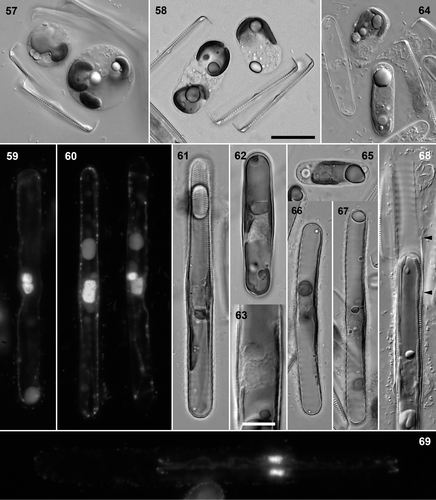
The zygotes now changed shape, becoming elongate, with a length–breadth ratio of just over 2:1 (, , , ). This was achieved without expansion. Young zygotes like those shown in (which are in the process of contraction after plasmogamy) had volumes of c. 8,000–11,000 µm3. Contracted spherical zygotes () had diameters of c. 12 µm and therefore volumes of c. 7200 µm3, whereas stages like those in and had volumes of c. 4,500–6,000 µm3 (calculated by approximating the shape to a cylinder capped by two hemispheres).
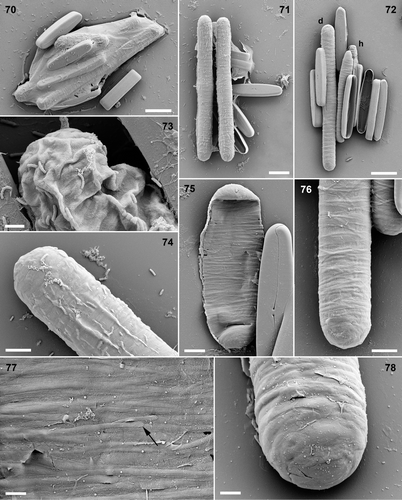
Figs 70–78. Pinnularia cf. gibba, SEM. Figs 70–74. Fixed, critical-point-dried material. . Organic envelope surrounding gametangia and expanded auxospores. . Expanded diploid auxospores. . Diploid auxospore (d), two haploid auxospores (h, one almost obscured) and four dehisced gametangia. . Detail of crumpled zygote with organic wall. . Expanded auxospore with slightly pleated organic layer overlying siliceous elements. . Oxidized auxospore walls. . Early stage in auxospore expansion with closely packed, parallel incunabular strips in the collapsed central zone; the auxospore poles are more robust. . Mature auxospore, with widely spaced incunabular strips separating to reveal the perizonium beneath. . Detail of incunabular strips; note that some have free ends (e.g. arrow). . Detail of auxospore pole, showing elliptical and polygonal scales of the incunabula. Scale bars: 20 µm (), 5 µm () or 2 µm ().
The metamorphosis of the zygotes was achieved during the formation of special incunabular elements, which appeared in LM as delicate, closely spaced transverse markings (shown at a later stage in ). This took place before the production of any perizonial bands (see below). By the time shape change was complete, the incunabular striations could be detected over the whole central part of the cell, with less easily differentiated (LM!) elements also covering the poles. The two superfluous meiotic nuclei disappeared during maturation of the zygote (not shown) and the chloroplasts moved to lie one at each end (, ).
In triplets and quadruplets, one of the gametangia was often at an earlier stage of sexual development than the others (, ) and its gametes usually reached maturity too late, after plasmogamy had occurred among the gametes produced by the other gametangia (). However, even where development was synchronous in triplets, the maximum number of normal diploid (dikaryotic) auxospores produced was still only two, despite the formation of six gametes (except where some aborted prematurely). Fusion never took place between the gametes produced by a ‘superfluous’ gametangium in triplets.
In the conditions used (a low-light, moderate temperature environment), cells had paired and were in meiotic prophase three days after compatible clones had been mixed; auxospores were present after a further day. However expanded auxospores were not present until day seven or eight. Fully formed and released initial cells were seen 10–12 days after cultures were mixed. Gametes and young zygotes seemed to be particularly sensitive to disturbance and many died – more than we have observed in other genera and species of raphid diatoms. This meant that many gametangia produced no viable auxospores, and others produced only one. For example, about half of the pairs, triplets and large groups in one (vigorous) cross between Craiglush clones 5 and 6 left no progeny, and 40% of the remainder produced only one diploid auxospore.
Auxospore development
We regarded the transition from zygotes to auxospores as being marked by the formation of the silicified primary perizonial band beneath the incunabula (see later). The number of nuclei was reduced to two, which remained unfused. The initial formation of the perizonium could be detected in LM by thickening of the auxospore wall (; contrast , ), and the auxospore then began bipolar expansion (, ), during which delicate secondary perizonial bands were produced on either side of the primary band (visible as periodic thickenings on the upper side of the top auxospore in ; see also ). The tip of the auxospore could sometimes be seen to be covered with several or many scale-like elements (), but discrete caps were not visible. The two surviving haploid nuclei remained closely associated but unfused at the centre of the auxospores throughout expansion (, , ) and the chloroplasts became displaced diagonally (), eventually coming to lie one on either side of the cell, as in vegetative cells (, , ).
Initially and during the early stages of expansion the orientations of the two auxospores bore little relation to each other or the gametangial thecae (, , , ), but the auxospores commonly became parallel to each other during late stages in expansion (, , ). Once auxospores had reached 100–130 µm (), expansion ceased, the two nuclei fused (, left), an acytokinetic mitosis took place with the elimination of one of the daughter nuclei (, right), and the initial epivalve was produced (, , ). Subsequent formation of the initial hypotheca completed the initial cell (presumably after a further acytokinetic mitosis, though this was not documented), which then escaped from the perizonium by sliding through one end before () or after () its first mitotic division. There were no contractions of the protoplast during formation of the initial valves, so that they were produced directly against the inside of the perizonium. Initial and post-initial valves had a very similar stria pattern to gametangial valves (; compare ), with a well-defined wide fascia.
Mucilage secretion continued to take place, apparently over the whole surface of the cells, during the development of zygotes into auxospores and during auxospore expansion () and must therefore have occurred between or through the incunabular strips and perizonium. The reorientation of the auxospores during expansion, to become parallel to each other (, ), was probably brought about passively, through the resistance of the mucilage envelope, causing the auxospores and gametangial thecae to become ever more densely and regularly packed within it.
Trikaryotic auxospores and haploid parthenogenesis
Trikaryotic and haploid auxospores were observed. They were uncommon relative to diploid auxospores (e.g. 1 triploid + 1 haploid were formed in only two out of 56 and three out of 43 pairs, triplets and larger aggregations of sexualized cells producing viable auxospores in crosses of clone 5 × clone 6 and clone 3 × clone 6, respectively) but they were almost always present in experimental crosses. In , a pair of gametangia has produced a zygote containing three chloroplasts, produced by simultaneous fusion of three gametes. The superfluous unfused gamete has rounded off and contracted. The stage illustrated is almost the same as that shown for two normal zygotes in . Trikaryotic auxospores developed like dikaryotic auxospores, but had three unfused nuclei at the centre () and three chloroplasts, and were wider (; compare , ). We never observed fusion of the three nuclei to form triploid initial cells, but we may have overlooked them: It is also possible that only two of the nuclei fuse at the end of expansion, producing an unusually large but diploid initial cell, though this would have three chloroplasts.
Rather more surprising than the trikaryotic auxospores was the frequent parthenogenetic development of unfused gametes. There was an initial phase of elongation in the unfertilized ‘pseudozygote’, with the single chloroplast migrating to one end (). Subsequently, the pseudozygote secreted a mucilage envelope like that of diploid auxospores (see below and compare with ) and began to expand. A single nucleus survived, out of the two that must originally have been present from meiosis II, and moved near the centre of the auxospore (), though it was noticeably less exactly centred than the paired nuclei of dikaryotic auxospores. The haploid auxospores expanded and produced incunabula and a perizonium (, , ), and some were observed to produce an initial epivalve, but no further development was documented. Haploid auxospores were smaller and narrower than their diploid equivalents (e.g. compare , with , with ) and reached less than 100 µm long (initial epivalves ranged from 70–96 µm: n = 4).
Most of our observations of unusual ploidies were made in experimental crosses, but we also observed one pair with a haploid and a triploid auxospore in incubated material from the Lake of Menteith.
Incunabula and perizonium of Craiglush Pinnularia
The fine transverse striation observed by LM in the incunabula of metamorphosing zygotes and expanding auxospores proved to comprise many very narrow, apparently non-porous, strips of material, which were wound around the cell (). The formation of the strips preceded formation of the primary perizonial band, demonstrated by direct observation in LM and also in SEM by stages in which the complete collapse of the incunabula showed that no perizonium was present internally (); the cell shown in measures c. 40 µm long and corresponds to the stages shown in Figs . In vivo, the incunabular strips appeared to be covered by organic wall layers (). In young auxospores, the strips were more or less parallel, but overlapped and sometimes crossed each other. At least some of the strips were not complete hoops around the auxospore, possessing smoothly rounded, symmetrical ends (). In expanded auxospores, the incunabular strips tended to separate and their orientations became more diverse. At the poles, the narrow strips graded into wider segments and irregular or circular scales (), which imbricated and corresponded to the layers visible in optical sections ().
As the incunabular strips became separated by the expansion of the auxospore within, transverse perizonial bands became visible (, ). The transverse perizonium consisted of a very long, cylindrical primary band (), flanked on each side by a series of secondary perizonial bands (). The primary band was robust, perforated sparsely by irregularly placed pores (), and its outer surface was slightly grooved, indicating that it was moulded during its formation by the overlying incunabula. It was symmetrical, bearing flat branching fimbriae at both ends (). The secondary bands were very delicate and were thicker proximally (i.e. nearer the primary band) and thinned distally (so that the auxospore had a very slightly corrugated profile: ) where they overlapped each other; they bore pores and branched fimbriae ().
Figs 79–84. Pinnularia cf. gibba, oxidized auxospore walls, SEM. . Part of an expanded auxospore, showing incunabular strips overlying the transverse perizonium. . Secondary transverse perizonial bands. Note the very slightly corrugated profile of the perizonium. . Centre of perizonium, showing the very wide, irregularly porous, and slightly grooved primary transverse band (its limits are indicated by arrows; the break at bottom left is an artefact). . Broken centre of auxospore showing the interior of the primary transverse perizonial band. . Fimbriate margin of the primary transverse perizonial band (p) and an adjacent secondary transverse perizonial band (s). . Secondary transverse perizonial bands with fimbriate margins. Scale bars: 2 µm.
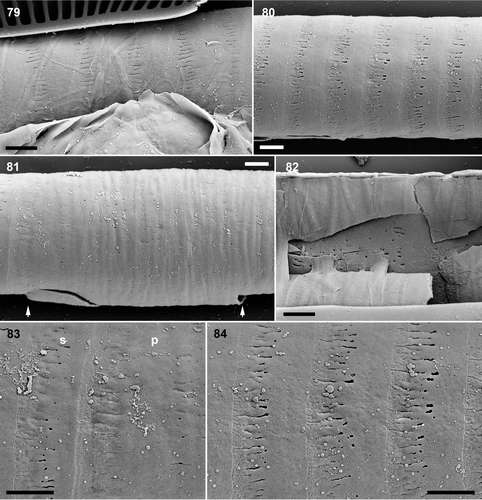
In SEM, we were unable to find any splits in either the primary perizonial band or the secondary bands. However, incunabula obscured the perizonium in many cases and we therefore looked for evidence of splits in LM. In some auxospores we detected a slight inward inflection of some of the perizonial bands along a line on one side of the auxospore (not illustrated). In one case this line was on the opposite side to the initial epivalve, as might be expected for a perizonial suture (cf. Rhoicosphenia Grunow: Mann, Citation1982). In addition, in burnt preparations, there seemed to be a longitudinal line of weakness along many auxospores, which again pointed to the presence of aligned splits in the transverse bands. However, we obtained no conclusive proof.
No longitudinal perizonium was seen in SEM. However, we observed several auxospores in LM in which an initial cell had begun to escape from the perizonium and lay in girdle view. In such cells, the side of the perizonium opposite the initial epivalve was clearly thicker than the other side, indicating the presence of longitudinal perizonial bands.
Unlike normal vegetative cells, which had flat valve faces and a clear separation of valve face and mantle, initial cells had convex valves (), as a result of their formation against the insides of the cylindrical auxospores. Both initial valves had apparently normal raphe systems (, , ). A series of indentations was present on each initial valve (e.g. ) because of moulding by the thickened proximal sides of the transverse perizonial bands. Valves formed after the first division of the initial cell had flat valve faces ().
Incunabula of Pinnularia acidojaponica
As in the Craiglush Pinnularia sp., expanding and mature auxospores of P. acidojaponica were cigar-like, although the ends collapsed inwards during preparation for SEM unless initial valves had already been formed (). The outermost part of the auxospore consisted of string-like incunabula (), which was briefly reported by Suzuki & Mayama (Citation1995) but is shown in detail here for the first time, following extra observations. The incunabular strips formed a dense wrapping around most of the auxospore and each strip was 0.1 to 0.3 µm wide (). At the poles, we did not observe any differentiated, more isodiametric incunabular elements like those of the Craiglush Pinnularia sp., and this correlates with the greater flimsiness of the poles in P. acidojaponica.
Figs 85–90. . Pinnularia cf. gibba, oxidized initial cells, SEM. . Divided initial cell: disrupted frustule with initial valves (left and right) and almost complete daughter-cell hypovalves. Note that the striae are not strictly alveolate. . Part of a divided initial cell, showing the imprint of the transverse perizonium on the initial valves (e.g. at arrows). Scale bar: 5 µm. . Pinnularia acidojaponica, auxospores, SEM. . Expanded auxospore, entirely surrounded by incunabular strips and containing a divided initial cell. . Detail of auxospore showing collapsed pole (unsupported by an underlying perizonium. . Incunabular strips. . Fragment of the perizonium, showing a few transverse bands and part of a longitudinal perizonial band (lp). Scale bar: 10 µm (), 5 µm () or 1µm ().
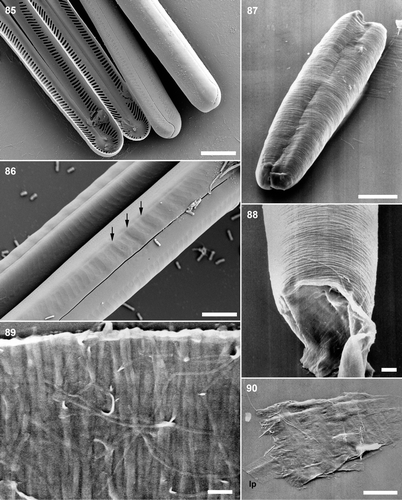
Transverse perizonial bands were observed beneath the incunabula in well-developed auxospores and could be revealed by manipulation. The perizonial bands were not porous and had fimbriate margins (), which were more regular on the pars exterior than on the pars interior. Open ends of the transverse perizonial bands were not observed. One longitudinal perizonial band, again without pores, lay inside the series of transverse bands. It was narrower than the transverse bands and bore equivalent fringes of fimbriae on either side (). The string-like incunabula persisted even after formation of both initial valves (). Incunabula and perizonium disintegrated when the initial cell divided and moved away.
EDS analysis indicated that the principal elements present in the strip-like incunabula were silicon and oxygen (). The incunabula also included much carbon, probably representing mucilage. Although the background of the specimen contained carbon because of the plastic sheet, the relative amount in the control was less than in the specimen ().
Discussion
Systematics
There is no pre-zygotic isolation mechanism separating Craiglush clones of Pinnularia cf. gibba from either of the Lake of Menteith clones or from the Loch Achray clone. In our first experiments, there appeared to be clear differences in the success of auxosporulation between ‘allopatric’ and ‘sympatric’ crosses: Initial cells produced from crosses between clones from different habitats did not divide, and in the Craiglush × Achray cross, the auxospores did not expand fully and aborted before producing initial valves; the positive controls (Craiglush × Craiglush crosses), on the other hand, yielded viable initial cells under the same conditions. It is possible, therefore, that the Menteith, Achray and Craiglush populations have acquired physiological adaptations to their local environments, so that F1 progeny between clones of different provenance have lower vitality. However, in our second experiments, there was little sign of reduced viability resulting from mating between clones of different provenance. Nevertheless, the slight differences in valve outline in culture and the small (<1 µm) but statistically significant differences in valve width point to genetic differences between the Menteith–Achray clones and the Craiglush clones. That such variation cannot be assumed unimportant is shown by Sellaphora capitata and S. blackfordensis, which were initially separated via a consistent small difference in width and later found to be reproductively isolated (through pre-zygotic mechanisms) and widely divergent in their ITS sequences (CitationMann, 1988a, 1989b; Behnke et al., Citation2004; Mann et al., Citation2004). We are therefore using molecular genetic markers to establish the extent of differentiation between the populations of P. cf. gibba.
Mating system and life history
Pinnularia cf. gibba is heterothallic: clones belong to one of two mating types and there is no trace of intraclonal reproduction. Pinnularia therefore joins a rapidly growing list of raphid pennate diatom genera in which heterothally has been demonstrated (earlier literature reviewed by Chepurnov et al., Citation2004; subsequent reports by Amato et al., Citation2005, in press; Chepurnov et al., Citation2005; Mann & Chepurnov, Citation2005). By analogy with other groups of organisms, the mating system probably covaries with the ecology and ‘growth form’ of diatom species; for example, in angiosperms, there is a broad correlation between inbreeding (or apomixis) and opportunistic, weedy habit (e.g. Barrett et al., Citation1996; Richards, Citation1997; Silvertown & Charlesworth, Citation2001). Our observations of epipelon in various Scottish lakes over many years indicate that Pinnularia cf. gibba and its immediate relatives are constantly present in relatively low numbers (when they occur), and we have seen no evidence (e.g. sudden increases or decreases in population density) to suggest that they are opportunistic, highly invasive species.
Several diatom genera have already been shown to be variable with respect to mating system: The freshwater genus Sellaphora contains obligately heterothallic, facultatively homothallic, freely homothallic, and autogamous species (Mann et al., Citation1999, Citation2004; our unpublished observations), and marine Achnanthes Bory species exhibit a similar range (Chepurnov & Mann, Citation1997; Sabbe et al., Citation2004). The genera Eunotia (Mann et al. Citation2003, Chepurnov et al. Citation2004) and Nitzschia are also variable (Trobajo et al., Citation2006). Mating systems must therefore evolve rapidly in many groups of raphid diatoms, relative to the morphological characteristics that are used to characterize genera, although there may be a predominant behaviour; for example, Pseudo-nitzschia H. Peragallo seems to be mostly heterothallic (e.g. Chepurnov et al., Citation2005), the single known exception being P. subcurvata (Hasle) G.A. Fryxell (Fryxell et al., Citation1991). Although we have cultured only a few (c. 15) Pinnularia species out of the hundreds that have been named, our observations (in preparation) show already that the genus includes homothallic and automictic species, as well as heterothallic species like P. cf. gibba. However, it is too early to judge which mating system is prevalent.
During 2 months in culture, P. cf. gibba clones decreased in length by 2–3 µm (, for clones 3–6). It seems unlikely that our low-light but moderate temperature regime would lead to gross underestimates of the rate of size reduction and so rates of 20 µm decline per year in nature are probably generous. Given that initial cells measure c. 115 µm and that the largest cells found to be capable of sexual reproduction are c. 60 µm, with many smaller cells being found in natural populations, we can therefore estimate that the life cycle of P. cf. gibba will occupy at least 3 years in nature, possibly as much as double this. Life cycles of similar length have been suggested to occur in other pennate diatoms (Mann, Citation1988b).
Sexual reproduction in a systematic context
Geitler (Citation1973) made a hierarchical classification of auxosporulation in pennate diatoms (see also Mann, Citation1993). In Geitler's system, P. cf. gibba would be placed in category IC: Two gametes are produced by each gametangium (and hence potentially two zygotes are formed by a gametangial pair), plasmogamy is more or less isogamous, and the orientations of the auxospores do not bear any fixed relationship to each other or to the gametangia because the gametes fuse within a relatively watery mucilage envelope. However, to reach this conclusion, it is necessary to observe early stages in auxosporulation, because space constraints within the copulation envelope lead subsequently to reorientation of the auxospores and gametangia, so that they often become parallel to each other (e.g. contrast with Figs ).
Type IC is not particularly common among the raphid diatoms surveyed by Geitler (Citation1973), who listed it in Caloneis, Rhoicosphenia Stauroneis Ehrenberg, Neidium Pfitzer, Brachysira Kützing (formerly included within Anomoeoneis Pfitzer) and Nitzschia angustata (W. Smith) Grunow (for independent reports for some of these genera, see Mann, Citation1982, Citation1989a, Citation1996a; Mann & Stickle Citation1996). Geitler's tentative categorization of Neidium has since been shown to be incorrect (Mann, Citation1984; Mann & Chepurnov, Citation2005), but his other examples seem to be valid and type IC behaviour has also been found in Achnanthes Bory (Idei Citation1991, Roshchin & Chepurnov Citation1999), Campylopyxis Medlin (Mann, Citation1990), Craticula Grun. (Mann & Stickle, Citation1991) and Dickieia Berk. ex Kütz. (Mann, Citation1994a). Two of the type IC diatoms, Caloneis and Pinnularia, are probably closely related (and may not be separate genera: Mann, Citation2001): The cell-wall structure is very similar in both (with highly characteristic multiseriate to alveolate striae), as is raphe structure; the chloroplasts are girdle-appressed plates (connected by a very narrow bridge in some species of both genera: e.g. Cox, Citation1996); and the pyrenoids are often invaginated (Tschermak-Woess, Citation1953; Edgar, Citation1980; Mann, Citation1996b), as in P. cf. gibba. Some of these characteristics are probably synapomorphies for a wider group of raphid diatoms than the Pinnulariaceae, but the stria structure seems to be a synapomorphy for the family. Consistent with this, the overall pattern of auxosporulation in P. cf. gibba is similar to that in Caloneis (Mann, Citation1989a), and both genera secrete a mucilage envelope around the gametangia during meiosis, generally causing the gametangia to separate and creating a space within which plasmogamy occurs. Nevertheless, there are differences. The mucilage envelope around C. silicula gametangia (and a few other freshwater Caloneis species) is more robust and differentiated than in P. cf. gibba, possessing a stiff cortical zone; the superfluous haploid nuclei degenerate before plasmogamy in C. silicula; there are discrete caps at the ends of Caloneis auxospores but apparently no silicified incunabular strips; and the initial epivalves in Caloneis have an aberrant raphe system (Mann, Citation1989a), as in the unrelated diatom Amphora Ehrenberg ex Kützing (Geitler, Citation1969).
Pairing, gametangium development and plasmogamy
The gametangia can apparently pair in almost any configuration, providing they are more or less side-by-side. Probably, therefore, recognition between cells can be mediated via any part of the cell surface.
The wastage of gametangia and gametes was surprisingly high in experimental crosses. Part of the loss (particularly the abortion of gametes in ‘normal’ pairings between two gametangia) is probably attributable to some fault in our culture conditions. However, the high proportion of triplets, reaching 10–30%, and the ease with which we could find haploid and triploid auxospores, require a different explanation. Once gametangia have entered meiosis, they apparently cannot revert to a vegetative state. Thus, because triplets never produce more than two auxospores (itself implying some kind of self–non-self recognition among gametes), each triplet represents an automatic one-third penalty for sexual reproduction. Furthermore, the low but not insignificant production of trikaryotic auxospores implies that there is only limited protection against polyspermy – perhaps only via the slight asynchrony of development of the gametangia in triplets (e.g. , , ). A similarly high rate of polyploid formation and haploid parthenogenesis has been observed in seminatural populations of Dickieia (Mann, Citation1994a), and the same types of ‘abnormal’ auxosporulation occur in cultures of several other pennate diatoms (Chepurnov et al., Citation2004). Our hypothesis is that effective mechanisms to prevent triplet formation and polyspermy have not evolved in P. cf. gibba and some other diatoms because population densities are not high enough in nature for triplets to arise at more than an infinitesimal rate (because ± simultaneous encounter between three sexualized cells is extremely unlikely). Three types of data are needed to test this idea: (i) dilution experiments, to examine whether the rate of triplet formation approaches zero when the densities of compatible sexualized cells are low; (ii) information on P. cf. gibba abundance in natural epipelic populations (anecdotally, in harvested epipelon from Scottish lakes, we have never yet observed such high densities of P. cf. gibba or related Pinnularia species as we routinely use in experimental crosses); and (iii) examination of plasmogamy in species that do reproduce sexually during periods of high cell density (blooms) in nature.
Persistence of superfluous haploid nuclei in the gametes and young zygotes has not often been recorded in diatoms. The exceptions are among Navicula species, where up to eight nuclei can be present in zygotes formed in species exhibiting type II auxosporulation (Poulíčková & Mann, Citation2006, review relevant literature). Poulíčková & Mann (Citation2006) suggest that the prolonged survival of superfluous nuclei in Navicula may help mask the effects of deleterious recessive genes in the gametes and suggested, therefore, that species in which this occurs will be predominantly outbreeding (deleterious genes would be rapidly purged by selection in habitual inbreeders: Crnokrak & Barrett Citation2002). The obligate heterothally of P. cf. gibba is consistent with this idea but examination of nuclear behaviour in freely inbreeding and automictic Pinnularia species will provide better tests of our hypothesis.
Zygotes and expanding auxospores of raphid diatoms are often dikaryotic in raphid diatoms (Chepurnov et al., Citation2004), as in P. cf. gibba. Indeed, karyogamy often seems to be the first outward sign that the auxospore has changed its developmental programme, from bipolar expansion to frustule formation. However, the fact that an initial epivalve can also be produced in haploid auxospores, as during haploid parthenogenesis in P. cf. gibba or in asexually produced auxospores (e.g. during apomixis in Eunotia sp. from South America and Achnanthes cf. subsessilis: Chepurnov et al., Citation2004; Sabbe et al. Citation2004) must mean that karyogamy is not an essential precursor to initial cell formation.
Incunabula and other secretions
It used to be thought that the only discrete silica structures present in the walls of diatom auxospores were (i) round, plate-like scales (occasionally with spines) in auxospores that expand isodiametrically, and (ii) the hoop-like bands and split-rings that make up the perizonium or properizonium in auxospores that expand anisometrically (von Stosch, Citation1962, Citation1982; Mann, Citation1982; Round et al., Citation1990). In some cases, plate-like scales and bands can be present in the same species, the scales being produced first (von Stosch Citation1962, Citation1982). Kaczmarska et al. (Citation2001) note, however, that the silica elements of auxospore casings can be much more varied than earlier work indicated, especially in raphid diatoms. For example, in Neidium and Biremis D.G. Mann & E.J. Cox, bipartite silica walls are formed around the zygote; the two halves of the wall later separate and persist as caps over the auxospore poles (Mann, Citation1984, Citation1993).
Until recently, however, nothing had been described resembling the incunabular strips we describe here from Pinnularia species. The first brief report was by Suzuki & Mayama (Citation1995) for P. acidojaponica and Trobajo et al. (Citation2006) have now reported finding a remarkable tangle of narrow, ± transverse strips within the incunabula of Nitzschia fonticola Grun. in Van Heurck. With hindsight (Trobajo et al., Citation2006), it is likely that incunabular strip systems were also seen in a Nitzschia species by Geitler (Citation1932), in which he illustrated irregularly banded auxospores at various stages of development. In early stages, the bands were very closely spaced, whereas later they became separated by hyaline areas. Geitler did not detect a separate perizonium and noted a ‘certain similarity’ between the irregular bands and the more regular perizonia seen by Karsten (e.g. 1899) in other Bacillariaceae. However, Geitler was clearly unconvinced that the two sets of structures were homologous; indeed, he considered that the transverse striations of Nitzschia ‘fonticola’ were more likely to represent folds than thickened strips. Geitler's bands stained with haematoxylin–eosin and must therefore have contained organic components. Unfortunately, Trobajo et al. (Citation2006) were unable to obtain unambiguous information about the elemental composition of their incunabular strips. Our observations show that the strips of P. acidojaponica are siliceous, and this is almost certain in P. cf. gibba also, because they resist oxidation with hot 96% HNO3. It is possible that the silica content varies between Nitzschia and Pinnularia, but the two sets of structures correspond in position and development and are probably homologous, whatever their exact chemical composition. The incunabular strips of N. fonticola seem to be less well bound to each other than the strips of Pinnularia, since they often pulled apart into separate bundles during auxospore expansion (Trobajo et al., Citation2006).
The incunabular strips cannot easily be reconciled with any of the types of silica element described by Kaczmarska et al. (Citation2001). As noted by Trobajo et al. (Citation2006) and confirmed here, the strips cannot be parts of the perizonium, because in both N. fonticola and Pinnularia, a normal transverse perizonium is produced by the auxospore underneath the incunabula. Unlike the much larger perizonial bands produced beneath them, which are formed sequentially during expansion, the system of fine incunabular strips is formed in its entirety before the auxospore starts to enlarge. Indeed, it would be geometrically impossible to secrete the strips through the barrier of the perizonium. Furthermore, unlike the perizonial bands, the strips are only roughly aligned, can cross each other, and seem to be redistributed passively as the auxospore elongates (although in P. acidojaponica the strips remain closely spaced even on mature auxospores). Since extension of the perizonium occurs only at the ends of the auxospore, whereas the incunabula seem to differentiate globally during auxospore expansion, it is likely that the strips (and also the caps of differentiated strip-like or scale-like elements in P. cf. gibba) are embedded together in a cohesive elastic matrix. Conceivably, the incunabular strips are homologous with the round scales present in the auxospore walls of some centric and araphid pennate diatoms (reviewed by Kaczmarska et al., Citation2001; see also Sato et al., Citation2004).
Even though few data are available, it is already clear that the incunabula vary among Pinnulariaceae. The P. cf. gibba incunabula possess robust ± isodiametric, scale-like elements at the auxospore poles, whereas the polar elements of P. acidojaponica incunabula are rather weak and scarcely differ from the strips covering the central part of the auxospore. Furthermore, the strips of P. acidojaponica are extremely narrow (0.1–0.3 μm) compared to those of P. cf. gibba (ca. 1 μm). Incunabular strip systems have not been detected in Caloneis species (Mann, Citation1989a) and Hashizume's (Citation1978, Citation1985) illustrations of Pinnularia viridis (Nitzsch) Ehrenberg show a well-developed perizonium, but no clear evidence of incunabular strips. However, in one photograph where an initial cell is shown escaping from the auxospore (Hashizume, Citation1985, ), the auxospore envelope clearly possesses a very robust cap at the poles, which is shown (op cit., ) to be external to the perizonium. Quite possibly, therefore, the incunabula of P. viridis contain silica elements of some kind, at least at the poles.
In diatoms, silica is always deposited within intracellular ‘silicon deposition vesicles’ and then exocytosed (Pickett-Heaps et al., Citation1990), and this must apply to the formation of the incunabular strips and the more isodiametric silica elements (scales) lying at the poles of Pinnularia auxospores. It is not surprising, therefore, that there is a slight decrease in the diameter of the zygote protoplast during the final phases of its maturation (cf. with 45, 46), as the incunabular strips are secreted and the zygote adopts its final sausage-like shape, prior to expansion.
Cells actively secrete organic material at all stages of auxosporulation in P. cf. gibba. Pairing is accomplished by active movement, involving raphe secretion; diffuse secretions are formed around the gametangia during meiosis; and the gametes produce new material both before and after dehiscence of the gametangia. Besides silica elements, the incunabula of the zygote also contain a wide outer zone of mucilage, which is probably secreted before the silica strips. This material seems to persist during auxospore expansion, but the extent and width of the sheaths around fully expanded auxospores and initial cells (, compare ) suggest that further mucilage is secreted during expansion and this must occur through the pores and slits in and between the fimbriae of the transverse perizonial bands. Later, raphe secretion resumes, allowing the initial cell to escape from the perizonium and surrounding incunabula. However, although secretion takes place during all stages of development, the consistency of the mucilage varies, from the relatively watery material within which the gametangia undergo meiosis and the gametes fuse, to the well-defined capsule present around the zygote. The chemistries of the secretions presumably show a corresponding variation, but there is no information on this for any diatom.
Perizonium
In its overall organization, the transverse perizonium of P. cf. gibba corresponds to that in other pennate diatoms (von Stosch, Citation1962, Citation1982; Round et al., Citation1990). However, P. cf. gibba is remarkable for the extreme differentiation of the primary band, which takes the form of a complete cylinder almost twice as long as broad. In Rhoicosphenia (Mann, Citation1982) and Lyrella (Mann & Stickle, Citation1993), by contrast, the central band is scarcely different from the bands on either side (apart from details of ornamentation and its bifacial nature), and even where the primary band is markedly wider than the other perizonial bands, as in Caloneis silicula (Mann, Citation1989a) and Navicula cryptocephala Kützing (Poulíčková & Mann, Citation2006), it is not an elongate tube as in P. cf. gibba. The significance of this variation is unclear. Since the primary function of the transverse perizonium seems to be to support anisometric expansion of the auxospore (e.g. von Stosch, Citation1962; Mann, Citation1994b), one might expect a relationship between the structure of the perizonium and the shape that the auxospore attains. However, correlations break down even among the few examples studied so far. Thus, wide primary bands are present in the linear auxospore of P. cf. gibba, the centrally expanded auxospore of C. silicula (Mann, Citation1989a), and the rhombic-lanceolate auxospore of N. cryptocephala (Poulíčková & Mann, Citation2006), while narrow primary bands occur in Neidium (linear auxospore: Mann, Citation1984), Craticula cuspidata (Kützing) D.G. Mann in Round et al. and Rhoicosphenia curvata (Kützing) Grunow (lanceolate auxospores: Cohn et al., Citation1989; Mann, Citation1982), and Lyrella atlantica (A.W.F. Schmidt) D.G. Mann (elliptical auxospore: Mann & Stickle, Citation1993).
Only Pinnularia and Nitzschia have been shown to possess incunabular strips and so one might hope to estimate the function of the strip system by looking for aspects of auxospore development that are common to P. cf. gibba and N. fonticola but not shared by other pennate diatoms. Nitzschia fonticola has an unusually broad primary perizonial band, exceeded (among the few diatoms studied) only by P. cf. gibba. Accordingly, we suggest that the strips help the zygote to maintain an elongate shape while it secretes an elongate primary perizonial band, which thereafter constrains auxospore expansion.
The possible absence of a perizonial suture, formed by the aligned split ends of the secondary transverse perizonial bands, needs confirmation. Perizonial sutures can often be detected in LM in raphid diatoms, and a suture is certainly present in Caloneis (Mann, Citation1989a). We have detected split secondary bands in other Pinnularia species (in preparation) and indeed, no pennate diatom has yet been found in which any of the non-primary perizonial bands are complete hoops. We therefore suspect that we were simply unlucky in the orientations of those few auxospores that we were able to study in SEM, and that our LM observations of some kind of longitudinal split in the perizonium are probably accurate. However, it is also possible that the split ends of the secondary bands (if present) are not fully aligned in P. cf. gibba, since we have observed this in another Pinnularia species (in press).
Acknowledgements
This research was supported by project GACR 522/03/0323 and GACR 206/07/0115 from the Czech Republic and a EU Framework 6 SYNTHESYS award (GB-TAF-643) to Aloisie Poulíčková. David Mann is grateful to the Royal Society for an equipment grant allowing purchase of a Polyvar photomicroscope. Frieda Christie provided invaluable help with scanning electron microscopy. We are also very grateful to Dr Michael Möller (Royal Botanic Garden Edinburgh, UK) for advice on DAPI staining protocols and fluorescence microscopy, and Dr Laurence Carvalho (Centre for Ecology and Hydrology, Edinburgh, UK) for advice and data on the three lakes we sampled.
References
- Amato , A , Orsini , L , D'alelio , D and Montresor , M . 2005 . Life cycle, size reduction patterns, and ultrastructure of the pennate planktonic diatom Pseudo-nitzschia delicatissima (Bacillariophyceae) . J. Phycol. , 41 : 542 – 556 .
- Amato , A , Kooistra , WHCF , Levialdi Ghiron , JH , Mann , DG , Pröschold , T and Montresor , M . 2007 . Reproductive isolation among sympatric cryptic species in marine diatoms. . Protist , 158 : 193 – 207 .
- Barker , P . 1875 . Conjugated state of Pinnularia hemiptera . Q, J. Microsc. Sci., new ser. , 15 : 105
- Barrett , SCH , Harder , LD and Worley , AC . 1996 . The comparative biology of pollination and mating in flowering plants . Phil. Trans. R. Soc. Lond. , B 351 : 1271 – 1280 .
- Bayer , MM , Droop , SJM and Mann , DG . 2001 . Digital imaging as a tool in phycological research, with especial reference to microalgae . Phycol. Res. , 49 : 263 – 274 .
- Behnke , A , Friedl , T , Chepurnov , VA and Mann , DG . 2004 . Reproductive compatibility and rDNA sequence analyses in the Sellaphora pupula species complex (Bacillariophyta) . J. Phycol. , 40 : 193 – 208 .
- Carter , HJ . 1865 . Conjugations of Navicula serians, N. rhomboides, and Pinnularia gibba . Ann. Mag. Nat. Hist. , 3, 15 : 161 – 175 .
- Chepurnov , VA and Mann , DG . 1997 . Variation in the sexual behaviour of natural clones of Achnanthes longipes (Bacillariophyta) . Eur. J. Phycol. , 32 : 147 – 154 .
- Chepurnov , VA , Mann , DG , Sabbe , K and Vyverman , W . 2004 . Experimental studies on sexual reproduction in diatoms . Int. Rev. Cytol. , 237 : 91 – 154 .
- Chepurnov , VA , Mann , DG , Sabbe , K , Vannerum , K , Casteleyn , G , Verleyen , I , Peperzak , L and Vyverman , W . 2005 . Sexual reproduction, mating system, chloroplast dynamics and abrupt cell size reduction in Pseudo-nitzschia pungens from the North Sea (Bacillariophyta) . Eur. J. Phycol. , 40 : 379 – 395 .
- Cohn , SA , Spurck , TP , Pickett-Heaps , JD and Edgar , LA . 1989 . Perizonium and initial valve formation in the diatom Navicula cuspidata (Bacillariophyceae) . J. Phycol. , 25 : 15 – 26 .
- Cox , EJ . 1996 . Identification of Freshwater Diatoms from Live Material , London, UK : Chapman & Hall .
- Crnokrak , P and Barrett , SCH . 2002 . Purging the genetic load: a review of the experimental evidence . Evolution , 56 : 2347 – 2358 .
- Edgar , LA . 1980 . Fine structure of Caloneis amphisbaena (Bacillariophyceae) . J. Phycol. , 16 : 62 – 72 .
- Fryxell , GA , Garza , SA and Roelke , DL . 1991 . Auxospore formation in an antarctic clone of Nitzschia subcurvata Hasle . Diatom Res. , 6 : 235 – 245 .
- Geitler , L . 1932 . Der Formwechsel der pennaten Diatomeen (Kieselalgen) . Arch. Protistenk. , 78 : 1 – 226 .
- Geitler , L . 1937 . Sexuelle Fortpflanzung der Diatomeen (Auxosporenbildung) . Tab. Biol. , 6 : 284 – 290 .
- Geitler , L . 1958 . Notizen über Rassenbildung, Fortpflanzung, Formwechsel und morphologische Eigentümlichkeiten bei pennaten Diatomeen . Österr. Bot. Z. , 105 : 408 – 442 .
- Geitler , L . 1969 . Notizen über die Auxosporenbildung einiger pennater Diatomeen . Österr. Bot. Z. , 117 : 265 – 275 .
- Geitler , L . 1973 . Auxosporenbildung und Systematik bei pennaten Diatomeen und die Cytologie von Cocconeis-Sippen . Österr. Bot. Z. , 122 : 299 – 321 .
- Guillard , RRL and Lorenzen , CL . 1972 . Yellow-green algae with chlorophyllide c . J. Phycol. , 8 : 10 – 14 .
- Hashizume , M . 1978 . The formation and germination of the auxospore of . Pinnularia. Iden (Heredity) , 32 : pls 3, 4 (in Japanese)
- Hashizume , M . 1985 . Desmids and Auxospores of Diatoms in Komegane , Komegane : Hashizume . (in Japanese)
- Hustedt , F . 1930 . “ Bacillariophyta ” . In Die Süsswasser-Flora Mitteleuropas , 2 , Edited by: Pascher , A . Vol. 10 , Jena, , Germany : G. Fischer .
- Idei , M . 1991 . “ Achnanthes brevipes C. Agardh var. intermedia (Kützing) Cleve ” . In An Illustrated Atlas of the Life History of Algae , Edited by: Hori , T . Vol. 261 , Tokyo, , Japan : Uchida Rokakuho Publishing Co., Ltd. .
- Idei , M and Mayama , S . 2001 . “ Pinnularia acidojaponica M. Idei et H. Kobayasi sp. nov. and P. valdetolerans Mayama et H. Kobayasi sp. nov. – new diatom taxa from Japanese extreme environments ” . In Lange-Bertalot-Festschrift , Edited by: Jahn , R , Kociolek , JP , Witkowski , A and Compère , P . 265 – 277 . Ruggell, , Liechtenstein : A.R.G. Gantner .
- Jones , HM , Simpson , GE , Stickle , AJ and Mann , DG . 2005 . Life history and systematics of Petroneis (Bacillariophyta), with special reference to British waters . Eur. J. Phycol. , 40 : 43 – 71 .
- Kaczmarska , I , Ehrman , JM and Bates , SS . 2001 . A review of auxospore structure, ontogeny and diatom phylogeny . Proceedings of the 16th International Diatom Symposium . 2001 , University of Athens, Greece. Edited by: Economou-Amilli , A . pp. 153 – 168 .
- Karsten , G . 1899 . Die Diatomeen der Kieler Bucht . Wiss. Meeresunters, n.F. (Kiel) , 4 : 17 – 205 .
- Krammer , K . 1992 . Pinnularia: eine Monographie der europäischen Taxa . Bibliotheca Diatomol. , 26 : 1 – 353 .
- Krammer , K . 2000 . “ The genus Pinnularia ” . In Diatoms of Europe , Edited by: Lange-Bertalot , H . Vol. 1 , 1 – 704 . Ruggell, , Liechtenstein : A.R.G. Gantner .
- Krammer , K and Lange-Bertalot , H . 1986 . “ Bacillariophyceae 1. Teil: Naviculaceae. In ” . In Süsswasserflora von Mitteleuropa , Edited by: Ettl , H , Gerloff , J , Heynig , H and Mollenhauer , D . Vol. 2/1 , Stuttgart, , Germany & New York, USA : G. Fischer .
- Mann , DG . 1982 . Structure, life history and systematics of Rhoicosphenia (Bacillariophyta). II. Auxospore formation and perizonium structure of Rh. curvata . J. Phycol. , 18 : 264 – 274 .
- Mann , DG . 1984 . Auxospore formation and development in Neidium (Bacillariophyta) . Br. Phycol. J. , 19 : 319 – 331 .
- Mann , DG . 1986 . Nitzschia subgenus, Nitzschia (Notes for a monograph of the Bacillariaceae 2) . Proceedings of the 8th International Diatom Symposium . 1986 . Edited by: Ricard , M . pp. 215 – 226 . Koenigstein, , Germany : O. Koeltz .
- Mann , DG . 1988a . The nature of diatom species: analysis of sympatric populations . Proceedings of the 9th International Diatom Symposium . 1988a , Biopress, Bristol, UK & O. Koeltz, Koenigstein, Germany. Edited by: Round , FE . pp. 317 – 327 .
- Mann , DG . 1988b . “ Why didn't Lund see sex in Asterionella? A discussion of the diatom life cycle in nature ” . In Algae and the Aquatic Environment , Edited by: Round , FE . 383 – 412 . Bristol, UK : Biopress .
- Mann , DG . 1989a . On auxospore formation in Caloneis and the nature of Amphiraphia (Bacillariophyta) . Plant Syst. Evol. , 163 : 43 – 52 .
- Mann , DG . 1989b . The species concept in diatoms: evidence for morphologically distinct, sympatric gamodemes in four epipelic species . Plant Syst. Evol. , 164 : 215 – 237 .
- Mann , DG . 1990 . “ Evidence from sexual reproduction and protoplast structure concerning the relationships of the heterovalvar diatom Campylopyxis ” . In Ouvrage dédié à la Mémoire du Professeur Henry Germain , Edited by: Ricard , M . 169 – 179 . Koenigstein, , Germany : Koeltz Scientific Publications .
- Mann , DG . 1993 . Patterns of sexual reproduction in diatoms . Hydrobiologia. , 269/270 : 11 – 20 .
- Mann , DG . 1994a . Auxospore formation, reproductive plasticity and cell structure in Navicula ulvacea and the resurrection of the genus Dickieia (Bacillariophyta) . Eur. J. Phycol. , 29 : 141 – 157 .
- Mann , DG . 1994b . “ The origins of shape and form in diatoms: the interplay between morphogenetic studies and systematics ” . In Shape and Form in Plants and Fungi , Edited by: Ingram , DS and Hudson , AJ . 17 – 38 . London, UK : Academic Press .
- Mann , DG . 1996a . The systematics of Stauroneis (Bacillariophyta): sexual reproduction and auxospore development in . S. anceps var. siberica. Nova Hedwigia, Beih. , 112 : 307 – 319 .
- Mann , DG . 1996b . “ Chloroplast morphology, movements and inheritance in diatoms ” . In Cytology, Genetics and Molecular Biology of Algae , Edited by: Chaudhary , BR and Agrawal , SB . 249 – 274 . Amsterdam, , The Netherlands : SPB Academic Publishing .
- Mann , DG . 2001 . A discussion of Caloneis and related genera . Diatom. , 17 : 29 – 36 .
- Mann , DG and Chepurnov , VA . 2005 . Auxosporulation, mating system, and reproductive isolation in Neidium (Bacillariophyta) . Phycologia. , 44 : 335 – 350 .
- Mann , DG and Stickle , AJ . 1991 . The genus . Craticula. Diatom Res. , 6 : 79 – 107 .
- Mann , DG and Stickle , AJ . 1993 . Life history and systematics of . Lyrella. Nova Hedwigia, Beih , 106 : 43 – 70 .
- Mann , DG and Stickle , AJ . 1996 . The systematics of Stauroneis (Bacillariophyta) II. The life history of S. phoenicenteron and related species . Diatom Res. , 10 : 277 – 297 .
- Mann , DG , Chepurnov , VA and Droop , SJM . 1999 . Sexuality, incompatibility, size variation, and preferential polyandry in natural populations and clones of Sellaphora pupula (Bacillariophyceae) . J. Phycol. , 35 : 152 – 170 .
- Mann , DG , Chepurnov , VA and Idei , M . 2003 . Mating system, sexual reproduction, and auxosporulation in the anomalous raphid diatom Eunotia (Bacillariophyta) . J. Phycol. , 39 : 1067 – 1084 .
- Mann , DG , Mcdonald , SM , Bayer , MM , Droop , SJM , Chepurnov , VA , Loke , RE , Ciobanu , A and Du Buf , JMH . 2004 . Morphometric analysis, ultrastructure and mating data provide evidence for five new species of Sellaphora (Bacillariophyceae) . Phycologia , 43 : 459 – 482 .
- Palmer , MA and Roy , DB . 2001 . “ An estimate of the extent of dystrophic, oligotrophic, mesotrophic and eutrophic standing fresh water in Great Britain ” . In JNCC Report 317 , Peterborough, , UK : Joint Nature Conservation Committee .
- Pfitzer , E . 1871 . Untersuchungen über Bau und Entwicklung der Bacillariaceen (Diatomaceen) . Bot. Abhandl. (ed. Hanstein) , 1 : 1 – 189 .
- Pickett-Heaps , JD , Schmid , A-MM and Edgar , LA . 1990 . The cell biology of diatom valve formation . Progr. Phycol. Res. , 7 : 1 – 168 .
- Poulíčková , A and Mann , DG . 2006 . Sexual reproduction in Navicula cryptocephala (Bacillariophyceae) . J. Phycol. , 42 : 872 – 886 .
- Poulíčková , A and Mann , DG . 2008 . Autogamous auxosporulation in Pinnularia nodosa (Bacillariophyceae) . J. Phycol. , 44 in press
- Richards , AJ . 1997 . Plant Breeding Systems , 2 , London, , UK : Chapman & Hall .
- Roshchin , AM and Chepurnov , VA . 1999 . Dioecy and monoecy in the pennate diatoms (with reference to the centric taxa) . Proceedings of the 14th International Diatom Symposium . 1999 . Edited by: Mayama , M , Idei , M and Koizumi , I . pp. 241 – 261 . Koenigstein, , Germany : Koeltz Scientific Books .
- Ross , R , Cox , EJ , Karayeva , NI , Mann , DG , Paddock , TBB , Simonsen , R and Sims , PA . 1979 . An emended terminology for the siliceous components of the diatom cell . Nova Hedwigia, Beih. , 64 : 513 – 530 .
- Round , FE . 1953 . An investigation of two benthic algal communities in Malham Tarn, Yorkshire . J. Ecol. , 41 : 174 – 179 .
- Round , FE , Crawford , RM and Mann , DG . 1990 . The Diatoms. Biology and Morphology of the Genera , Cambridge, UK : Cambridge University Press .
- Sabbe , K , Chepurnov , VA , Vyverman , W and Mann , DG . 2004 . Apomixis in Achnanthes (Bacillariophyceae); development of a model system for diatom reproductive biology . Eur. J. Phycol. , 39 : 327 – 341 .
- Sato , S , Nagumo , T and Tanaka , J . 2004 . Auxospore formation and the morphology of the initial cell of the marine araphid diatom Gephyria media (Bacillariophyceae) . J. Phycol. , 40 : 684 – 691 .
- Schmidt , A . 1876 . Atlas der Diatomaceenkunde, part 11. , Leipzig, , Germany : R. Reisland .
- Schumann , J . 1869 . Beiträge zur Naturgeschichte der Diatomeen . Verh. zool.-bot. Ges. Wien , 19 : 693 – 722 .
- Silvertown , J and Charlesworth , D . 2001 . Introduction to Plant Population Biology , 4 , Oxford, UK : Blackwell Scientific Publications .
- Suzuki , S and Mayama , S . 1995 . Auxospore of Pinnularia sp. which has long been identified as Pinnularia braunii var. amphicephala (Mayer) Hustedt in Japan and the size reduction of the vegetative valve . Diatom , 11 : 76 – 78 .
- Thwaites , GHK . 1847 . On conjugation in the Diatomaceae. . Ann. Mag. Nat. Hist., ser. 1 , 20 : 9–11 – 343–344 .
- Trobajo , R , Mann , DG , Chepurnov , VA , Clavero , E and Cox , EJ . 2006 . Taxonomy, life cycle and auxosporulation of Nitzschia fonticola (Bacillariophyta) . J. Phycol. , 42 : 1353 – 1372 .
- Tschermak-Woess , E . 1953 . Über auffallende Strukturen in den Pyrenoiden einiger Naviculoiden . Österr. Bot. Z. , 100 : 160 – 178 .
- Underwood , GJC and Paterson , DM . 2003 . The importance of extracellular carbohydrate production by marine epipelic diatoms . Adv. Bot. Res. , 40 : 183 – 240 .
- Vanlandingham , SL . 1978 . Catalogue of the fossil and recent genera and species of diatoms and their synonyms. Part 6: Neidium through Rhoicosigma . J. Cramer, Vaduz, Liechtenstein ,
- Von Stosch , HA . 1962 . Über das Perizonium der Diatomeen . Vortr. Gesamtgeb. Bot. , 1 : 43 – 52 .
- Von Stosch , HA . 1982 . On auxospore envelopes in diatoms . Bacillaria , 5 : 127 – 156 .
- Wingfield , RA , Murphy , KJ , Hollingsworth , P and Gaywood , MJ . 2004 . “ The ecology of Najas flexilis ” . In Scottish Natural Heritage Commissioned Report No. 017 , Edinburgh, , UK : Scottish Natural Heritage .